Semi-Supervised Deep Learning-Based Image Registration Method with Volume Penalty for Real-Time Breast Tumor Bed Localization
Abstract
1. Introduction
1.1. Problem Statement
1.2. Related Work
1.3. Contribution
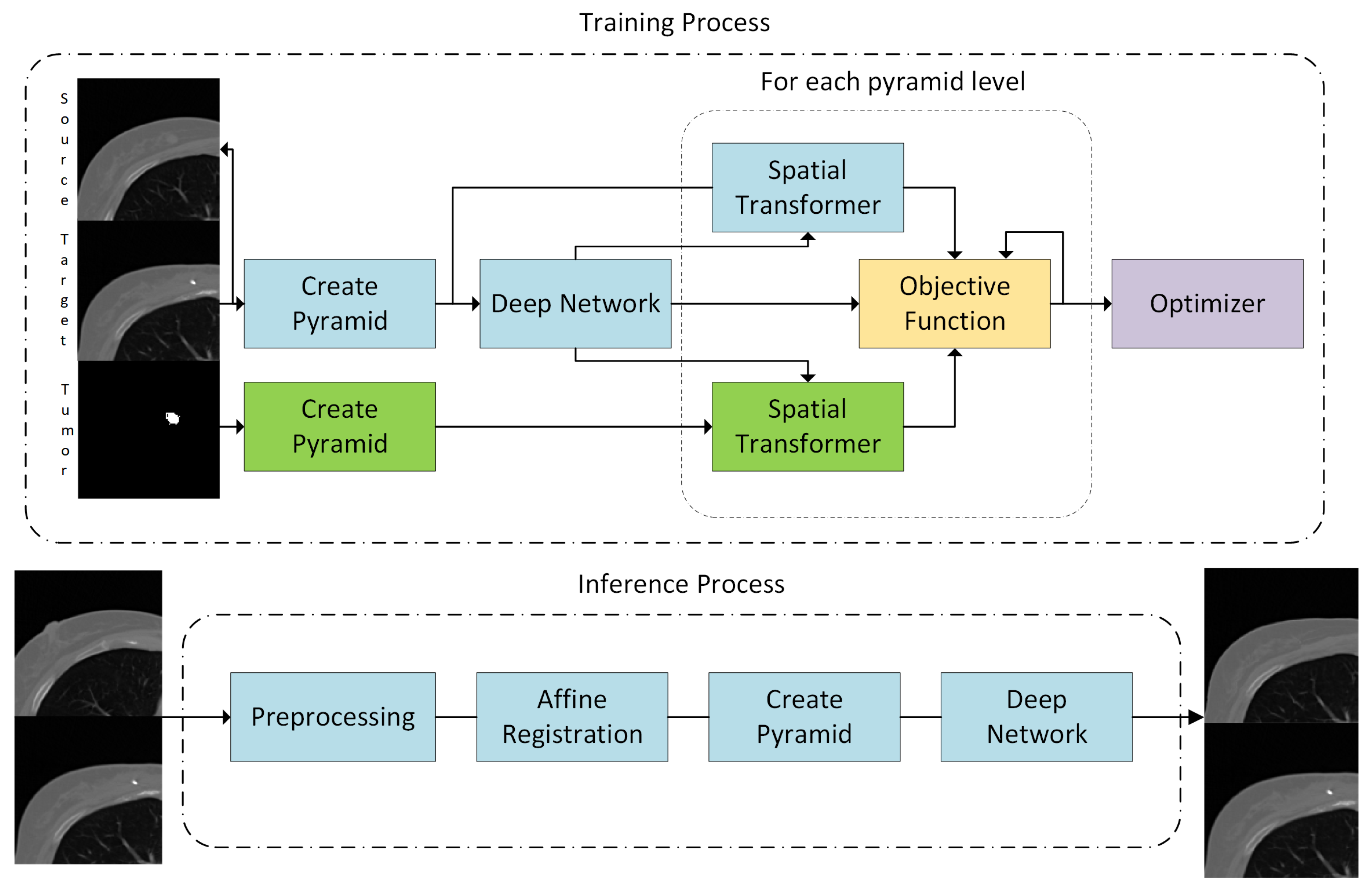
2. Methods
2.1. Overview and Preprocessing
2.2. Affine Registration
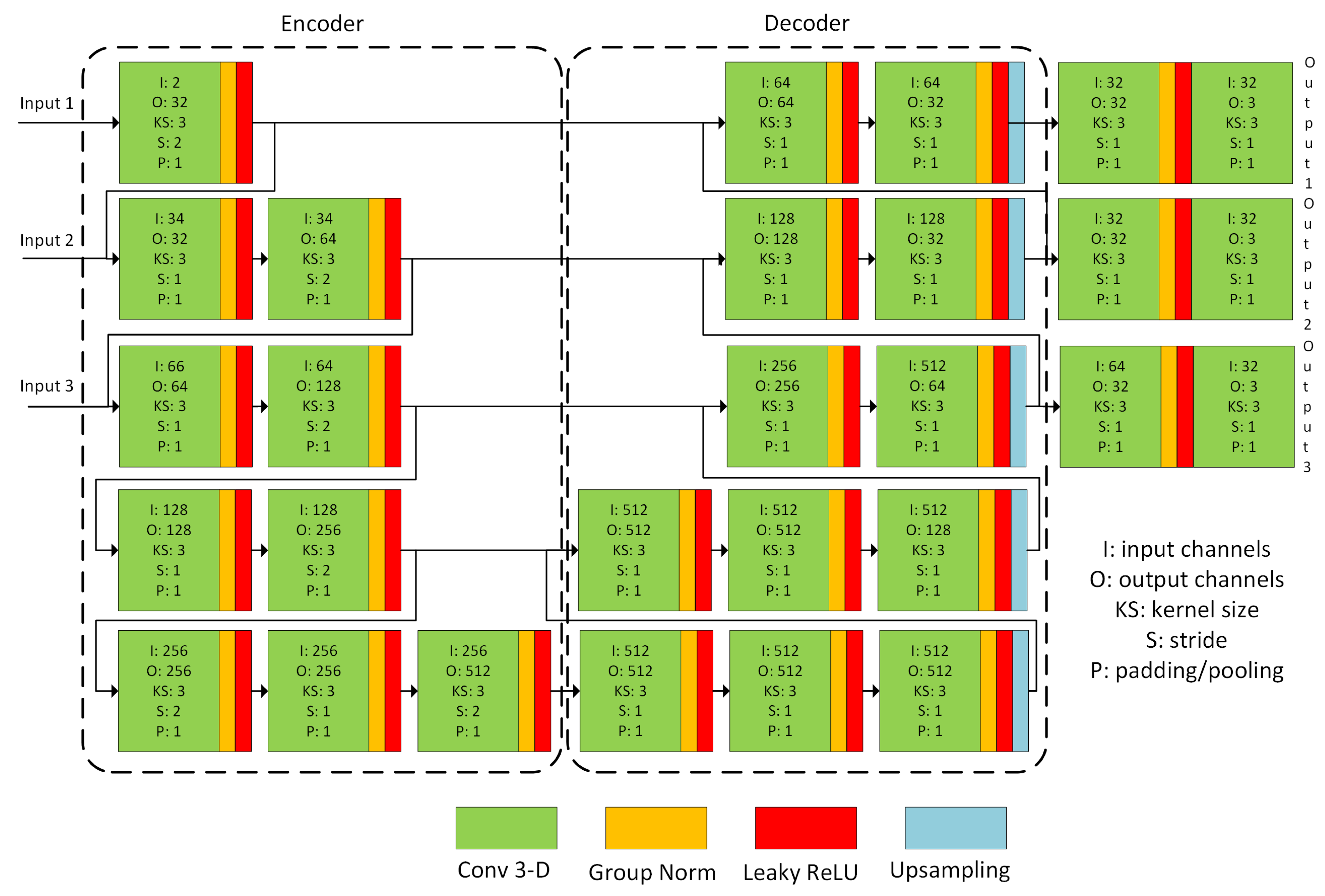
2.3. Nonrigid Registration Network
2.4. Unsupervised Training
2.5. Volume Penalty
2.6. Symmetric Registration
2.7. Dataset and Experimental Setup
3. Results
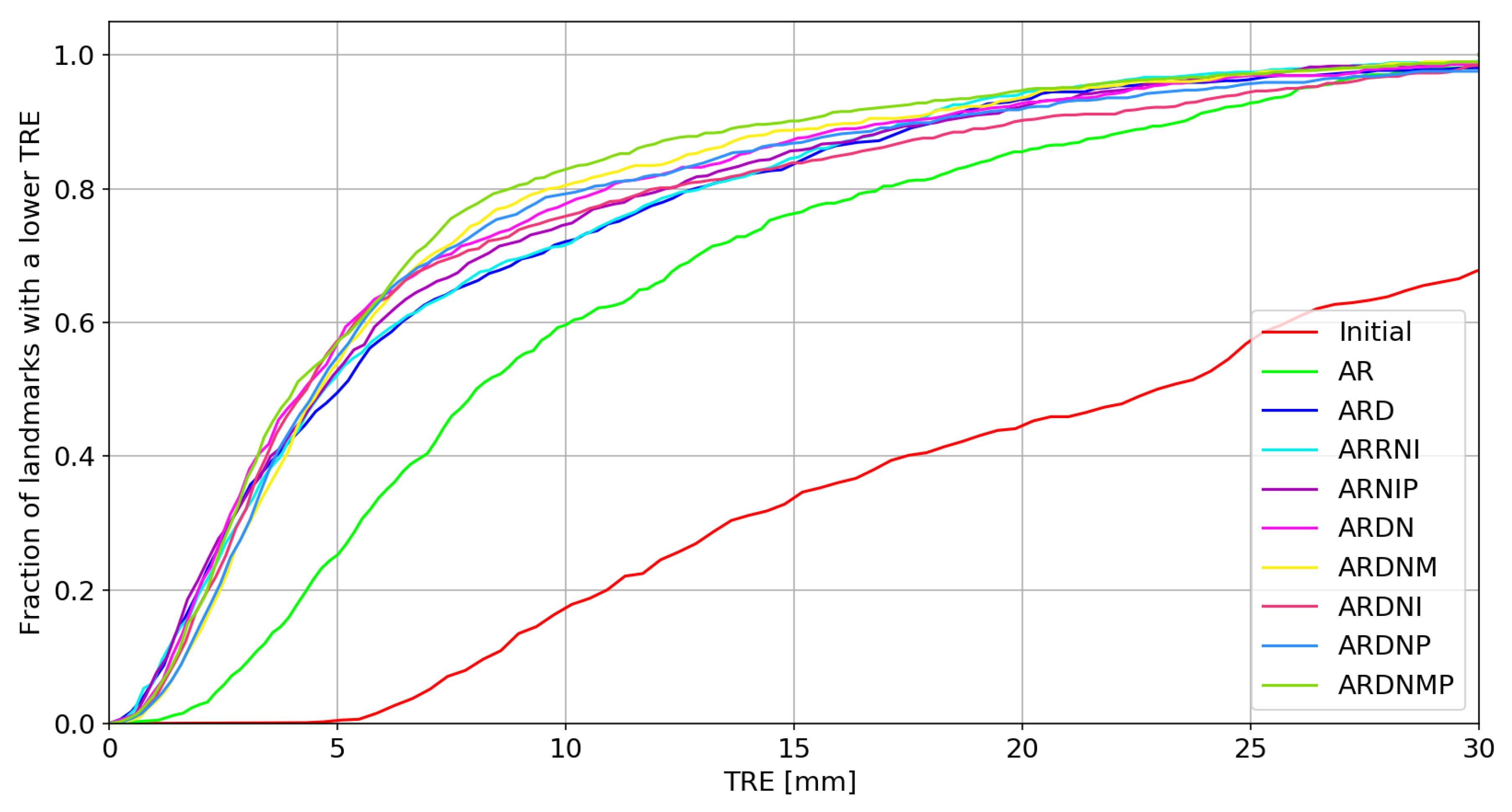
3.1. Target Registration Error
3.2. Tumor Volume Ratio
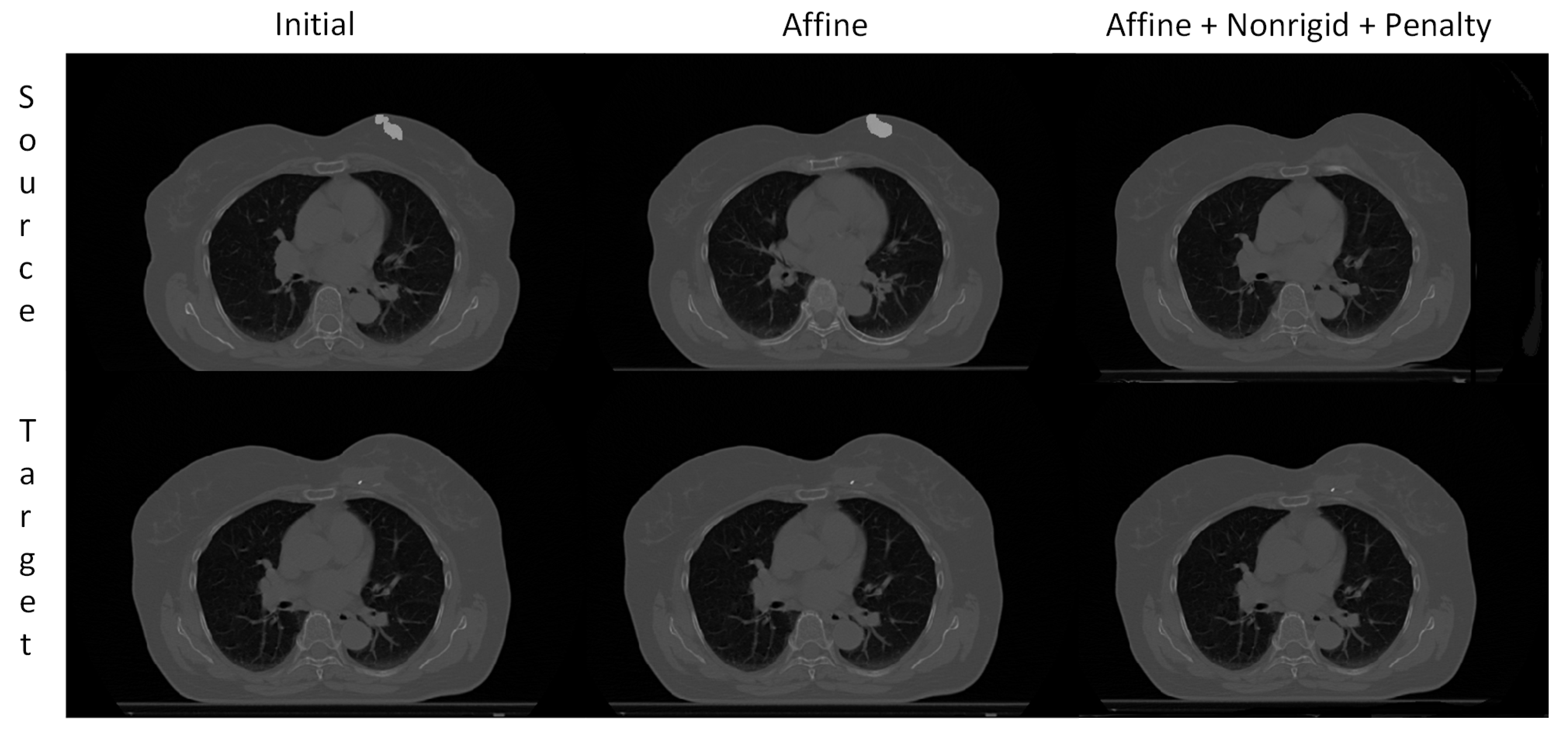
3.3. Visual Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCS | Breast-Conserving Surgery |
| BTB | Breast Tumor Bed |
| CPU | Central Processing Unit |
| CT | Computed Tomography |
| DL | Deep Learning |
| GPU | Graphics Processing Unit |
| IR | Image Registration |
| MRI | Magnetic Resonance Images |
| NCC | Normalized Cross-Correlation |
| RT | Radiation Therapy |
| TRE | Target Registration Error |
| TVR | Tumor Volume Ratio |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.; Bray, F.; Siegel, R.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.; Deutsch, M.; Fisher, E.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Mutic, S.; Dempsey, J. The ViewRay System: Magnetic Resonance-Guided and Controlled Radiotherapy. Semin. Radiat. Oncol. 2014, 24, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Eppenhof, K.; Maspero, M.; Savenije, M.; de Boer, J.; van der Voort van Zyp, J.; Raaymakers, B.; Raaijmakers, A.; Veta, M.; van den Berg, C.; Pluim, J. Fast contour propagation for MR-guided prostate radiotherapy using convolutional neural networks. Med. Phys. 2020, 47, 1238–1248. [Google Scholar] [CrossRef]
- Wodzinski, M.; Skalski, A.; Ciepiela, I.; Kuszewski, T.; Kedzierawski, P.; Gajda, J. Improving oncoplastic breast tumor bed localization for radiotherapy planning using image registration algorithms. Phys. Med. Biol. 2018, 63, 035024. [Google Scholar] [CrossRef] [PubMed]
- Periaswamy, S.; Farid, H. Medical image registration with partial data. Med. Image Anal. 2006, 10, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Nithiananthan, S.; Schafer, S.; Mirota, D.; Stayman, J.; Zbijewski, W.; Reh, D.; Gallia, G.; Siewerdsen, J. Extra-dimensional Demons: A method for incorporating missing tissue in deformable image registration. Med. Phys. 2012, 39, 5718–5731. [Google Scholar] [CrossRef]
- Risholm, P.; Samset, E.; Wells, W., III. Validation of a nonrigid registration framework that accommodates tissue resection. Prog. Biomed. Opt. Imaging Proc. SPIE 2010, 7623, 762319. [Google Scholar]
- Kirova, Y.; Castro Pena, P.; Hijal, T.; Fournier-Bidoz, N.; Laki, F.; Sigal-Zafrani, B.; Dendale, R.; Bollet, M.; Campana, F.; Fourquet, A. Improving the definition of tumor bed boost with the use of surgical clips and image registration in breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1352–1355. [Google Scholar] [CrossRef]
- Kovner, F.; Agay, R.; Merimsky, O.; Stadler, J.; Klausner, J.; Inbar, M. Clips and scar as the guidelines for breast radiation boost after lumpectomy. Eur. J. Surg. Oncol. 1999, 25, 483–486. [Google Scholar] [CrossRef]
- Benda, R.; Yasuda, G.; Sethi, A.; Gabram, S.; Hinerman, R.; Mendenhall, N. Breast boost: Are we missing the target? A dosimetric comparison of two boost techniques. Cancer 2003, 97, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ma, J.; Xiao, G.; Shao, Z.; Guo, X. A review of multimodal image matching: Methods and applications. Inf. Fusion 2021, 73, 22–71. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, X.; Fan, A.; Jiang, J.; Yan, J. Image Matching from Handcrafted to Deep Features: A Survey. Int. J. Comput. Vis. 2021, 129, 23–79. [Google Scholar] [CrossRef]
- Vercauteren, T.; Pennec, X.; Perchant, A.; Ayache, N. Diffeomorphic demons: Efficient non-parametric image registration. NeuroImage 2009, 45, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Sotiras, A.; Davatzikos, C.; Paragios, N. Deformable medical image registration: A survey. IEEE Trans. Med Imaging 2013, 32, 1153–1190. [Google Scholar] [CrossRef] [PubMed]
- Haskins, G.; Kruger, U.; Yan, P. Deep learning in medical image registration: A survey. Mach. Vis. Appl. 2020, 31, 1–18. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Zhao, A.; Sabuncu, M.; Guttag, J.; Dalca, A. VoxelMorph: A Learning Framework for Deformable Medical Image Registration. IEEE Trans. Med. Imaging 2019, 38, 1788–1800. [Google Scholar] [CrossRef] [PubMed]
- Dalca, A.; Balakrishnan, G.; Guttag, J.; Sabuncu, M. Unsupervised learning of probabilistic diffeomorphic registration for images and surfaces. Med. Image Anal. 2019, 57, 226–236. [Google Scholar] [CrossRef]
- Zhang, J. Inverse-Consistent Deep Networks for Unsupervised Deformable Image Registration. arXiv 2018, arXiv:1809.03443. [Google Scholar]
- Mok, T.; Chung, A. Large Deformation Diffeomorphic Image Registration with Laplacian Pyramid Networks. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Cham, Switzerland, 2020; pp. 1–10. [Google Scholar]
- Dalca, A.; Hering, A.; Hansen, L.; M., H. The Learn2Reg Challenge. 2020. Available online: https://learn2reg.grand-challenge.org (accessed on 15 January 2021).
- Fan, J.; Cao, X.; Wang, Q.; Yap, P.T.; Shen, D. Adversarial learning for mono- or multi-modal registration. Med. Image Anal. 2019, 58, 101545. [Google Scholar] [CrossRef]
- De Vos, B.; Berendsen, F.; Viergever, M.; Sokooti, H.; Staring, M.; Išgum, I. A deep learning framework for unsupervised affine and deformable image registration. Med. Image Anal. 2019, 52, 128–143. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xan, H.; Xu, Z.; Niethammer, M. Networks for Joint Affine and Non-parametric Image Registration. In Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), Long Beach, CA, USA, 16–20 June 2019; pp. 4224–4233. [Google Scholar]
- Heinrich, M.; Hansen, L. Highly Accurate and Memory Efficient Unsupervised Learning-Based Discrete CT Registration Using 2.5D Displacement Search. MICCAI 2020 2020, 12263 LNCS, 190–200. [Google Scholar]
- Paszke, A.; Gross, S.; Massa, F.; Lerer, A.; Bradbury, J.; Chanan, G.; Killeen, T.; Lin, Z.; Gimelshein, N.; Antiga, L.; et al. PyTorch: An Imperative Style, High-Performance Deep Learning Library. In Advances in Neural Information Processing Systems 32; Curran Associates, Inc.: Red Hook, NY, USA, 2019; pp. 8024–8035. [Google Scholar]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In International Conference on Medical Image Computing and Computer-Assisted Intervention; Springer: Cham, Switzerland, 2015; pp. 234–241. [Google Scholar]
- Wodzinski, M.; Müller, H. DeepHistReg: Unsupervised Deep Learning Registration Framework for Differently Stained Histology Samples. Comput. Methods Programs Biomed. 2021, 198, 105799. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, K. Group Normalization. arXiv 2018, arXiv:1803.08494. [Google Scholar]
- Vercauteren, T.; Pennec, X.; Perchant, A.; Ayache, N. Non-parametric diffeomorphic image registration with the demons algorithm. Int. Conf. Med. Image Comput. Comput. Assist. Interv. 2007, 4792 LNCS, 319–326. [Google Scholar]
- Wodzinski, M. The Source Code. 2021. Available online: https://github.com/lNefarin/BreastReg (accessed on 13 June 2021).
- Boveiri, H.; Khayami, R.; Javidan, R.; Mehdizadeh, A. Medical Image Registration Using Deep Neural Networks: A Comprehensive Review. Comput. Electr. Eng. 2020, 87, 106767. [Google Scholar] [CrossRef]
- Pesce, E.; Joseph Withey, S.; Ypsilantis, P.P.; Bakewell, R.; Goh, V.; Montana, G. Learning to detect chest radiographs containing pulmonary lesions using visual attention networks. Med. Image Anal. 2019, 53, 26–38. [Google Scholar] [CrossRef]
- Faisan, S.; Passat, N.; Noblet, V.; Chabrier, R.; Meyer, C. Topology preserving warping of 3-D binary images according to continuous one-to-One mappings. IEEE Trans. Image Process. 2011, 20, 2135–2145. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.; Deng, W. Deep visual domain adaptation: A survey. Neurocomputing 2018, 312, 135–153. [Google Scholar] [CrossRef]
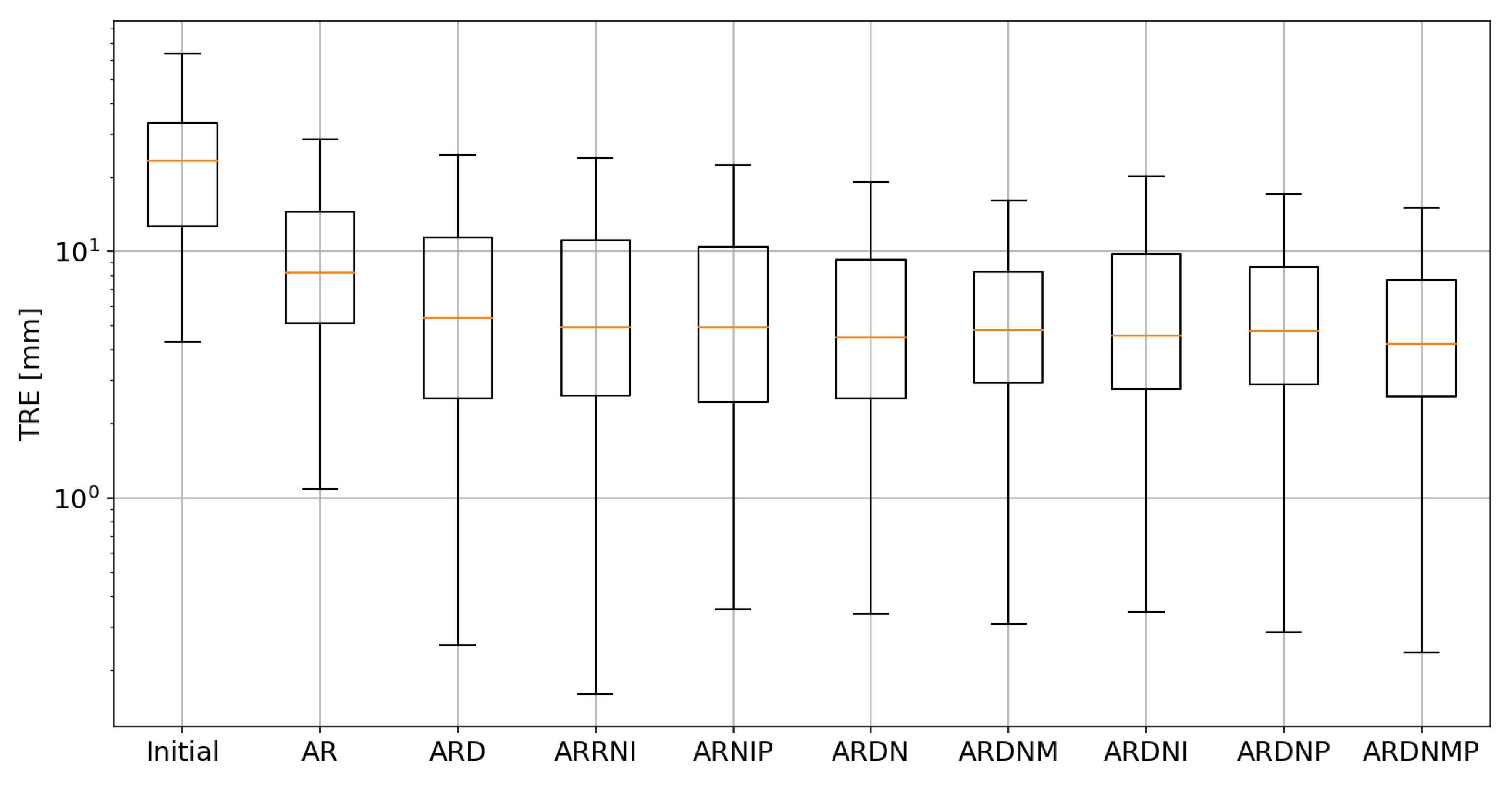
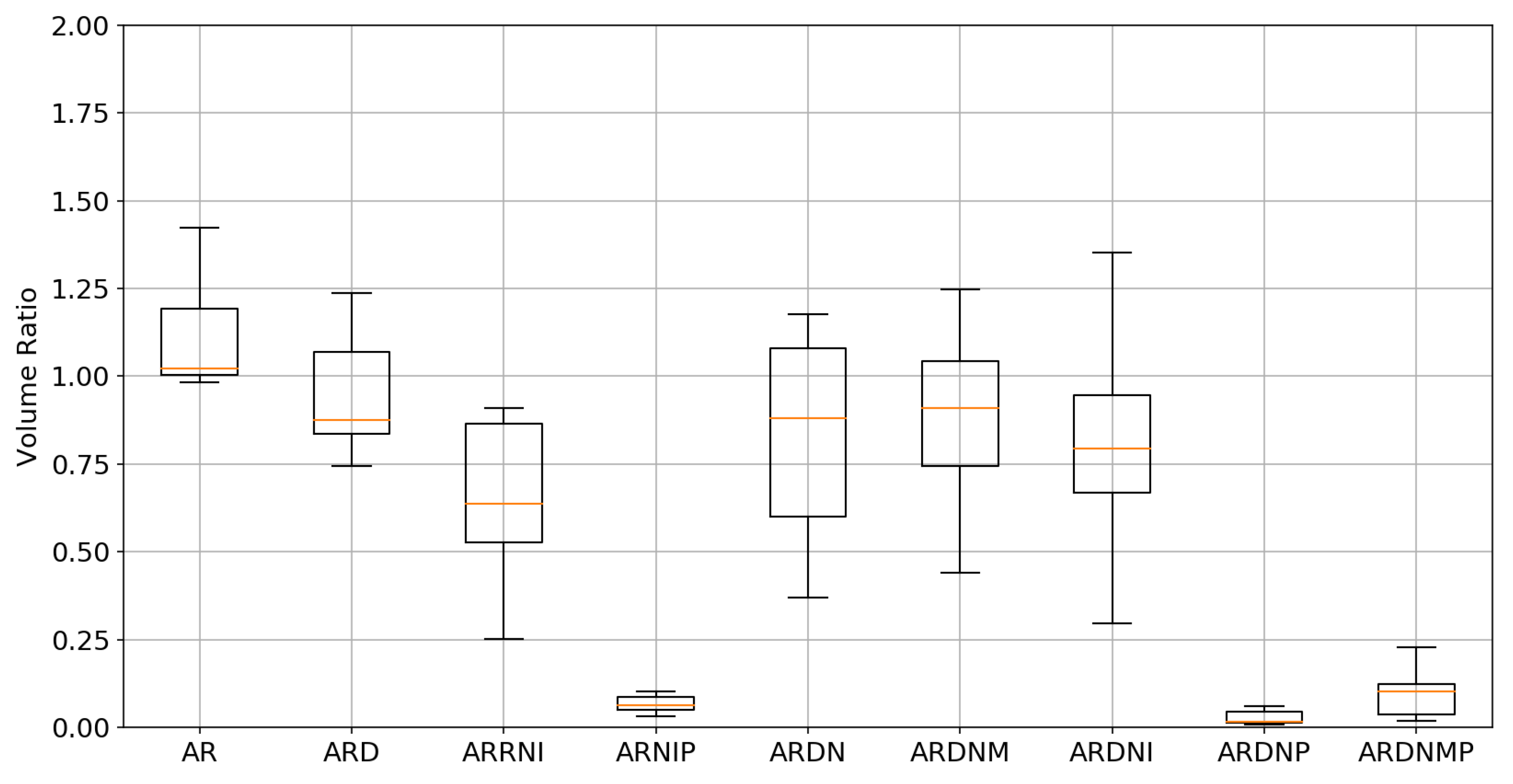

| Experiment | Average TRE [mm] | Median TRE [mm] | Average TVR | Median TVR | Average Time [s] |
|---|---|---|---|---|---|
| Initial | 24.47 | 23.32 | 1.00 | 1.00 | - |
| AR | 10.88 | 8.22 | 1.10 | 1.02 | 0.34 |
| ARD | 7.86 | 5.35 | 0.96 | 0.88 | 51.18 |
| ARRNI | 7.60 | 4.95 | 0.69 | 0.63 | 4.15 |
| ARNIP | 7.50 | 4.92 | 0.07 | 0.06 | 4.78 |
| ARDN | 7.45 | 4.75 | 0.82 | 0.88 | 0.52 |
| ARDNM | 7.07 | 4.80 | 0.88 | 0.90 | 0.54 |
| ARDNI | 7.78 | 4.56 | 0.81 | 0.79 | 0.51 |
| ARDNP | 7.15 | 4.49 | 0.03 | 0.01 | 0.53 |
| ARDNMP | 6.51 | 4.22 | 0.10 | 0.10 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wodzinski, M.; Ciepiela, I.; Kuszewski, T.; Kedzierawski, P.; Skalski, A. Semi-Supervised Deep Learning-Based Image Registration Method with Volume Penalty for Real-Time Breast Tumor Bed Localization. Sensors 2021, 21, 4085. https://doi.org/10.3390/s21124085
Wodzinski M, Ciepiela I, Kuszewski T, Kedzierawski P, Skalski A. Semi-Supervised Deep Learning-Based Image Registration Method with Volume Penalty for Real-Time Breast Tumor Bed Localization. Sensors. 2021; 21(12):4085. https://doi.org/10.3390/s21124085
Chicago/Turabian StyleWodzinski, Marek, Izabela Ciepiela, Tomasz Kuszewski, Piotr Kedzierawski, and Andrzej Skalski. 2021. "Semi-Supervised Deep Learning-Based Image Registration Method with Volume Penalty for Real-Time Breast Tumor Bed Localization" Sensors 21, no. 12: 4085. https://doi.org/10.3390/s21124085
APA StyleWodzinski, M., Ciepiela, I., Kuszewski, T., Kedzierawski, P., & Skalski, A. (2021). Semi-Supervised Deep Learning-Based Image Registration Method with Volume Penalty for Real-Time Breast Tumor Bed Localization. Sensors, 21(12), 4085. https://doi.org/10.3390/s21124085






