Activity-Aware Vital Sign Monitoring Based on a Multi-Agent Architecture
Abstract
:1. Introduction
- To build an activity recognition component that uses the accelerometer data obtained from different wearable sensors in order to discriminate between different activities of daily living;
- To automatically extract threshold ranges for different monitored vital signs, from the group of users when performing different physical activities, which are used for evaluating the health status of a target user;
- To implement a multi-agent architecture that is easily adaptable to the sensor network and hardware from different manufacturers, which can process signals from different body sensors, recognize the physical activity, evaluate the user’s health status based on the recognized activity, and alarm when necessary, using ontology-based models to address the data heterogeneity.
2. Background and Related Work
2.1. Vital Sign Monitoring
2.2. Physical Activity Recognition
2.3. Multi-Agent Systems
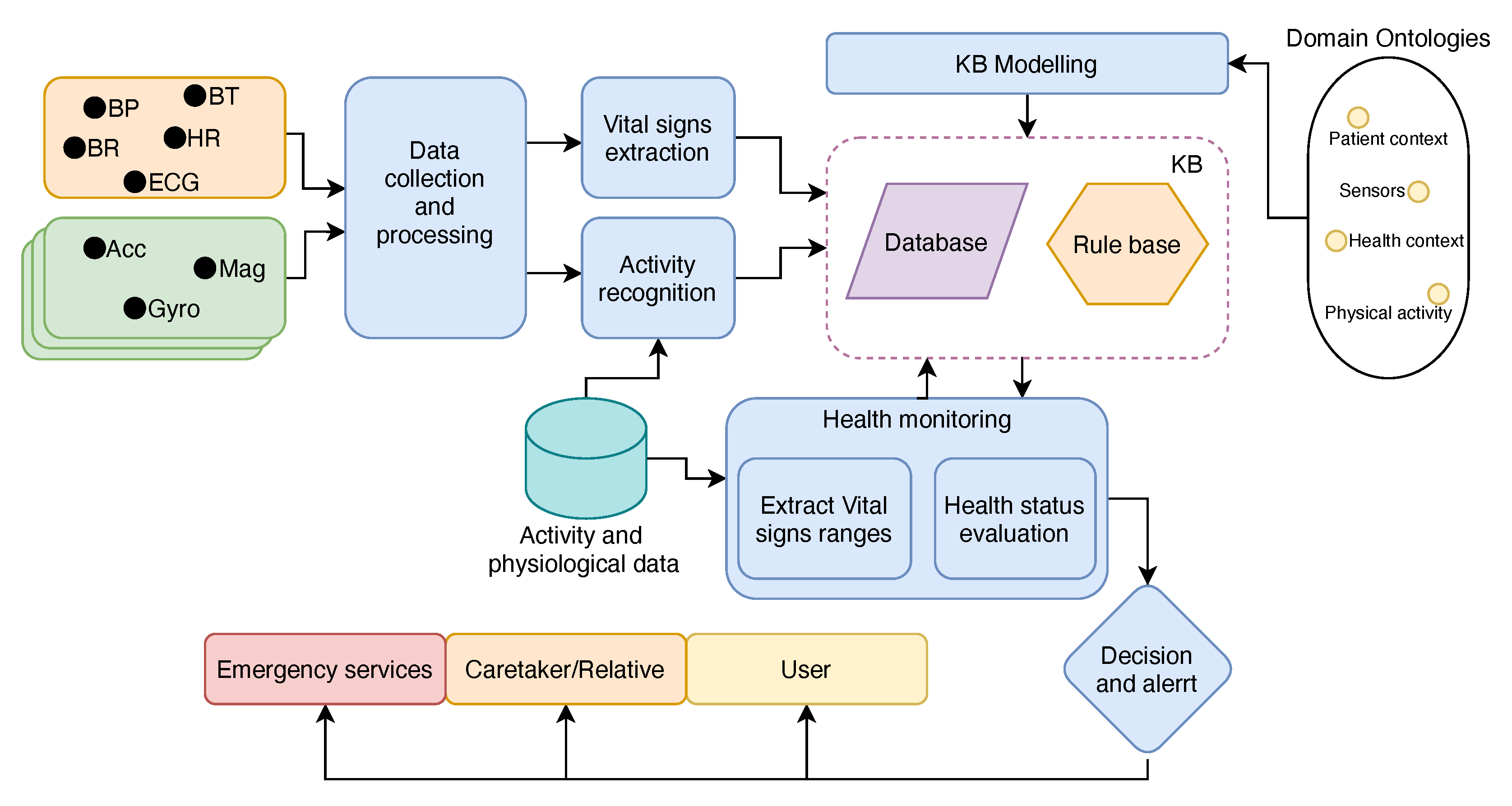
3. Proposed Architecture
3.1. Knowledge Representation and Semantic Modeling
3.2. MAS
3.3. Vital Sign Ranges
3.4. Vital Sign Extraction
3.4.1. HR Agent
3.4.2. BR agent
3.5. Activity Recognition
3.5.1. Signal Processing
- xyz_t—feature vector from the original signal in the time domain;
- xyz_f—feature vector from the FFT components of the original signal;
- xyz_tf—aggregated features from xyz_t and xyz_f;
- mag_t—feature vector from the computed magnitude of the original signal in the time domain;
- mag_f—feature vector from the FFT components of mag_t;
- mag_tf—aggregated features from mag_t and mag_f.
3.5.2. Activity Classification
3.6. Team of Agents
4. Experimental Results and Discussion
4.1. Dataset
4.2. Activity Recognition
4.2.1. Classifiers’ Results
4.2.2. Feature Reduction
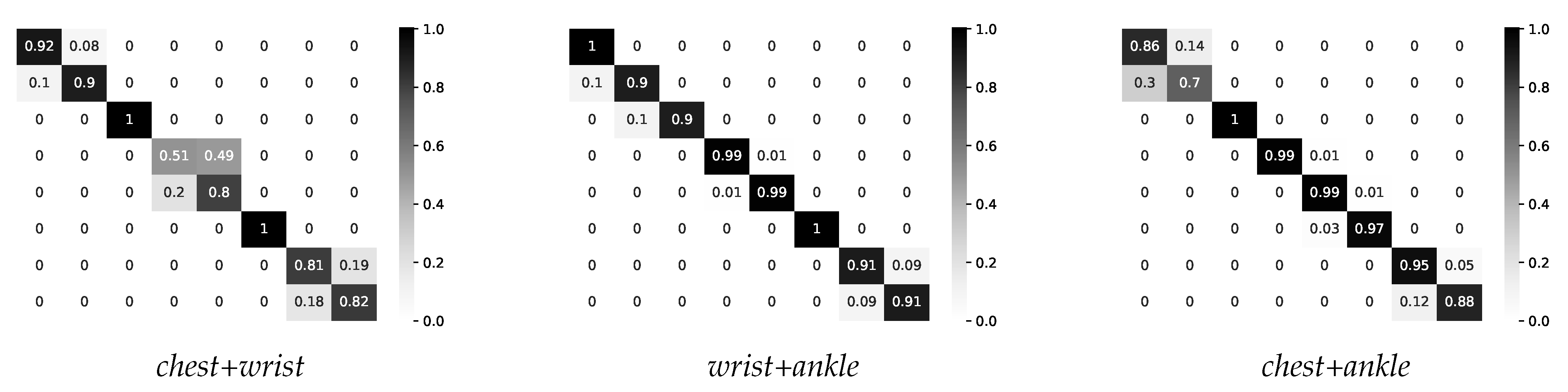
4.2.3. Two-Layer Multimodal Fusion
4.3. Activity-Aware Vital Sign Thresholds
4.4. Health Status Monitoring
5. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Tanaka, T.; Fujita, T.; Sonoda, K.; Nii, M.; Kanda, K.; Maenaka, K.; Alex Chan Chun Kit; Okochi, S.; Higuchi, K. Wearable health monitoring system by using fuzzy logic heart-rate extraction. In Proceedings of the World Automation Congress 2012, Puerto Vallarta, Mexico, 24–28 June 2012; pp. 1–4. [Google Scholar]
- Lee, D.H.; Rabbi, A.; Choi, J.; Fazel-Rezai, R. Development of a Mobile Phone Based e-Health Monitoring Application. Int. J. Adv. Comput. Sci. Appl. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Kannan, S. Wheats: A Wearable Personal Healthcare and Emergency Alert and Tracking System. Eur. J. Sci. Res. 2012, 85, 382–393. [Google Scholar]
- Watanabe, T.; Saito, H. Tests of wireless wearable sensor system in joint angle measurement of lower limbs. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar] [CrossRef]
- Felisberto, F.; Costa, N.; Fdez-Riverola, F.; Pereira, A. Unobstructive Body Area Networks (BAN) for Efficient Movement Monitoring. Sensors 2012, 12, 12473–12488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anania, G.; Tognetti, A.; Carbonaro, N.; Tesconi, M.; Cutolo, F.; Zupone, G.; Rossi, D.D. Development of a novel algorithm for human fall detection using wearable sensors. In Proceedings of the 2008 IEEE Sensors, Lecce, Italy, 26–29 October 2008. [Google Scholar] [CrossRef]
- Baek, W.S.; Kim, D.M.; Bashir, F.; Pyun, J.Y. Real life applicable fall detection system based on wireless body area network. In Proceedings of the 2013 IEEE 10th Consumer Communications and Networking Conference (CCNC), Las Vegas, NV, USA, 11–14 January 2013. [Google Scholar] [CrossRef]
- Felisberto, F.; Fdez.-Riverola, F.; Pereira, A. A Ubiquitous and Low-Cost Solution for Movement Monitoring and Accident Detection Based on Sensor Fusion. Sensors 2014, 14, 8961–8983. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, K.; Pitchers, S.; Barnard, M. Body area networks: Towards a wearable future. In Proceedings of the Wireless World Research Forum (WWRF) Kick off Meeting, Munich, Germany, 6–7 March 2001; pp. 6–7. [Google Scholar]
- Subbe, C.P.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admissions. Qjm 2001, 94, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Hart, J. Normal resting pulse rate ranges. J. Nurs. Educ. Pract. 2015, 5. [Google Scholar] [CrossRef]
- Nealen, P.M. Exercise and lifestyle predictors of resting heart rate in healthy young adults. J. Hum. Sport Exerc. 2016, 11, 348–357. [Google Scholar] [CrossRef] [Green Version]
- Whyte, G.; Sharma, S. Practical ECG for Exercise Science and Sports Medicine; Human Kinetics: Champaign, IL, USA, 2010. [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee report 2008: To the Secretary of Health and Human Services. Nutr. Rev. 2008, 67, 114–120. [Google Scholar] [CrossRef]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M. ACSM’s Guidelines for Exercise Testing and Prescription; Chapter General Principles of Exercise Prescription; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2017. [Google Scholar]
- Barrett, K. Ganong’s Review of Medical Physiology; McGraw-Hill Medical McGraw-Hill Distributor: New York, NY, USA; London, UK, 2012. [Google Scholar]
- Loring, S.H.; Mead, J.; Waggener, T.B. Determinants of breathing frequency during walking. Respir. Physiol. 1990, 82, 177–188. [Google Scholar] [CrossRef]
- Raßler, B.; Kohl, J. Coordination of Breathing and Walking at Different Treadmill Speed and Slope Levels and its Effects on Respiratory Rate and Minute Ventilation. In The Physiology and Pathophysiology of Exercise Tolerance; Springer: New York, NY, USA, 1996; pp. 139–145. [Google Scholar] [CrossRef]
- Nicolò, A.; Massaroni, C.; Passfield, L. Respiratory Frequency during Exercise: The Neglected Physiological Measure. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef]
- Mcllroy, M.B. The respiratory response to exercise. Pediatrics 1963, 32, 680–682. [Google Scholar]
- Liu, G.Z.; Guo, Y.W.; Zhu, Q.S.; Huang, B.Y.; Wang, L. Estimation of Respiration Rate from Three-Dimensional Acceleration Data Based on Body Sensor Network. Telemed. E-Health 2011, 17, 705–711. [Google Scholar] [CrossRef]
- Jetté, M.; Sidney, K.; Blümchen, G. Metabolic equivalents (METS) in exercise testing, exercise prescription, and evaluation of functional capacity. Clin. Cardiol. 1990, 13, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Micucci, D.; Mobilio, M.; Napoletano, P. UniMiB SHAR: A Dataset for Human Activity Recognition Using Acceleration Data from Smartphones. Appl. Sci. 2017, 7, 1101. [Google Scholar] [CrossRef] [Green Version]
- Paul, P.; George, T. An effective approach for human activity recognition on smartphone. In Proceeding of the 2015 IEEE International Conference on Engineering and Technology (ICETECH), Coimbatore, India, 20–20 March 2015. [Google Scholar] [CrossRef]
- Banos, O.; Damas, M.; Pomares, H.; Rojas, I. On the Use of Sensor Fusion to Reduce the Impact of Rotational and Additive Noise in Human Activity Recognition. Sensors 2012, 12, 8039–8054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, A.; Stricker, D. Creating and benchmarking a new dataset for physical activity monitoring. In Proceedings of the 5th International Conference on PErvasive Technologies Related to Assistive Environments—PETRA12; ACM Press: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Cottone, P.; Re, G.L.; Maida, G.; Morana, M. Motion sensors for activity recognition in an ambient-intelligence scenario. In Proceedings of the 2013 IEEE International Conference on Pervasive Computing and Communications Workshops (PERCOM Workshops), San Diego, CA, USA, 18–22 March 2013. [Google Scholar] [CrossRef]
- Htike, K.K.; Khalifa, O.O.; Ramli, H.A.M.; Abushariah, M.A.M. Human activity recognition for video surveillance using sequences of postures. In Proceedings of the The Third International Conference on e-Technologies and Networks for Development (ICeND2014), Beirut, Lebanon, 29 April–1 May 2014. [Google Scholar] [CrossRef]
- Pires, I.M.; Garcia, N.M.; Pombo, N.; Flórez-Revuelta, F.; Spinsante, S.; Teixeira, M.C. Identification of activities of daily living through data fusion on motion and magnetic sensors embedded on mobile devices. Pervasive Mob. Comput. 2018, 47, 78–93. [Google Scholar] [CrossRef]
- Cleland, I.; Kikhia, B.; Nugent, C.; Boytsov, A.; Hallberg, J.; Synnes, K.; McClean, S.; Finlay, D. Optimal Placement of Accelerometers for the Detection of Everyday Activities. Sensors 2013, 13, 9183–9200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sztyler, T.; Stuckenschmidt, H. On-body localization of wearable devices: An investigation of position-aware activity recognition. In Proceedings of the 2016 IEEE International Conference on Pervasive Computing and Communications (PerCom), Sydney, NSW, Australia, 14–19 March 2016. [Google Scholar] [CrossRef]
- Ordóñez, F.; Roggen, D. Deep Convolutional and LSTM Recurrent Neural Networks for Multimodal Wearable Activity Recognition. Sensors 2016, 16, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.; Lim, J.; Noh, K.J.; Kim, G.; Jeong, H. Sensor Data Acquisition and Multimodal Sensor Fusion for Human Activity Recognition Using Deep Learning. Sensors 2019, 19, 1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, R.A.; Pathan, A.S.K. The state-of-the-art wireless body area sensor networks: A survey. Int. J. Distrib. Sens. Netw. 2018, 14, 155014771876899. [Google Scholar] [CrossRef] [Green Version]
- Hasan, K.; Biswas, K.; Ahmed, K.; Nafi, N.S.; Islam, M.S. A comprehensive review of wireless body area network. J. Netw. Comput. Appl. 2019, 143, 178–198. [Google Scholar] [CrossRef]
- Mahdiani, S.; Jeyhani, V.; Peltokangas, M.; Vehkaoja, A. Is 50 Hz high enough ECG sampling frequency for accurate HRV analysis? In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015. [Google Scholar] [CrossRef]
- Kwon, O.; Jeong, J.; Kim, H.B.; Kwon, I.H.; Park, S.Y.; Kim, J.E.; Choi, Y. Electrocardiogram Sampling Frequency Range Acceptable for Heart Rate Variability Analysis. Healthc. Inform. Res. 2018, 24, 198. [Google Scholar] [CrossRef] [PubMed]
- van Gent, P.; Farah, H.; van Nes, N.; van Arem, B. HeartPy: A novel heart rate algorithm for the analysis of noisy signals. Transp. Res. Part F Traffic Psychol. Behav. 2019, 66, 368–378. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Bhattacherjee, S.; Pal, S. Extraction of respiration signal from ECG for respiratory rate estimation. In Proceedings of the Michael Faraday IET International Summit 2015, Kolkata, India, 12–13 September 2015; pp. 336–340. [Google Scholar]
- Bersch, S.; Azzi, D.; Khusainov, R.; Achumba, I.; Ries, J. Sensor Data Acquisition and Processing Parameters for Human Activity Classification. Sensors 2014, 14, 4239–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ni, Q.; Patterson, T.; Cleland, I.; Nugent, C. Dynamic detection of window starting positions and its implementation within an activity recognition framework. J. Biomed. Inform. 2016, 62, 171–180. [Google Scholar] [CrossRef]
- Khusainov, R.; Azzi, D.; Achumba, I.; Bersch, S. Real-Time Human Ambulation, Activity, and Physiological Monitoring: Taxonomy of Issues, Techniques, Applications, Challenges and Limitations. Sensors 2013, 13, 12852–12902. [Google Scholar] [CrossRef] [Green Version]
- Lima, W.S.; Souto, E.; El-Khatib, K.; Jalali, R.; Gama, J. Human Activity Recognition Using Inertial Sensors in a Smartphone: An Overview. Sensors 2019, 19, 3213. [Google Scholar] [CrossRef] [Green Version]
- Banos, O.; Villalonga, C.; Garcia, R.; Saez, A.; Damas, M.; Holgado-Terriza, J.A.; Lee, S.; Pomares, H.; Rojas, I. Design, implementation and validation of a novel open framework for agile development of mobile health applications. BioMed. Eng. OnLine 2015, 14, S6. [Google Scholar] [CrossRef] [Green Version]
- Preece, S.J.; Goulermas, J.Y.; Kenney, L.P.J.; Howard, D. A Comparison of Feature Extraction Methods for the Classification of Dynamic Activities From Accelerometer Data. IEEE Trans. Biomed. Eng. 2009, 56, 871–879. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, M.; Ordieres-Meré, J. Comparison of data preprocessing approaches for applying deep learning to human activity recognition in the context of industry 4.0. Sensors 2018, 18, 2146. [Google Scholar] [CrossRef] [Green Version]
- Castro, D.; Coral, W.; Rodriguez, C.; Cabra, J.; Colorado, J. Wearable-based human activity recognition using an iot approach. J. Sens. Actuator Netw. 2017, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.T.; Billah, M.M.; Hossain, M.F. Random forests based recognition of human activities and postural transitions on smartphone. In Proceedings of the 2016 5th International Conference on Informatics, Electronics and Vision (ICIEV), Dhaka, Bangladesh, 13–14 May 2016. [Google Scholar] [CrossRef]
- Shakshuki, E.; Reid, M. Multi-Agent System Applications in Healthcare: Current Technology and Future Roadmap. Procedia Comput. Sci. 2015, 52, 252–261. [Google Scholar] [CrossRef] [Green Version]
- Su, C.J. Mobile multi-agent based, distributed information platform (MADIP) for wide-area e-health monitoring. Comput. Ind. 2008, 59, 55–68. [Google Scholar] [CrossRef]
- Fuentes-Fernández, R.; Guijarro, M.; Pajares, G. A Multi-Agent System Architecture for Sensor Networks. Sensors 2009, 9, 10244–10269. [Google Scholar] [CrossRef]
- Villarrubia, G.; Bajo, J.; Paz, J.D.; Corchado, J. Monitoring and Detection Platform to Prevent Anomalous Situations in Home Care. Sensors 2014, 14, 9900–9921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmid, M.; Kazar, O.; Benharzallah, S.; Kahloul, L.; Merizig, A. An Intelligent and Secure Health Monitoring System Based on Agent. In Proceedings of the 2020 IEEE International Conference on Informatics, IoT, and Enabling Technologies (ICIoT), Doha, Qatar, 2–5 February 2020. [Google Scholar] [CrossRef]
- Armentia, A.; Gangoiti, U.; Priego, R.; Estévez, E.; Marcos, M. Flexibility Support for Homecare Applications Based on Models and Multi-Agent Technology. Sensors 2015, 15, 31939–31964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strang, T.; Linnhoff-Popien, C. A Context Modeling Survey. In Proceedings of the First International Workshop on Advanced Context Modelling, Reasoning And Management at UbiComp 2004, Nottingham, UK, 7 September 2004. [Google Scholar]
- Goldfain, A.; Smith, B.; Arabandi, S.; Brochhausen, M.; Hogan, W. Vital Sign Ontology. In Proceedings of the 14th Annual Bio-Ontologies Meeting, Vienna, Austria, 15–16 July 2011. [Google Scholar]
- Kim, H.; Mentzer, J.; Taira, R. Developing a Physical Activity Ontology to Support the Interoperability of Physical Activity Data. J. Med Internet Res. 2019, 21, e12776. [Google Scholar] [CrossRef] [PubMed]
- Compton, M.; Barnaghi, P.; Bermudez, L.; García-Castro, R.; Corcho, O.; Cox, S.; Graybeal, J.; Hauswirth, M.; Henson, C.; Herzog, A.; et al. The SSN ontology of the W3C semantic sensor network incubator group. J. Web Semant. 2012, 17, 25–32. [Google Scholar] [CrossRef]
- Villalonga, C.; Pomares, H.; Rojas, I.; Banos, O. MIMU-Wear: Ontology-based sensor selection for real-world wearable activity recognition. Neurocomputing 2017, 250, 76–100. [Google Scholar] [CrossRef]
- Rhayem, A.; Mhiri, M.B.A.; Gargouri, F. HealthIoT Ontology for Data Semantic Representation and Interpretation Obtained from Medical Connected Objects. In Proceedings of the 2017 IEEE/ACS 14th International Conference on Computer Systems and Applications (AICCSA), Hammamet, Tunisia, 30 October–3 November 2017. [Google Scholar] [CrossRef]
- Ivascu, T. An Energy Efficient Intelligent Wireless Body Area Network for Real-Time Vital Signs Monitoring. In Proceedings of the 2015 17th International Symposium on Symbolic and Numeric Algorithms for Scientific Computing (SYNASC), Timisoara, Romania, 21–24 September 2015. [Google Scholar] [CrossRef]
- Van Gent, P.; Farah, H.; Nes, N.; van Arem, B. Heart rate analysis for human factors: Development and validation of an open source toolkit for noisy naturalistic heart rate data. In Proceedings of the 6th HUMANIST Conference, The Hague, The Netherlands, 13–14 June 2018; pp. 173–178. [Google Scholar]
- Orphanidou, C.; Bonnici, T.; Charlton, P.; Clifton, D.; Vallance, D.; Tarassenko, L. Signal Quality Indices for the Electrocardiogram and Photoplethysmogram: Derivation and Applications to Wireless Monitoring. IEEE J. Biomed. Health Inform. 2014, 1. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Hara, Y.; Findley, T.; Yoncmoto, K. A useful method for measuring daily physical activity by a three-direction monitor. Scand. J. Rehabil. Med. 1997, 29, 37. [Google Scholar]
- Ravi, N.; Dandekar, N.; Mysore, P.; Littman, M.L. Activity recognition from accelerometer data. Aaai 2005, 5, 1541–1546. [Google Scholar]
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software. ACM SIGKDD Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Witten, I.; Frank, E.; Hall, M.A.; Pal, C.J. Data Mining: Practical Machine Learning Tools and Techniques; Elsevier LTD: Oxford, UK, 2017. [Google Scholar]
- Banos, O.; Garcia, R.; Holgado-Terriza, J.A.; Damas, M.; Pomares, H.; Rojas, I.; Saez, A.; Villalonga, C. mHealthDroid: A Novel Framework for Agile Development of Mobile Health Applications. In Ambient Assisted Living and Daily Activities; Springer International Publishing: Cham, Switzerland, 2014; pp. 91–98. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sport. Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.D.; Tran, K.P.; Zeng, X.; Koehl, L.; Tartare, G. Wearable Sensor Data Based Human Activity Recognition using Machine Learning: A new approach. In Proceedings of the ISSAT International Conference on Data Science in Business, Finance and Industry, Da Nang, Vietnam, 3–5 July 2019. [Google Scholar]
- Khowaja, S.A.; Yahya, B.N.; Lee, S.L. Hierarchical classification method based on selective learning of slacked hierarchy for activity recognition systems. Expert Syst. Appl. 2017, 88, 165–177. [Google Scholar] [CrossRef]
- ud din Tahir, S.B.; Jalal, A.; Kim, K. Wearable Inertial Sensors for Daily Activity Analysis Based on Adam Optimization and the Maximum Entropy Markov Model. Entropy 2020, 22, 579. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Yun, J.M.; Choi, S. Multi-modal Convolutional Neural Networks for Activity Recognition. In Proceedings of the 2015 IEEE International Conference on Systems, Man, and Cybernetics, Hong Kong, China, 9–12 October 2015. [Google Scholar] [CrossRef]
- Ha, S.; Choi, S. Convolutional neural networks for human activity recognition using multiple accelerometer and gyroscope sensors. In Proceedings of the 2016 International Joint Conference on Neural Networks (IJCNN), Vancouver, BC, Canada, 24–29 July 2016. [Google Scholar] [CrossRef]
- Lyu, L.; He, X.; Law, Y.W.; Palaniswami, M. Privacy-Preserving Collaborative Deep Learning with Application to Human Activity Recognition. In Proceedings of the 2017 ACM on Conference on Information and Knowledge Management; ACM: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Jalal, A.; Batool, M.; Kim, K. Stochastic Recognition of Physical Activity and Healthcare Using Tri-Axial Inertial Wearable Sensors. Appl. Sci. 2020, 10, 7122. [Google Scholar] [CrossRef]
- Chowdhury, A.K.; Tjondronegoro, D.; Chandran, V.; Trost, S.G. Physical Activity Recognition Using Posterior-Adapted Class-Based Fusion of Multiaccelerometer Data. IEEE J. Biomed. Health Inform. 2018, 22, 678–685. [Google Scholar] [CrossRef] [PubMed]













| Physical Activity | MET |
|---|---|
| Light-intensity activities | <3 |
| standing | 1.3 |
| sitting | 1.3 |
| lying down | 1.0 |
| Moderate-intensity activities | 3–6 |
| walking | 3.5 |
| climbing stairs | 4.0 |
| cycling | 4.0 |
| Vigorous-intensity activities | >6 |
| jogging | 7.0 |
| running | 8.0 |
| Physical Activity | Accuracy | Precision | Recall |
|---|---|---|---|
| standing | 0.72 | 0.53 | 0.72 |
| sitting | 0.36 | 0.56 | 0.36 |
| lying | 1.0 | 1.0 | 1.0 |
| walking | 0.82 | 0.84 | 0.82 |
| climbing stairs | 0.84 | 0.81 | 0.84 |
| cycling | 0.97 | 0.99 | 0.97 |
| jogging | 0.88 | 0.85 | 0.88 |
| running | 0.84 | 0.87 | 0.84 |
| IF intensity=light THEN fuse chest sensor AND wrist sensor |
| IF intensity=moderate THEN fuse wrist sensor AND ankle sensor |
| IF intensity=vigorous THEN fuse chest sensor AND ankle sensor |
| Physical Activity | Accuracy | Precision | Recall |
|---|---|---|---|
| standing | 0.92 | 0.90 | 0.92 |
| sitting | 0.90 | 0.92 | 0.90 |
| lying | 1.0 | 1.0 | 1.0 |
| walking | 0.99 | 0.99 | 0.99 |
| climbing stairs | 0.99 | 0.99 | 0.98 |
| cycling | 1.0 | 1.0 | 1.0 |
| jogging | 0.95 | 0.89 | 0.95 |
| running | 0.88 | 0.94 | 0.88 |
| IF activity=walking THEN normal HR range is 70–125 bpm |
| IF activity=running THEN altered HR range is 100–135 bpm |
| IF activity=cycling THEN normal BR range is 18–30 brpm |
| IF activity=sitting THEN emergency BR range is <8 brpm |
| Physical Activity | Subject 1 | Subject 2 | Subject 3 | Subject 4 | Subject 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR | BR | HR | BR | HR | BR | HR | BR | HR | BR | |
| standing | 60–105 | 9–30 | 60–90 | 9–23 | 60–105 | 9–30 | 60–100 | 9–30 | 60–110 | 9–30 |
| sitting | 55–95 | 6–22 | 55–80 | 8–22 | 55–80 | 6–22 | 55–80 | 6–22 | 55–80 | 6–22 |
| lying | 45–90 | 6–18 | 55–80 | 6–18 | 45–70 | 6–18 | 45–70 | 6–18 | 45–70 | 6–18 |
| walking | 70–125 | 12–30 | 65–125 | 12–30 | 70–125 | 12–30 | 70–100 | 12–30 | 85–110 | 18–30 |
| climbing stairs | 100–160 | 25–38 | 100–145 | 25–38 | 100–145 | 24–40 | 100–145 | 25–38 | 105–125 | 28–37 |
| cycling | 60–120 | 18–30 | 60–125 | 16–28 | 90–145 | 18–30 | 90–145 | 18–30 | 75–120 | 18–27 |
| jogging | 105–160 | 29–33 | 105–160 | 29–33 | 105–145 | 28–33 | 110–160 | 29–33 | 110–160 | 28–33 |
| running | 150–185 | 21–38 | 150–185 | 20–37 | 150–185 | 21–38 | 150–185 | 23–34 | 150–185 | 21–38 |
| Physical Activity | Subject 6 | Subject 7 | Subject 8 | Subject 9 | Subject 10 | |||||
| HR | BR | HR | BR | HR | BR | HR | BR | HR | BR | |
| standing | 75–105 | 9–30 | 60–105 | 9–30 | 60–105 | 9–30 | 65–105 | 9–28 | 60–105 | 9–30 |
| sitting | 55–80 | 6–22 | 60–85 | 6–22 | 55–80 | 6–22 | 60–85 | 6–18 | 55–95 | 6–22 |
| lying | 45–70 | 6–18 | 45–70 | 6–18 | 45–70 | 6–18 | 45–70 | 12–18 | 45–90 | 6–18 |
| walking | 70–125 | 18–30 | 70–125 | 12–28 | 70–125 | 18–30 | 70–115 | 14–30 | 70–125 | 12–30 |
| climbing stairs | 100–145 | 25–38 | 100–145 | 25–38 | 100–145 | 25–38 | 110–145 | 25–38 | 100–145 | 25–38 |
| cycling | 90–145 | 18–30 | 90–145 | 18–30 | 90–145 | 18–30 | 80–135 | 22–30 | 90–145 | 18–30 |
| jogging | 110–160 | 28–33 | 110–160 | 28–33 | 110–160 | 28–33 | 95–150 | 28–33 | 110–160 | 28–33 |
| running | 150–185 | 21–38 | 150–185 | 21–38 | 150–185 | 21–38 | 155–185 | 21–38 | 150–185 | 21–38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivașcu, T.; Negru, V. Activity-Aware Vital Sign Monitoring Based on a Multi-Agent Architecture. Sensors 2021, 21, 4181. https://doi.org/10.3390/s21124181
Ivașcu T, Negru V. Activity-Aware Vital Sign Monitoring Based on a Multi-Agent Architecture. Sensors. 2021; 21(12):4181. https://doi.org/10.3390/s21124181
Chicago/Turabian StyleIvașcu, Todor, and Viorel Negru. 2021. "Activity-Aware Vital Sign Monitoring Based on a Multi-Agent Architecture" Sensors 21, no. 12: 4181. https://doi.org/10.3390/s21124181
APA StyleIvașcu, T., & Negru, V. (2021). Activity-Aware Vital Sign Monitoring Based on a Multi-Agent Architecture. Sensors, 21(12), 4181. https://doi.org/10.3390/s21124181






