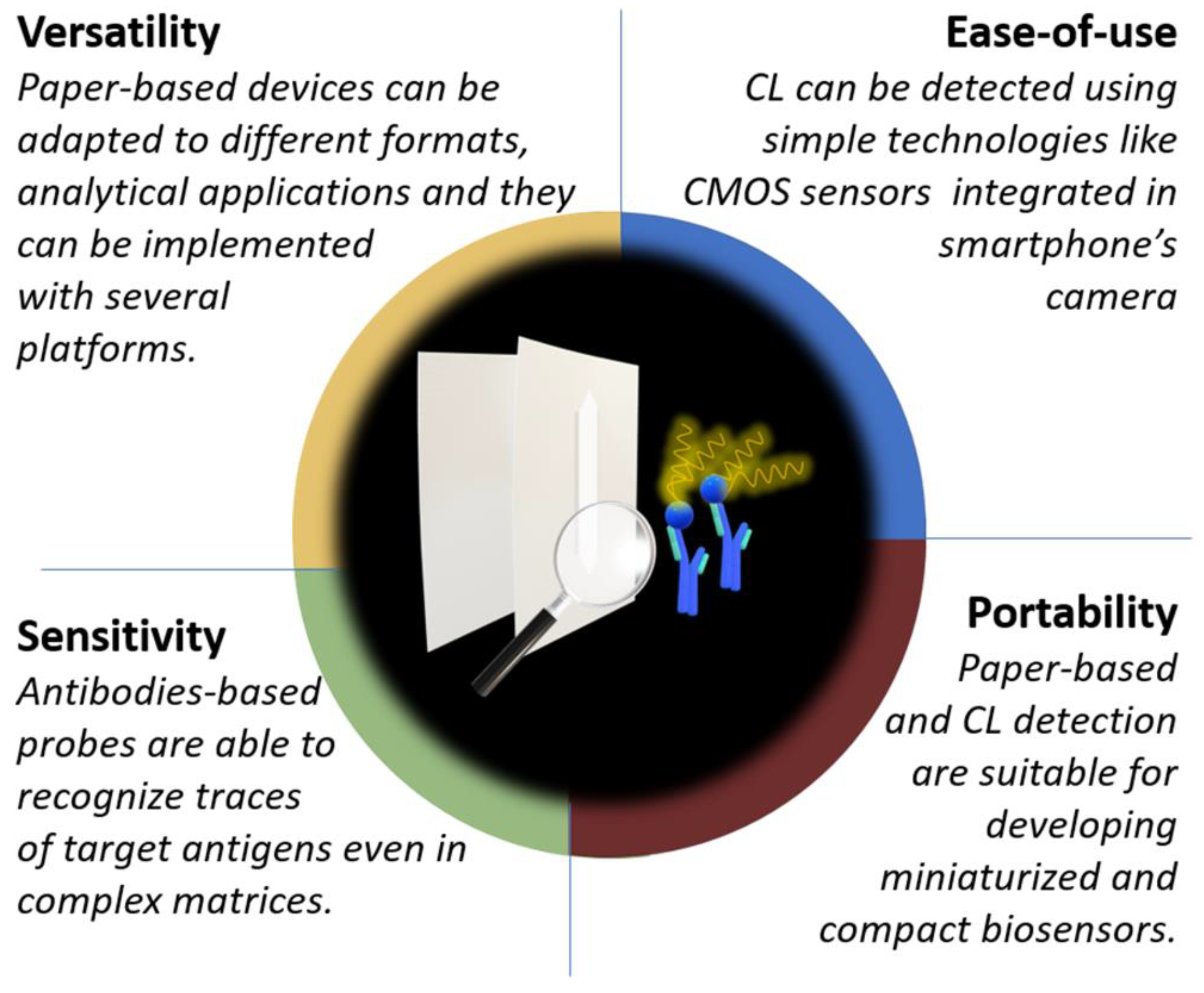
Paper-Based Immunosensors with Bio-Chemiluminescence Detection
Abstract
:1. Introduction
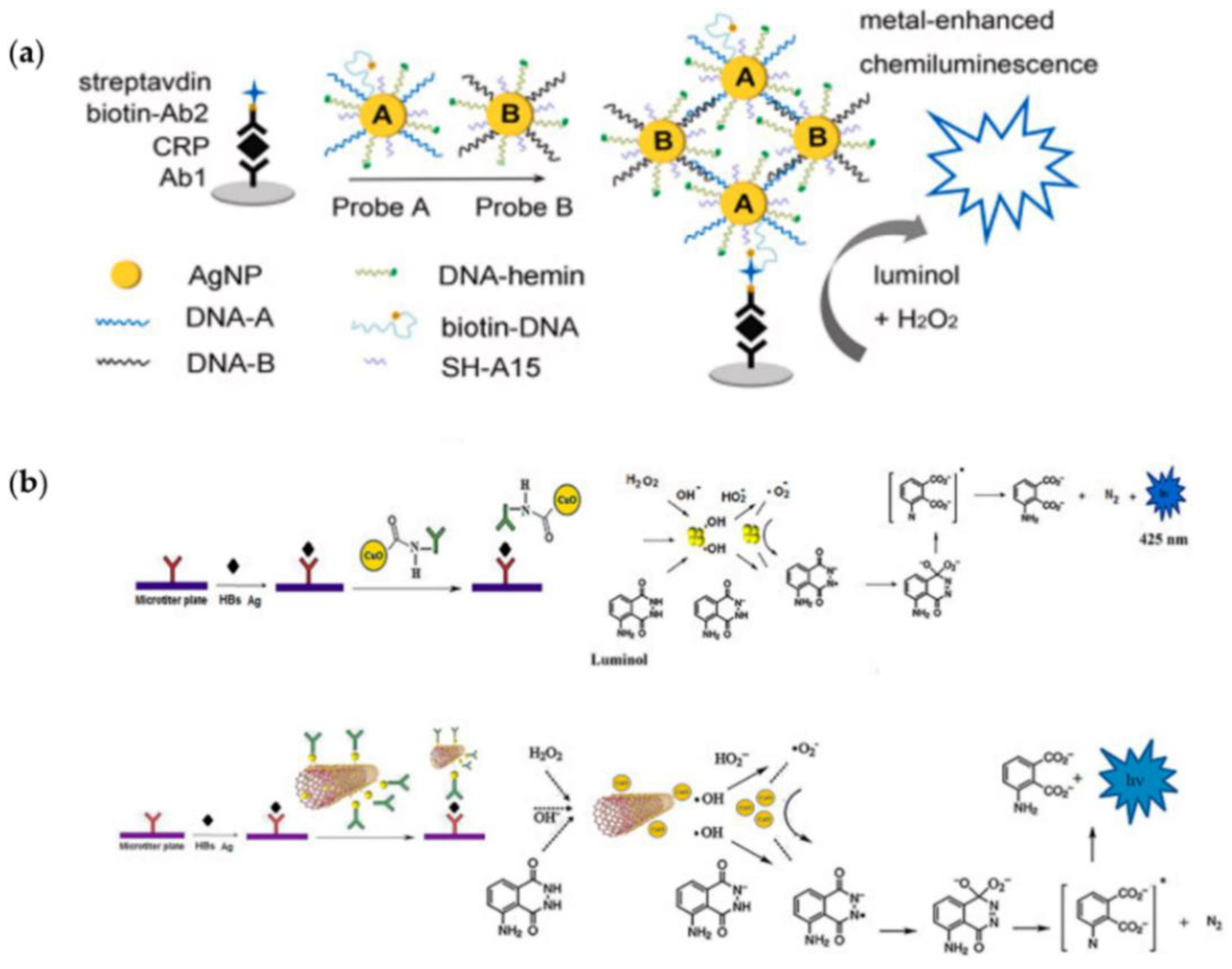
2. Nanomaterials for Signal Enhancement of Chemiluminescence
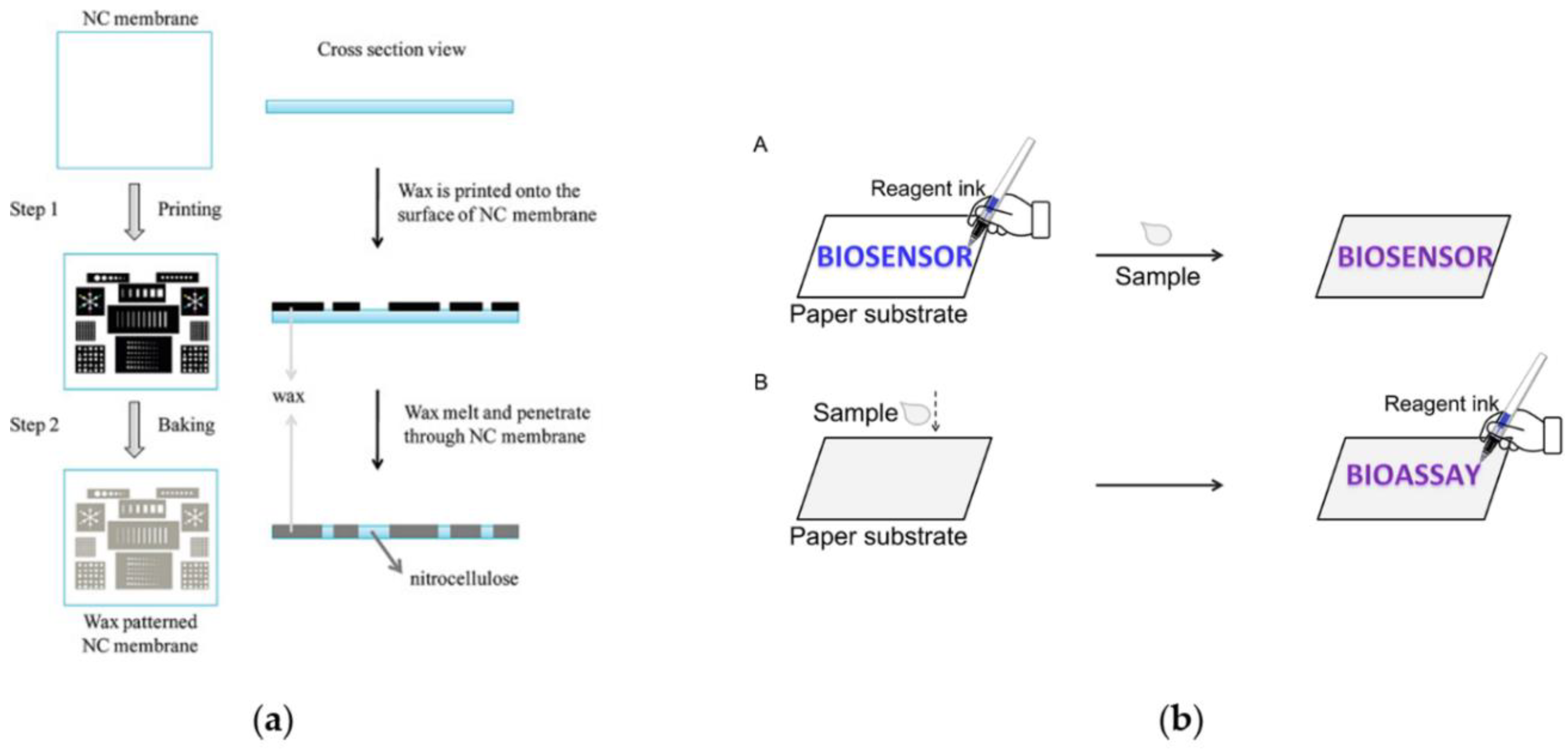
3. Fluid Control and Fluid Handling
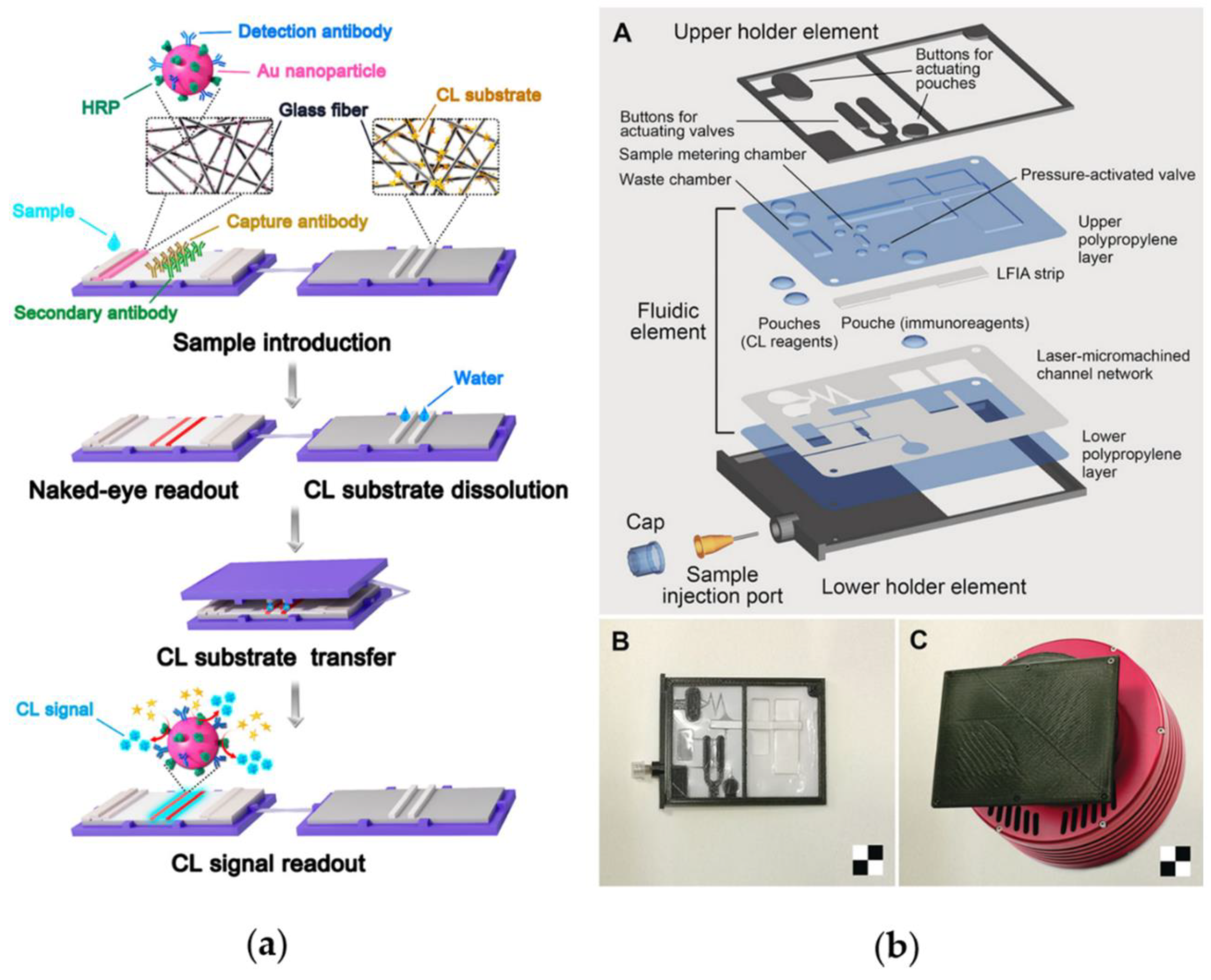
4. Progress in Reagents Storage and Self-Contained Devices for POC Application
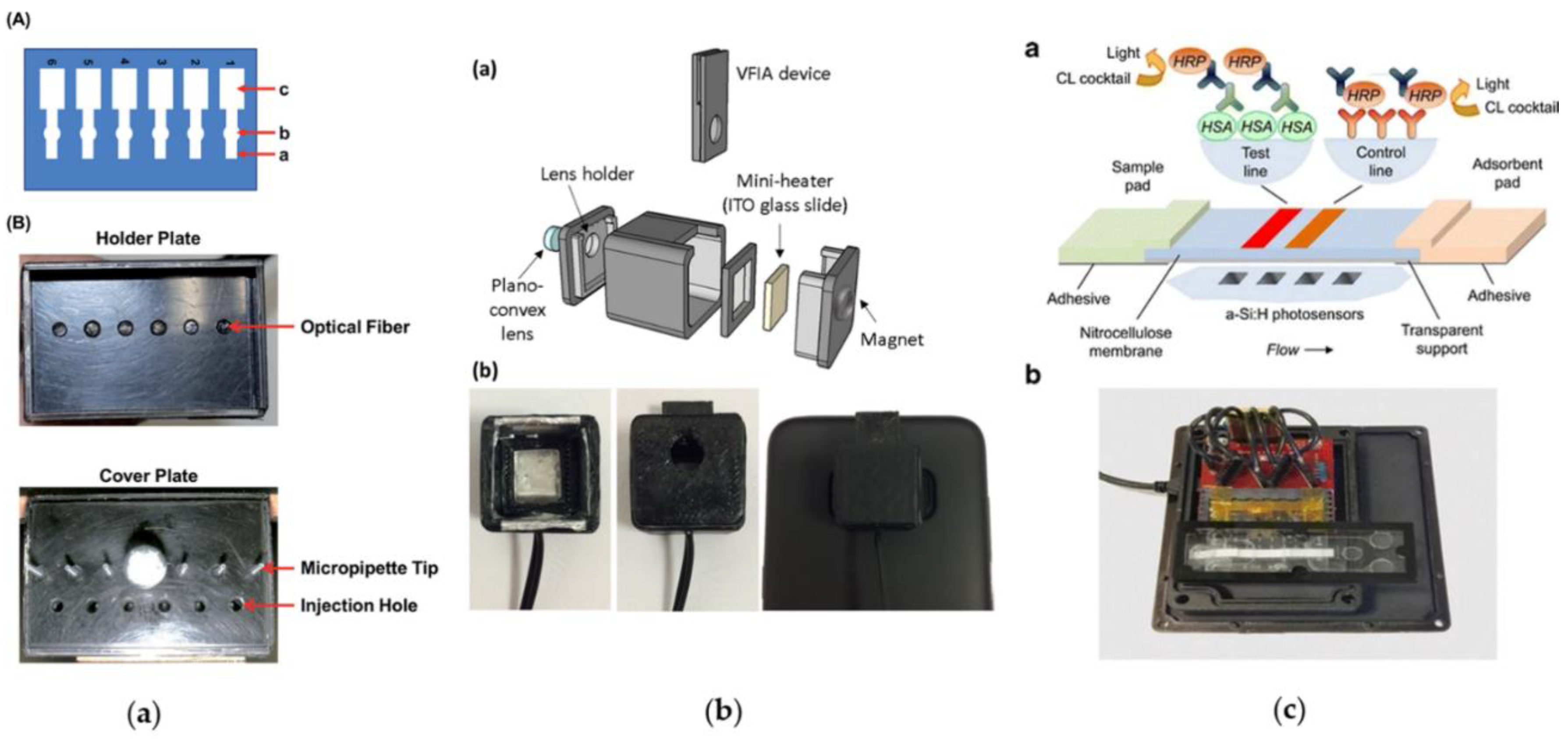
5. Light Detection Technologies Integrated with CL Paper-Based Immunoassay Devices
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, F.; You, M.; Li, S.; Hu, J.; Liu, C.; Gong, Y.; Yang, H.; Xu, F. Paper-based point-of-care immunoassays: Recent advances and emerging trends. Biotechnol. Adv. 2020, 39, 107442. [Google Scholar] [CrossRef]
- Zhu, G.; Yin, X.; Jin, D.; Zhang, B.; Gu, Y.; An, Y. Paper-based immunosensors: Current trends in the types and applied detection techniques. Trends Anal. Chem. 2019, 111, 100–117. [Google Scholar] [CrossRef]
- Ozer, T.; McMahon, C.; Henry, C.S. Advances in Paper-Based Analytical Devices. Annu. Rev. Anal. Chem. 2020, 13, 85–109. [Google Scholar] [CrossRef] [Green Version]
- Al Mughairy, B.; Al-Lawati, H.A. Recent analytical advancements in microfluidics using chemiluminescence detection systems for food analysis. Trends Analyt. Chem. 2020, 124, 115802. [Google Scholar] [CrossRef]
- Akyazi, T.; Basabe-Desmonts, L.; Benito-Lopez, F. Review on microfluidic paper-based analytical devices towards commercialisation. Anal. Chim. Acta 2018, 1001, 1–17. [Google Scholar] [CrossRef]
- Gai, H.; Li, Y.; Yeung, E.S. Optical detection systems on microfluidic chips. In Microfluidics; Lin, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 304, pp. 171–201. [Google Scholar]
- Kuswandi, B.; Huskens, J.; Verboom, W. Optical sensing systems for microfluidic devices: A review. Anal. Chim. Acta 2007, 601, 141–155. [Google Scholar] [CrossRef]
- Baker, C.A.; Duong, C.T.; Grimley, A.; Roper, M.G. Recent advances in microfluidic detection systems. Bioanalysis 2009, 1, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, M.; Namasivayam, V.; Lin, R.; Pal, R.; Burns, M.A. Microfabricated reaction and separation systems. Curr. Opin. Biotechnol. 2001, 12, 92–98. [Google Scholar] [CrossRef]
- Roda, A.; Guardigli, M. Analytical chemiluminescence and bioluminescence: Latest achievements and new horizons. Anal. Bioanal. Chem. 2012, 402, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.I.A.O.; Jin-Ming, L.I.N. Advances and applications of chemiluminescence immunoassay in clinical diagnosis and foods safety. Chin. J. Anal. Chem. 2015, 43, 929–938. [Google Scholar]
- Gámiz-Gracia, L.; García-Campaña, A.M.; Huertas-Pérez, J.F.; Lara, F.J. Chemiluminescence detection in liquid chromatography: Applications to clinical, pharmaceutical, environmental and food analysis—A review. Anal. Chim. Acta 2009, 640, 7–28. [Google Scholar] [CrossRef]
- Fereja, T.H.; Hymete, A.; Gunasekaran, T. A Recent Review on Chemiluminescence Reaction, Principle and Application on Pharmaceutical Analysis. ISRN Spectrosc. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Roda, A.; Mirasoli, M.; Michelini, E.; Di Fusco, M.; Zangheri, M.; Cevenini, L.; Roda, B.; Simoni, P. Progress in chemical luminescence-based biosensors: A critical review. Biosens. Bioelectron. 2016, 76, 164–179. [Google Scholar] [CrossRef]
- Roda, A.; Guardigli, M.; Pasini, P.; Mirasoli, M.; Michelini, E.; Musiani, M. Bio-and chemiluminescence imaging in analytical chemistry. Anal. Chim. Acta 2005, 541, 25–35. [Google Scholar] [CrossRef]
- Créton, R.; Jaffe, L.F. Chemiluminescence microscopy as a tool in biomedical research. Biotechniques 2001, 31, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Zomer, G. The nature of chemiluminescent reactions. In Chemiluminescence and Bioluminescence; Springer: Berlin/Heidelberg, Germany, 2010; pp. 51–90. [Google Scholar]
- Marzocchi, E.; Grilli, S.; Della Ciana, L.; Prodi, L.; Mirasoli, M.; Roda, A. Chemiluminescent detection systems of horseradish peroxidase employing nucleophilic acylation catalysts. Anal. Biochem. 2008, 377, 189–194. [Google Scholar] [CrossRef]
- White, E.H.; Rapaport, E.; Seliger, H.H.; Hopkins, T.A. The chemi-and bioluminescence of firefly luciferin: An efficient chemical production of electronically excited states. Bioorg. Chem. 1971, 1, 92–122. [Google Scholar] [CrossRef]
- Niwa, K.; Ichino, Y.; Kumata, S.; Nakajima, Y.; Hiraishi, Y.; Kato, D.I.; Viviani, V.R.; Ohmiya, Y. Quantum yields and kinetics of the firefly bioluminescence reaction of beetle luciferases. Photochem. Photobiol. 2010, 86, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Wouters, S.F.A.; Vugs, W.J.P.; Arts, R.; de Leeuw, N.M.; Teeuwen, R.W.H.; Merkx, M. Bioluminescent Antibodies through Photoconjugation of Protein G–Luciferase Fusion Proteins. Bioconjugate Chem. 2020, 31, 656–662. [Google Scholar] [CrossRef]
- Hwang, B.B.; Engel, L.; Goueli, S.A.; Zegzouti, H. A homogeneous bioluminescent immunoassay to probe cellular signaling pathway regulation. Commun. Biol. 2020, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alsulami, T.; Nath, N.; Flemming, R.; Wang, H.; Zhou, W.; Yu, J.H. Development of a novel homogeneous immunoassay using the engineered luminescent enzyme NanoLuc for the quantification of the mycotoxin fumonisin B1. Biosens. Bioelectron. 2021, 177, 112939. [Google Scholar] [CrossRef] [PubMed]
- Azim, M.A.; Hasan, M.; Ansari, I.H.; Nasreen, F. Chemiluminescence immunoassay: Basic mechanism and applications. Bangladesh J. Nuclear Med. 2015, 18, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Hasanzadeh, M.; Shadjou, N. Advanced nanomaterials for use in electrochemical and optical immunoassays of carcinoembryonic antigen. A review. Microchim. Acta 2017, 184, 389–414. [Google Scholar] [CrossRef]
- Fu, L.M.; Wang, Y.N. Detection methods and applications of microfluidic paper-based analytical devices. Trends Analyt. Chem. 2018, 107, 196–211. [Google Scholar] [CrossRef]
- Arakawa, T.; Prestrelski, S.J.; Kenney, W.C.; Carpenter, J.F. Factors affecting short-term and long-term stabilities of proteins. Adv. Drug Deliv. Rev. 2001, 46, 307–326. [Google Scholar] [CrossRef]
- Miller, K.D.; Barnette, R.; Light, R.W. Stability of adenosine deaminase during transportation. Chest 2004, 126, 1933–1937. [Google Scholar] [CrossRef]
- Jahanshahi-Anbuhi, S.; Pennings, K.; Leung, V.; Liu, M.; Carrasquilla, C.; Kannan, B.; Li, Y.; Pelton, R.; Brennan, J.D.; Filipe, C.D. Pullulan encapsulation of labile biomolecules to give stable bioassay tablets. Angew. Chem. Int. Ed. Engl. 2014, 53, 6155–6158. [Google Scholar] [CrossRef]
- Hall, M.P.; Kincaid, V.A.; Jost, E.A.; Smith, T.P.; Hurst, R.; Forsyth, S.K.; Fitzgerald, C.; Ressler, V.T.; Zimmermann, K.; Lazar, D.; et al. Toward a Point-of-Need Bioluminescence-Based Immunoassay Utilizing a Complete Shelf-Stable Reagent. Anal. Chem. 2021, 93, 5177–5184. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, C. Research progress on chemiluminescence immunoassay combined with novel technologies. Trends Analyt. Chem. 2020, 124, 115780. [Google Scholar] [CrossRef]
- Cinquanta, L.; Fontana, D.E.; Bizzaro, N. Chemiluminescent immunoassay technology: What does it change in autoantibody detection? Auto Immun. Highlights 2017, 8, 9. [Google Scholar] [CrossRef]
- Roda, A.; Arduini, F.; Mirasoli, M.; Zangheri, M.; Fabiani, L.; Colozza, N.; Marchegiani, E.; Simoni, P.; Moscone, D. A challenge in biosensors: Is it better to measure a photon or an electron for ultrasensitive detection? Biosens. Bioelectron. 2020, 155, 112093. [Google Scholar] [CrossRef]
- Su, Y.; Xie, Y.; Hou, X.; Lv, Y. Recent advances in analytical applications of nanomaterials in liquid-phase chemiluminescence. Appl. Spectrosc. Rev. 2014, 49, 201–232. [Google Scholar] [CrossRef]
- Han, S.; Zhang, Z.; Li, S.; Qi, L.; Xu, G. Chemiluminescence and electrochemiluminescence applications of metal nanoclusters. Sci. China Chem. 2016, 59, 794–801. [Google Scholar] [CrossRef]
- Li, N.; Liu, D.; Cui, H. Metal-nanoparticle-involved chemiluminescence and its applications in bioassays. Anal. Bioanal. Chem. 2014, 406, 5561–5571. [Google Scholar] [CrossRef]
- Han, G.R.; Ki, H.; Kim, M.G. Automated, universal, and mass-producible paper-based lateral flow biosensing platform for high-performance point-of-care testing. ACS Appl. Mater. Interfaces 2019, 12, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Guo, L.; Hu, Y.; Li, Z.; Liu, J.; He, J.; Cui, H. Multiplexed chemiluminescence determination of three acute myocardial infarction biomarkers based on microfluidic paper-based immunodevice dual amplified by multifunctionalized gold nanoparticles. Talanta 2020, 207, 120346. [Google Scholar] [CrossRef]
- Zong, C.; Zhang, D.; Jiang, F.; Yang, H.; Liu, S.; Li, P. Metal-enhanced chemiluminescence detection of C-reaction protein based on silver nanoparticle hybrid probes. Talanta 2019, 199, 164–169. [Google Scholar] [CrossRef]
- Khataee, A.; Hasanzadeh, A.; Iranifam, M.; Joo, S.W. A novel flow-injection chemiluminescence method for determination of baclofen using L-cysteine capped CdS quantum dots. Sens. Actuators B Chem. 2015, 215, 272–282. [Google Scholar] [CrossRef]
- Chen, H.; Lin, L.; Li, H.; Lin, J.-M. Quantum dots-enhanced chemiluminescence: Mechanism and application. Coord. Chem. Rev. 2014, 263–264, 86–100. [Google Scholar] [CrossRef]
- Deng, S.; Lei, J.; Huang, Y.; Cheng, Y.; Ju, H. Electrochemiluminescent quenching of quantum dots for ultrasensitive immunoassay through oxygen reduction catalyzed by nitrogen-doped graphene-supported hemin. Anal. Chem. 2013, 85, 5390–5396. [Google Scholar] [CrossRef]
- Zhao, P.; Zhou, L.; Nie, Z.; Xu, X.; Li, W.; Huang, Y.; He, K.; Yao, S. Versatile electrochemiluminescent biosensor for protein-nucleic acid interaction based on the unique quenching effect of deoxyguanosine-5’-phosphate on electrochemiluminescence of CdTe/ZnS quantum dots. Anal. Chem. 2013, 85, 6279–6286. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, X.; Yu, Y.; Zou, G. A Monochromatic Electrochemiluminescence Sensing Strategy for Dopamine with Dual-Stabilizers-Capped CdSe Quantum Dots as Emitters. Anal. Chem. 2014, 86, 2784–2788. [Google Scholar] [CrossRef]
- Jie, G.; Zhao, Y.; Niu, S. Amplified electrochemiluminescence detection of cancer cells using a new bifunctional quantum dot as signal probe. Biosens. Bioelectron. 2013, 50, 368–372. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shena, W.; Gao, Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Chen, Y.; Chu, W.; Liu, W.; Guo, X.; Jin, Y.; Li, B. Paper-based chemiluminescence immunodevice for the carcinoembryonic antigen by employing multi-enzyme carbon nanosphere signal enhancement. Microchim. Acta 2018, 185, 187. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ge, S.; Wang, S.; Yan, M.; Ge, L.; Yu, J. Highly sensitive chemiluminescence immunoassay on chitosan membrane modified paper platform using TiO2 nanoparticles/multiwalled carbon nanotubes as label. Luminescence 2013, 28, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Ehsani, M.; Chaichi, M.J.; Hosseini, S.N. Comparison of CuO nanoparticle and CuO/MWCNT nanocomposite for amplification of chemiluminescence immunoassay for detection of the hepatitis B surface antigen in biological samples. Sens. Actuators B Chem. 2017, 247, 319–328. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Cui, H.; Lai, C.Z.; Liu, L.J. Gold nanoparticle-catalyzed luminol chemiluminescence and its analytical applications. Anal. Chem. 2005, 77, 3324–3329. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, B. Carbon dots-initiated luminol chemiluminescence in the absence of added oxidant. Carbon 2015, 82, 459–469. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, Y.; Pan, R.; Li, M.; Wang, R.; Chen, S.; Fu, S.; Man, C. A novel, simple and low-cost paper-based analytical device for colorimetric detection of Cronobacter spp. Anal. Chim. Acta 2018, 1036, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kang, B.H.; Jeong, K.H. Based biochip assays and recent developments: A review. BioChip J. 2018, 12, 1–10. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D. Paper-based electrochemical devices in biomedical field: Recent advances and perspectives. In Past, Present, and Future Challenges of Biosensors and Bioanalytical Tools in Analytical Chemistry: A Tribute to Professor Marco Mascini; Palchetti, I., Hansen, P.D., Mascini, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 385–413. [Google Scholar]
- LuLu, Y.; Shi, W.; Qin, J.; Lin, B. Fabrication and Characterization of Paper-Based Microfluidics Prepared in Nitrocellulose Membrane by Wax Printing. Anal. Chem. 2010, 82, 329–335. [Google Scholar] [CrossRef]
- Liu, S.; Cao, R.; Wu, J.; Guan, L.; Li, M.; Liu, J.; Tian, J. Directly writing barrier-free patterned biosensors and bioassays on paper for low-cost diagnostics. Sens. Actuators B Chem. 2019, 285, 529–535. [Google Scholar] [CrossRef]
- Yang, H.; Kong, Q.; Wang, S.; Xu, J.; Bian, Z.; Zheng, X.; Ma, C.; Ge, S.; Yu, J. Hand-drawn&written pen-on-paper electrochemiluminescence immunodevice powered by rechargeable battery for low-cost point-of-care testing. Biosens. Bioelectron. 2014, 61, 21–27. [Google Scholar] [PubMed]
- Wang, S.; Ge, L.; Song, X.; Yu, J.; Ge, S.; Huang, J.; Zeng, F. Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens. Bioelectron. 2012, 31, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ge, L.; Song, X.; Yan, M.; Ge, S.; Yu, J.; Zeng, F. Simple and covalent fabrication of a paper device and its application in sensitive chemiluminescence immunoassay. Analyst 2012, 137, 3821–3827. [Google Scholar] [CrossRef]
- Zhao, M.; Li, H.; Liu, W.; Guo, Y.; Chu, W. Plasma treatment of paper for protein immobilization on paper-based chemiluminescence immunodevice. Biosens. Bioelectron. 2016, 79, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cassano, C.L.; Xu, X.; Fan, Z.H. Laminated paper-based analytical devices (LPAD) with origami-enabled chemiluminescence immunoassay for cotinine detection in mouse serum. Anal. Chem. 2013, 85, 10270–10276. [Google Scholar] [CrossRef]
- Ge, L.; Wang, S.; Song, X.; Ge, S.; Yu, J. 3D origami-based multifunction-integrated immunodevice: Low-cost and multiplexed sandwich chemiluminescence immunoassay on microfluidic paper-based analytical device. Lab Chip 2012, 12, 3150–3158. [Google Scholar] [CrossRef] [PubMed]
- Montali, L.; Calabretta, M.M.; Lopreside, A.; D’Elia, M.; Guardigli, M.; Michelini, E. Multienzyme chemiluminescent foldable biosensor for on-site detection of acetylcholinesterase inhibitors. Biosens. Bioelectron. 2020, 162, 112232. [Google Scholar] [CrossRef]
- Deng, J.; Jiang, X. Advances in Reagents Storage and Release in Self-Contained Point-of-Care Devices. Adv. Mater. Technol. 2019, 4, 1800625. [Google Scholar] [CrossRef] [Green Version]
- Hitzbleck, M.; Delamarche, E. Reagents in microfluidics: An ‘in’and ‘out’challenge. Chem. Soc. Rev. 2013, 42, 8494–8516. [Google Scholar] [CrossRef]
- Robert, H.; Carlos, D.M. Simple and ultrastable all-inclusive pullulan tablets for challenging bioassays. Chem. Sci. 2016, 7, 2342–2346. [Google Scholar]
- Martinez, A.W. Microfluidic paper-based analytical devices: From POCKET to paper-based ELISA. Bioanalysis 2011, 3, 2589–2592. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yiu, B.; Obermeyer, J.; Filipe, C.D.; Brennan, J.D.; Pelton, R. Effects of temperature and relative humidity on the stability of paper-immobilized antibodies. Biomacromolecules 2012, 13, 559–564. [Google Scholar] [CrossRef]
- Kim, D.; Herr, A.E. Protein immobilization techniques for microfluidic assays. Biomicrofluidics 2013, 7, 041501. [Google Scholar] [CrossRef] [Green Version]
- Holstein, C.A.; Chevalier, A.; Bennett, S.; Anderson, C.E.; Keniston, K.; Olsen, C.; Li, B.; Bales, B.; Moore, D.R.; Fu, E.; et al. Immobilizing affinity proteins to nitrocellulose: A toolbox for paper-based assay developers. Anal. Bioanal. Chem. 2016, 408, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Tian, W.; Shen, W. Polysaccharides as protectants for paper-based analytical devices with antibody. Talanta 2017, 165, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Yang, M.; Wu, J.; Zhang, W.; Jiang, X. A self-contained chemiluminescent lateral flow assay for point-of-care testing. Anal. Chem. 2018, 90, 9132–9137. [Google Scholar] [CrossRef] [PubMed]
- Zangheri, M.; Mirasoli, M.; Guardigli, M.; Di Nardo, F.; Anfossi, L.; Baggiani, C.; Simoni, P.; Benassai, M.; Roda, A. Chemiluminescence-based biosensor for monitoring astronauts’ health status during space missions: Results from the International Space Station. Biosens. Bioelectron. 2019, 129, 260–268. [Google Scholar] [CrossRef]
- Mirasoli, M.; Bonvicini, F.; Dolci, L.S.; Zangheri, M.; Gallinella, G.; Roda, A. Portable chemiluminescence multiplex biosensor for quantitative detection of three B19 DNA genotypes. Anal. Bioanal. Chem. 2013, 405, 1139–1143. [Google Scholar] [CrossRef]
- Mirasoli, M.; Buragina, A.; Dolci, L.S.; Simoni, P.; Anfossi, L.; Giraudi, G.; Roda, A. Chemiluminescence-based biosensor for fumonisins quantitative detection in maize samples. Biosens. Bioelectron. 2012, 32, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Sciutto, G.; Zangheri, M.; Anfossi, L.; Guardigli, M.; Prati, S.; Mirasoli, M.; Di Nardo, F.; Baggiani, C.; Mazzeo, R.; Roda, A. Miniaturized Biosensors to Preserve and Monitor Cultural Heritage: From Medical to Conservation Diagnosis. Angew. Chem. Int. Ed. Engl. 2018, 130, 7507–7511. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Zangheri, M.; Lopreside, A.; Marchegiani, E.; Montali, L.; Simoni, P.; Roda, A. Precision medicine, bioanalytics and nanomaterials: Toward a new generation of personalized portable diagnostics. Analyst 2020, 145, 2841–2853. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, M.M.; Montali, L.; Lopreside, A.; Fragapane, F.; Iacoangeli, F.; Roda, A.; Bocci, V.; D’Elia, M.; Michelini, E. Ultrasensitive on-field luminescence detection using a low-cost silicon photomultiplier (SiPM) device. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Alahmad, W.; Uraisin, K.; Nacapricha, D.; Kaneta, T. A miniaturized chemiluminescence detection system for a microfluidic paper-based analytical device and its application to the determination of chromium (III). Anal. Methods 2016, 8, 5414–5420. [Google Scholar] [CrossRef]
- Zangheri, M.; Di Nardo, F.; Anfossi, L.; Giovannoli, C.; Baggiani, C.; Roda, A.; Mirasoli, M. A multiplex chemiluminescent biosensor for type B-fumonisins and aflatoxin B1 quantitative detection in maize flour. Analyst 2015, 140, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Roda, A.; Mirasoli, M.; Dolci, L.S.; Buragina, A.; Bonvicini, F.; Simoni, P.; Guardigli, M. Portable device based on chemiluminescence lensless imaging for personalized diagnostics through multiplex bioanalysis. Anal. Chem. 2011, 83, 3178–3185. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Álvarez-Diduk, R.; Michelini, E.; Roda, A.; Merkoçi, A. Nano-lantern on paper for smartphone-based ATP detection. Biosens. Bioelectron. 2020, 150, 111902. [Google Scholar] [CrossRef]
- Roda, A.; Calabretta, M.M.; Calabria, D.; Caliceti, C.; Cevenini, L.; Lopreside, A.; Zangheri, M. Smartphone-Based Biosensors. In Past, Present and Future Challenges of Biosensors and Bioanalytical Tools in Analytical Chemistry: A Tribute to Professor Marco Mascini; Elsevier: Amsterdam, The Netherlands, 2017; Volume 77, p. 237. [Google Scholar]
- Roda, A.; Zangheri, M.; Calabria, D.; Mirasoli, M.; Caliceti, C.; Quintavalla, A.; Lombardo, M.; Trombini, C.; Simoni, P. A simple smartphone-based thermochemiluminescent immunosensor for valproic acid detection using 1, 2-dioxetane analogue-doped nanoparticles as a label. Sens. Actuators B Chem. 2019, 279, 327–333. [Google Scholar] [CrossRef]
- Xue, L.; Yu, Q.; Griss, R.; Schena, A.; Johnsson, K. Bioluminescent Antibodies for Point-of-Care Diagnostics. Angew. Chem. Int. Ed. Engl. 2017, 129, 7218–7222. [Google Scholar] [CrossRef] [Green Version]
- Ni, Y.; Arts, R.; Merkx, M. Ratiometric Bioluminescent Sensor Proteins Based on Intramolecular Split Luciferase Complementation. ACS Sens. 2019, 4, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Caputo, D.; de Cesare, G.; Dolci, L.S.; Mirasoli, M.; Nascetti, A.; Roda, A.; Scipinotti, R. Microfluidic chip with integrated a-Si: H photodiodes for chemiluminescence-based bioassays. IEEE Sens. J. 2013, 13, 2595–2602. [Google Scholar] [CrossRef]
- Pereira, A.T.; Pimentel, A.C.; Chu, V.; Prazeres, D.M.F.; Conde, J.P. Chemiluminescent detection of horseradish peroxidase using an integrated amorphous silicon thin-film photosensor. IEEE Sens. J. 2009, 9, 1282–1290. [Google Scholar] [CrossRef]
- Wang, X.; Amatatongchai, M.; Nacapricha, D.; Hofmann, O.; de Mello, J.C.; Bradley, D.D.; de Mello, A.J. Thin-film organic photodiodes for integrated on-chip chemiluminescence detection–application to antioxidant capacity screening. Sens. Actuators B Chem. 2009, 140, 643–648. [Google Scholar] [CrossRef]
- Wojciechowski, J.R.; Shriver-Lake, L.C.; Yamaguchi, M.Y.; Füreder, E.; Pieler, R.; Schamesberger, M.; Winder, C.; Prall, H.J.; Sonnleitner, M.; Ligler, F.S.; et al. Organic photodiodes for biosensor miniaturization. Anal. Chem. 2009, 81, 3455–3461. [Google Scholar] [CrossRef]
- Shim, J.S.; Ahn, C.H. Optical immunosensor using carbon nanotubes coated with a photovoltaic polymer. Biosens. Bioelectron. 2012, 34, 208–214. [Google Scholar] [CrossRef]
- Lin, C.C.; Ko, F.H.; Chen, C.C.; Yang, Y.S.; Chang, F.C.; Wu, C.S. Miniaturized metal semiconductor metal photocurrent system for biomolecular sensing via chemiluminescence. Electrophoresis 2009, 30, 3189–3197. [Google Scholar] [CrossRef]
- Mirasoli, M.; Nascetti, A.; Caputo, D.; Zangheri, M.; Scipinotti, R.; Cevenini, L.; de Cesare, G.; Roda, A. Multiwell cartridge with integrated array of amorphous silicon photosensors for chemiluminescence detection: Development, characterization and comparison with cooled-CCD luminograph. Anal. Bioanal. Chem. 2014, 406, 5645–5656. [Google Scholar] [CrossRef]
- Nascetti, A.; Mirasoli, M.; Marchegiani, E.; Zangheri, M.; Costantini, F.; Porchetta, A.; Iannascoli, N.; Lovecchio, N.; Caputo, D.; de Cesare, G.; et al. Integrated chemiluminescence-based lab-on-chip for detection of life markers in extraterrestrial environments. Biosens. Bioelectron. 2019, 123, 195–203. [Google Scholar] [CrossRef]
- Zangheri, M.; Di Nardo, F.; Mirasoli, M.; Anfossi, L.; Nascetti, A.; Caputo, D.; De Cesare, G.; Guardigli, M.; Roda, A. Chemiluminescence lateral flow immunoassay cartridge with integrated amorphous silicon photosensors array for human serum albumin detection in urine samples. Anal. Bioanal. Chem. 2016, 408, 8869–8879. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Nanomaterial | Enhancement Mechanism Exploiting Luminol/H2O2 CL System | Features | Ref. |
|---|---|---|---|
| Metal nanoparticles (AgNPs, AuNPs, PtNPs, etc.) | MNPs catalyze the decomposition of H2O2 leading to the formation of a hydroxyl radical, which reacts with a luminol anion and HO2−. These species are involved in the production of a luminol radical and a superoxide anion leading to light emission. The radical generation and electron transfer processes take place on the surface of the MNPs, which are therefore responsible for the facilitation of these processes. |
| [50] |
| Quantum dots | Quantum dots act first by decomposing H2O2 to generate free radicals and then promoting CL by energy transfer and electron transfer annihilation effects. |
| [41] |
| Carbon nanomaterials(carbon nanoparticles (CNPs), graphene, graphene oxide (GO) and carbon nanotubes (CNTs)) | A possible mechanism involves the reaction between carbon materials with π-rich electronic structures and luminol allowing the formation of the activated transition complex. This complex may accelerate electron-transfer processes during the luminol-dissolved oxygen CL reaction. |
| [51] |
| Detector for CL Paper-Based Immunosensor | Detection Principle | Pros | Cons |
|---|---|---|---|
| Photomultiplier tube (PMT) | Each PMT can detect just one wavelength. It captures emitted photons and its photocathode layer, then converts these photons to electricity. Dynodes are then used to multiply this charge multi-fold, making it readable for the instrument. |
|
|
| Charged coupled device (CCD) | In a CCD image sensor, pixels represent the basic building blocks, and they are composed of p-doped metal–oxide-semiconductor (MOS) capacitors. Pixels allow the conversion of incoming photons into electron charges at the semiconductor-oxide interface; the CCD is then used to read out these charges. |
|
|
| Complementary metal-oxide semiconductor (CMOS) sensor | CMOS has emerged as alternative to CCD. Differently from CCD, each pixel sensor unit has a photodetector. |
|
|
| Thin-film photosensors | There is a wide variety of photosensors based on different materials but they are typically based on a p–n junction that converts light photons into current. The absorbed photons make electron–hole pairs in the depletion region. |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabretta, M.M.; Zangheri, M.; Calabria, D.; Lopreside, A.; Montali, L.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Mirasoli, M.; Michelini, E. Paper-Based Immunosensors with Bio-Chemiluminescence Detection. Sensors 2021, 21, 4309. https://doi.org/10.3390/s21134309
Calabretta MM, Zangheri M, Calabria D, Lopreside A, Montali L, Marchegiani E, Trozzi I, Guardigli M, Mirasoli M, Michelini E. Paper-Based Immunosensors with Bio-Chemiluminescence Detection. Sensors. 2021; 21(13):4309. https://doi.org/10.3390/s21134309
Chicago/Turabian StyleCalabretta, Maria Maddalena, Martina Zangheri, Donato Calabria, Antonia Lopreside, Laura Montali, Elisa Marchegiani, Ilaria Trozzi, Massimo Guardigli, Mara Mirasoli, and Elisa Michelini. 2021. "Paper-Based Immunosensors with Bio-Chemiluminescence Detection" Sensors 21, no. 13: 4309. https://doi.org/10.3390/s21134309
APA StyleCalabretta, M. M., Zangheri, M., Calabria, D., Lopreside, A., Montali, L., Marchegiani, E., Trozzi, I., Guardigli, M., Mirasoli, M., & Michelini, E. (2021). Paper-Based Immunosensors with Bio-Chemiluminescence Detection. Sensors, 21(13), 4309. https://doi.org/10.3390/s21134309









