Smartphone Screen Integrated Optical Breathalyzer
Abstract
1. Introduction
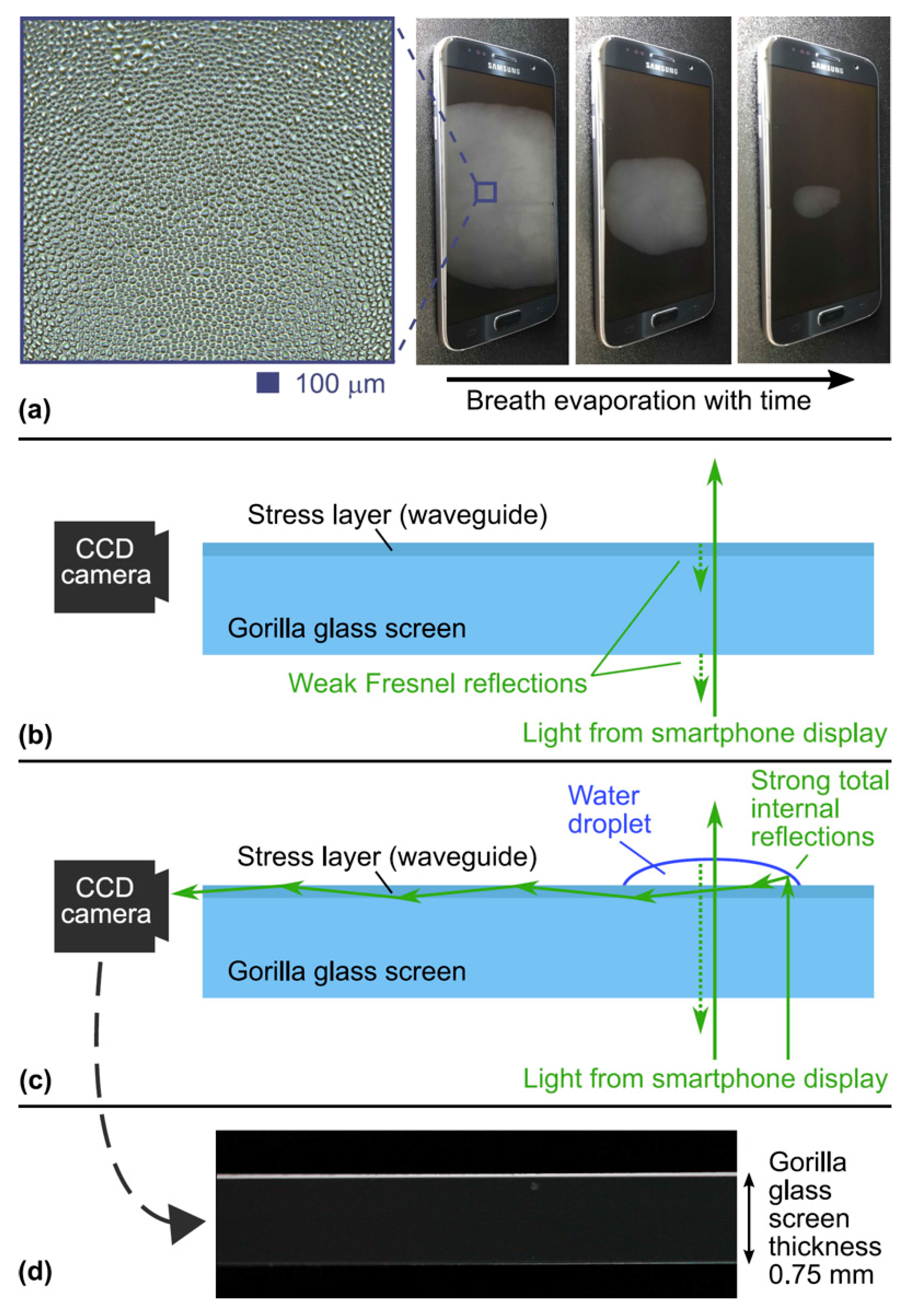
2. Breathalyzer Principle
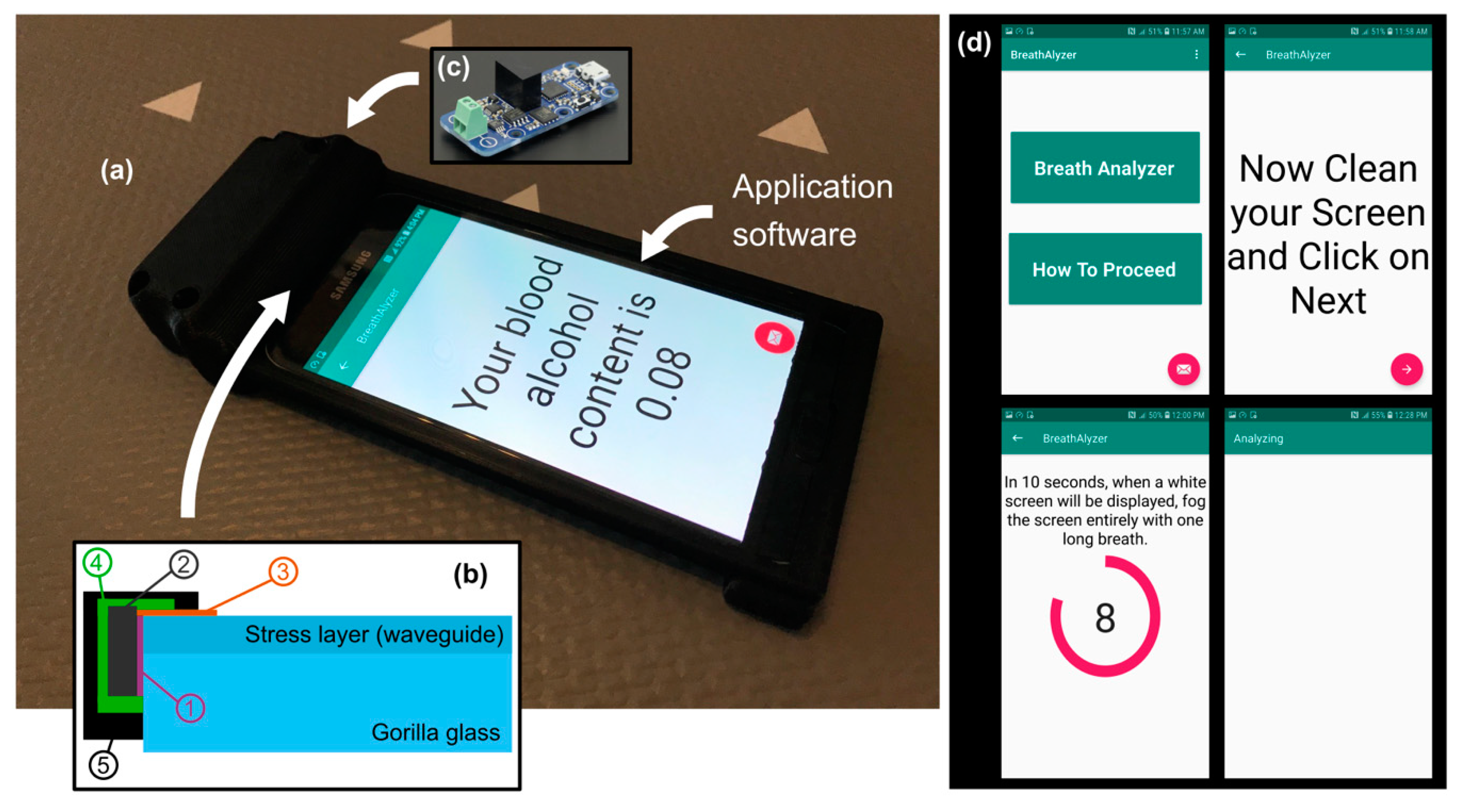
3. Breathalyzer Prototype
4. Results and Discussion
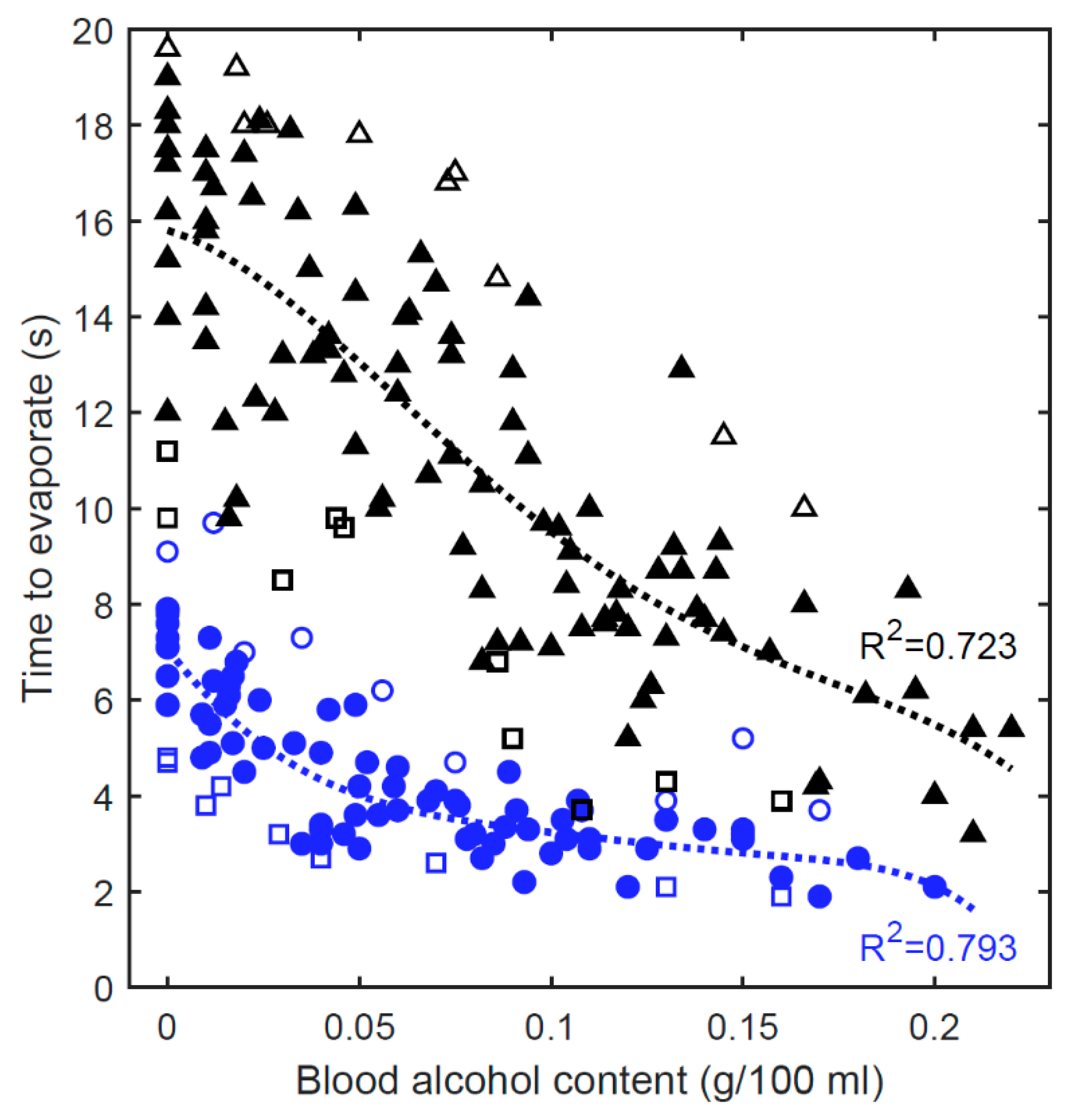
4.1. Laboratory Tests
4.2. Real-World Tests
4.2.1. Breathing Condition
4.2.2. Ambient Conditions
4.2.3. Postprocessed Results
4.3. Discussion
5. Conclusions and Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aseev, O.; Tuzson, B.; Looser, H.; Scheidegger, P.; Liu, C.; Morstein, C.; Niederhauser, B.; Emmenegger, L. High-precision ethanol measurement by mid-IR laser absorption spectroscopy for metrological applications. Opt. Express 2019, 27, 5314–5325. [Google Scholar] [CrossRef] [PubMed]
- Bihar, E.; Deng, Y.; Miyake, T.; Saadaoui, M.; Malliaras, G.G.; Rolandi, M. A Disposable paper breathalyzer with an alcohol sensing organic electrochemical transistor. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Ljungblad, J.; Hök, B.; Ekström, M. Development and evaluation of algorithms for breath alcohol screening. Sensors 2016, 16, 469. [Google Scholar] [CrossRef] [PubMed]
- Jurič, A.; Fijačko, A.; Bakulić, L.; Orešić, T.; Gmajnički, I. Evaluation of breath alcohol analysers by comparison of breath and blood alcohol concentrations. Arch. Ind. Hyg. Toxicol. 2018, 69, 69–76. [Google Scholar] [CrossRef]
- Jonsson, A.; Hök, B.; Andersson, L.; Hedenstierna, G. Methodology investigation of expirograms for enabling contact free breath alcohol analysis. J. Breath Res. 2009, 3, 036002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hok, B.; Pettersson, H.; Andersson, A.K.; Haasl, S.; Akerlund, P. Breath analyzer for alcolocks and screening devices. IEEE Sens. J. 2009, 10, 10–15. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, J.; Cheong, W.H.; Lee, I.J.; Lee, H.; Im, H.G.; Kong, H.; Bae, B.S.; Park, J.U. Alcohol gas sensors capable of wireless detection using In2O3/Pt nanoparticles and Ag nanowires. Sens. Actuators B Chem. 2018, 259, 825–832. [Google Scholar] [CrossRef]
- Thungon, P.D.; Kakoti, A.; Ngashangva, L.; Goswami, P. Advances in developing rapid, reliable and portable detection systems for alcohol. Biosens. Bioelectron. 2017, 97, 83–99. [Google Scholar] [CrossRef]
- National Center for Statistics and Analysis. 2018 Fatal Motor Vehicle Crashes: Overview; Traffic Safety Facts Research Note. Report No. DOT HS 812 826; National Highway Traffic Safety Administration: Washington, DC, USA, 2019.
- Vanlaar, W.; Robertson, R.; Marcoux, K.; Mayhew, D.; Brown, S.; Boase, P. Trends in alcohol-impaired driving in Canada. Accid. Anal. Prev. 2012, 48, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, V.A.; Kwo, P.Y.; Li, T.K. Effect of food and food composition on alcohol elimination rates in healthy men and women. J. Clin. Pharmacol. 2001, 41, 1345–1350. [Google Scholar] [CrossRef]
- Cromer, J.R.; Cromer, J.A.; Maruff, P.; Snyder, P.J. Perception of alcohol intoxication shows acute tolerance while executive functions remain impaired. Exp. Clin. Psychopharmacol. 2010, 18, 329. [Google Scholar] [CrossRef]
- Elder, R.W.; Voas, R.; Beirness, D.; Shults, R.A.; Sleet, D.A.; Nichols, J.L.; Compton, R. Effectiveness of ignition interlocks for preventing alcohol-impaired driving and alcohol-related crashes: A Community Guide systematic review. Am. J. Prev. Med. 2011, 40, 362–376. [Google Scholar] [CrossRef]
- Min, A.; Lee, D.; Shih, P.C. Potentials of Smart Breathalyzer: Interventions for Excessive Drinking Among College Students. In Transforming Digital Worlds, iConference 2018; Chowdhury, G., McLeod, J., Gillet, V., Willett, P., Eds.; Lecture Notes in Computer Science; Springer: Cham, Switzerland, 2018; Volume 10766, pp. 195–206. [Google Scholar]
- Liu, C.; Bonaccurso, E.; Butt, H.J. Evaporation of sessile water/ethanol drops in a controlled environment. Phys. Chem. Chem. Phys. 2008, 10, 7150–7157. [Google Scholar] [CrossRef]
- Marcuse, D. Launching light into fiber cores from sources located in the cladding. J. Light. Technol. 1988, 6, 1273–1279. [Google Scholar] [CrossRef]
- Snyder, A.W.; Love, J. Optical Waveguide Theory; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Lapointe, J.; Gagné, M.; Li, M.J.; Kashyap, R. Making smart phones smarter with photonics. Opt. Express 2014, 22, 15473–15483. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, J.; Parent, F.; de Lima Filho, E.S.; Loranger, S.; Kashyap, R. Toward the integration of optical sensors in smartphone screens using femtosecond laser writing. Opt. Lett. 2015, 40, 5654–5657. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, J.; Kashyap, R. A simple technique to overcome self-focusing, filamentation, supercontinuum generation, aberrations, depth dependence and waveguide interface roughness using fs laser processing. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, J.; Bérubé, J.P.; Ledemi, Y.; Dupont, A.; Fortin, V.; Messaddeq, Y.; Vallée, R. Nonlinear increase, invisibility, and sign inversion of a localized fs-laser-induced refractive index change in crystals and glasses. Light Sci. Appl. 2020, 9, 1–12. [Google Scholar] [CrossRef]
- Dubowski, K.M. Alcohol determination in the clinical laboratory. Am. J. Clin. Pathol. 1980, 74, 747–750. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Majumder, S.; Deen, M.J. Smartphone sensors for health monitoring and diagnosis. Sensors 2019, 19, 2164. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Tong, H.; Ji, P. Activity recognition with smartphone sensors. Tsinghua Sci. Technol. 2014, 19, 235–249. [Google Scholar]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Canning, J.; Yu, Z.; Ast, S.; Rutledge, P.J.; Wong, J.K.H.; Jamalipour, A.; Crossley, M.J. Time-resolved and temperature tuneable measurements of fluorescent intensity using a smartphone fluorimeter. Analyst 2017, 142, 1953–1961. [Google Scholar] [CrossRef]
- Stieger, S.; Reips, U.D. Well-being, smartphone sensors, and data from open-access databases: A mobile experience sampling study. Field Methods 2019, 31, 277–291. [Google Scholar] [CrossRef]
- Hossain, M.A.; Canning, J.; Yu, Z. Fluorescence-based determination of olive oil quality using an endoscopic smart mobile spectrofluorimeter. IEEE Sens. J. 2019, 20, 4156–4163. [Google Scholar] [CrossRef]
- Lapointe, J.; Bérubé, J.P.; Ledemi, Y.; Dupont, A.; Fortin, V.; Messaddeq, Y.; Vallée, R. Miniaturized and invisible laser-inscribed photonic circuits. In Proceedings of the Photonics North, Niagara Falls, ON, Canada, 26–28 May 2020. [Google Scholar]
- Crocombe, R.A. Portable spectroscopy. Appl. Spectrosc. 2018, 72, 1701–1751. [Google Scholar] [CrossRef]
- Abasi, S.; Minaei, S.; Jamshidi, B.; Fathi, D. Development of an Optical Smart Portable Instrument for Fruit Quality Detection. IEEE Trans. Instrum. Meas. 2020, 70, 1–9. [Google Scholar] [CrossRef]
- Nelis, J.; Elliott, C.; Campbell, K. “The Smartphone’s Guide to the Galaxy”: In Situ Analysis in Space. Biosensors 2018, 8, 96. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapointe, J.; Bécotte-Boutin, H.-S.; Gagnon, S.; Levasseur, S.; Labranche, P.; D’Auteuil, M.; Abdellatif, M.; Li, M.-J.; Vallée, R. Smartphone Screen Integrated Optical Breathalyzer. Sensors 2021, 21, 4076. https://doi.org/10.3390/s21124076
Lapointe J, Bécotte-Boutin H-S, Gagnon S, Levasseur S, Labranche P, D’Auteuil M, Abdellatif M, Li M-J, Vallée R. Smartphone Screen Integrated Optical Breathalyzer. Sensors. 2021; 21(12):4076. https://doi.org/10.3390/s21124076
Chicago/Turabian StyleLapointe, Jerome, Hélène-Sarah Bécotte-Boutin, Stéphane Gagnon, Simon Levasseur, Philippe Labranche, Marc D’Auteuil, Manel Abdellatif, Ming-Jun Li, and Réal Vallée. 2021. "Smartphone Screen Integrated Optical Breathalyzer" Sensors 21, no. 12: 4076. https://doi.org/10.3390/s21124076
APA StyleLapointe, J., Bécotte-Boutin, H.-S., Gagnon, S., Levasseur, S., Labranche, P., D’Auteuil, M., Abdellatif, M., Li, M.-J., & Vallée, R. (2021). Smartphone Screen Integrated Optical Breathalyzer. Sensors, 21(12), 4076. https://doi.org/10.3390/s21124076






