Texture Analysis of Dried Droplets for the Quality Control of Medicines
Abstract
1. Introduction
2. Materials and Methods
2.1. Solution Preparation
2.2. Drop Evaporation
2.3. Image Acquisition
2.4. Texture Analysis
2.4.1. Angular Second Moment (ASM)
2.4.2. Moment of Inertia
2.4.3. Correlation
2.4.4. Inverse Difference Moment (IDM)
2.4.5. Entropy
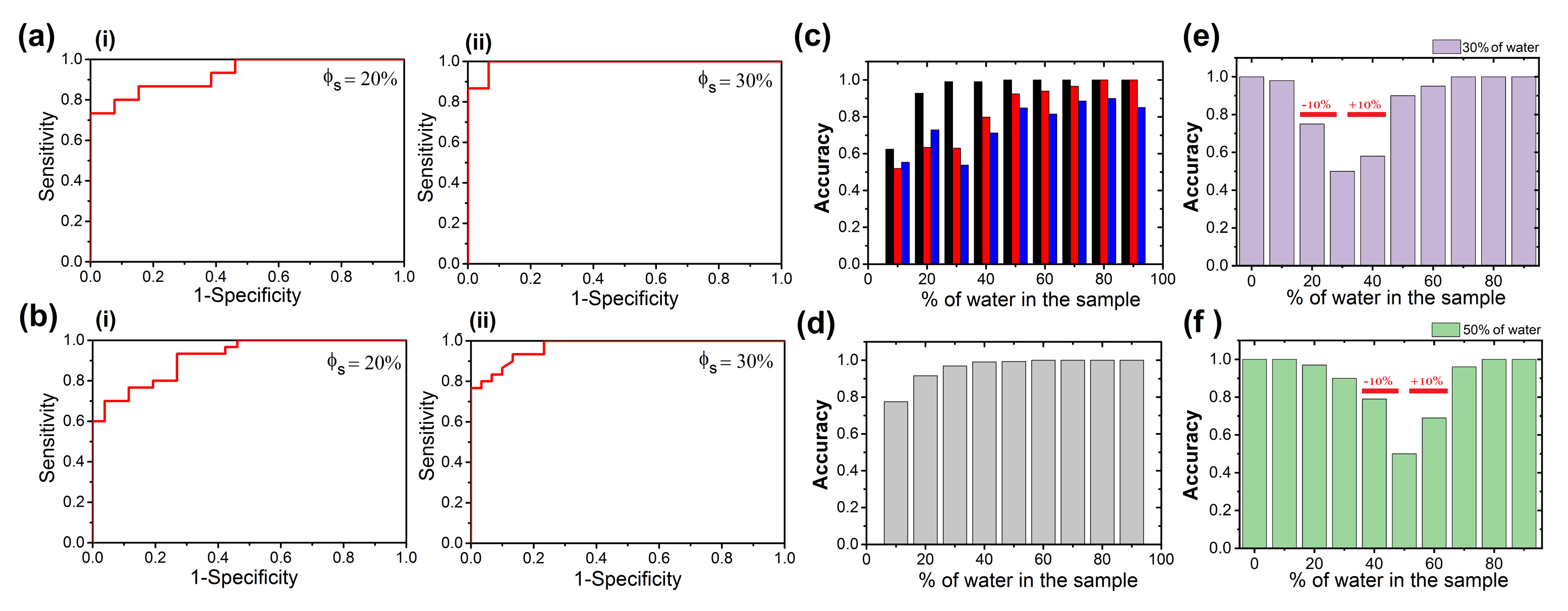
2.5. The Receiver Operating Characteristic (ROC) Curve
3. Results
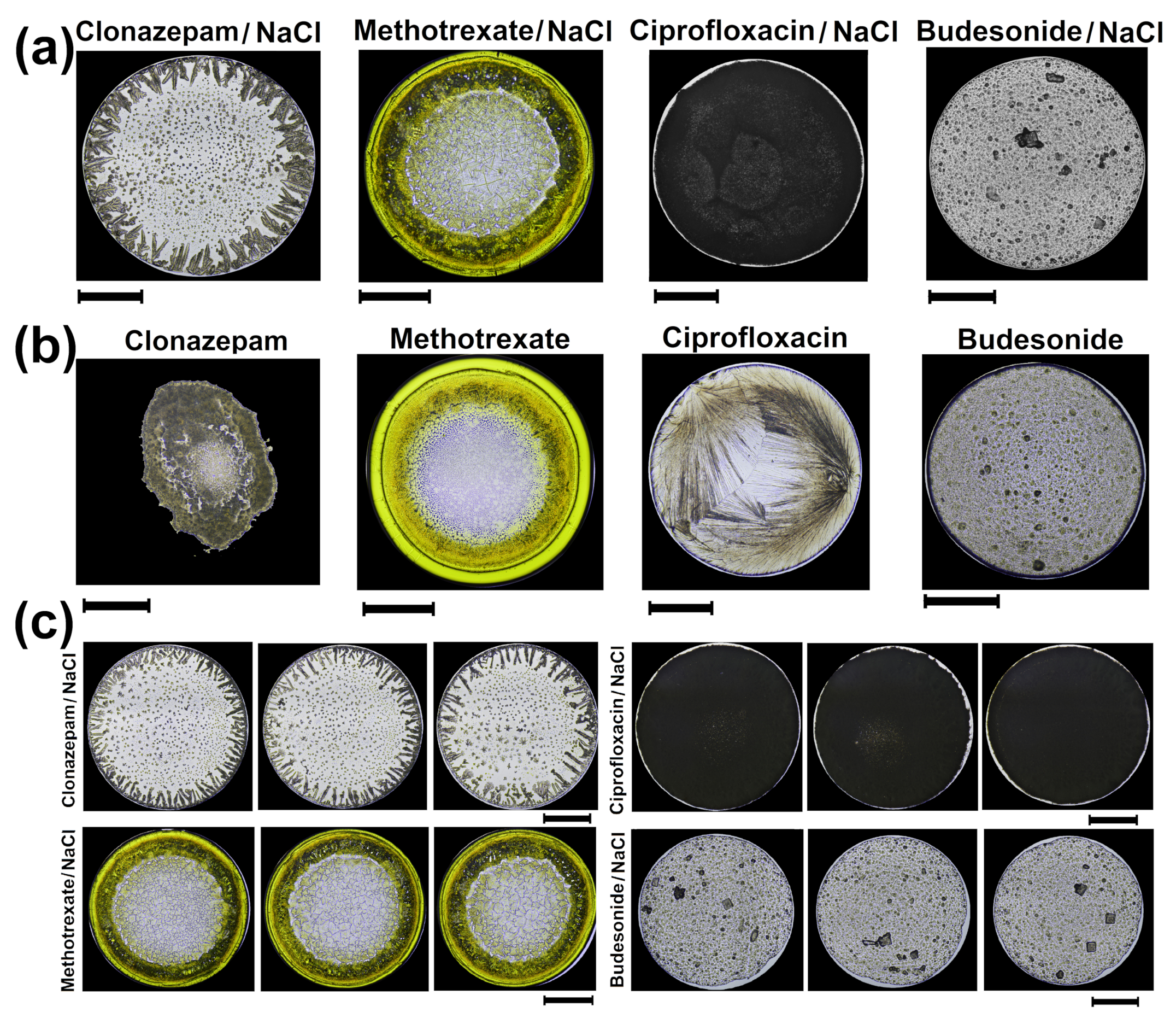
3.1. Pattern Formation in Medicines by Droplet Evaporation
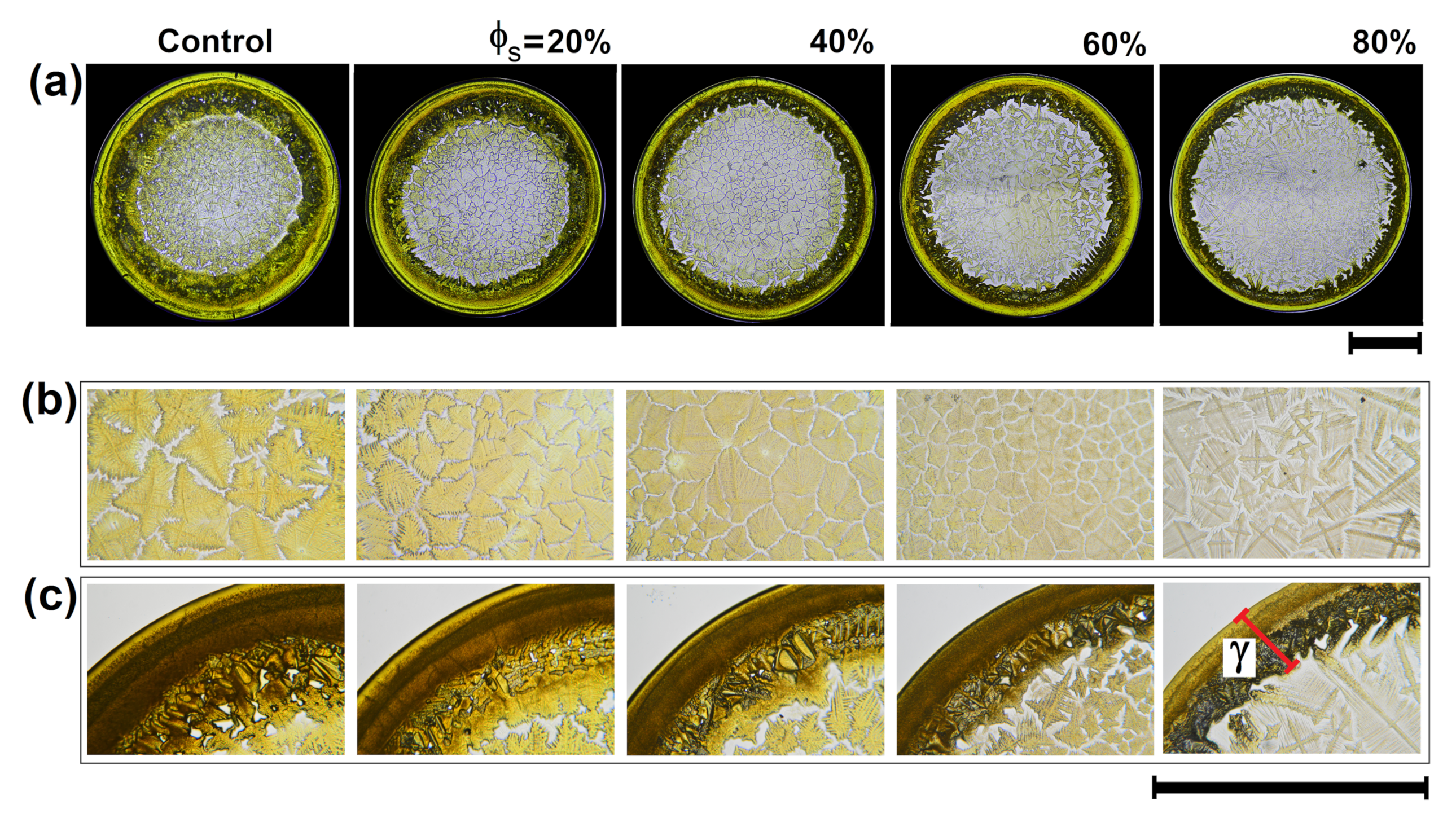
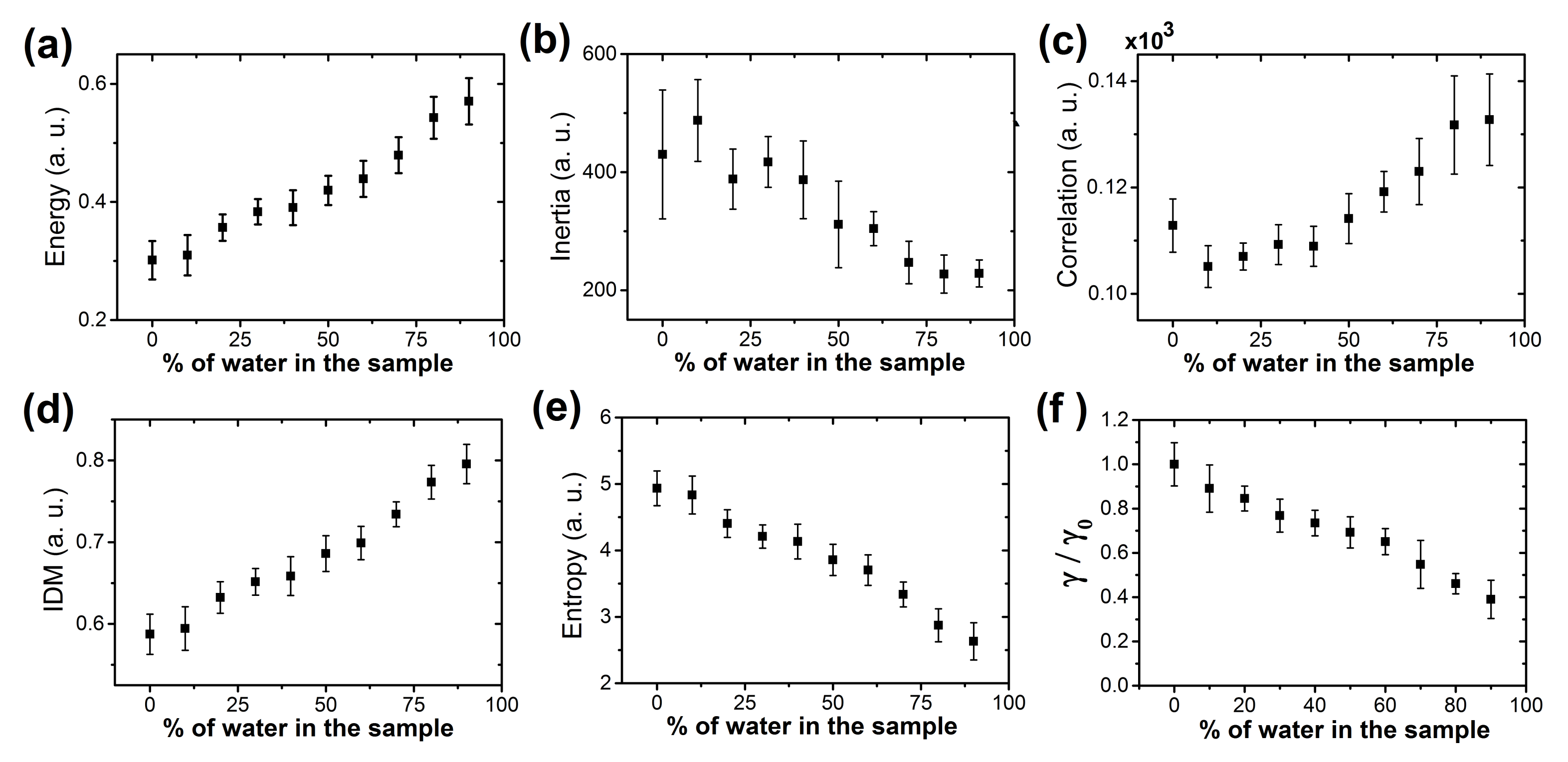
3.2. Texture Analysis and Classification of Dry Droplet Patterns
3.3. Patterns Formation of Dried Droplets of Medicines: Physical Mechanisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Moffat, A.; Assi, S.; Watt, R. Identifying counterfeit medicines using near infrared spectroscopy. J. Near Infrared Spectrosc. 2010, 18, 1–15. [Google Scholar] [CrossRef]
- Rodionova, O.; Houmøller, L.; Pomerantsev, A.; Geladi, P.; Burger, J.; Dorofeyev, V.; Arzamastsev, A. NIR spectrometry for counterfeit drug detection: A feasibility study. Anal. Chim. Acta 2005, 549, 151–158. [Google Scholar] [CrossRef]
- De Veij, M.; Vandenabeele, P.; Hall, K.; Fernandez, F.; Green, M.; White, N.; Dondorp, A.; Newton, P.; Moens, L. Fast detection and identification of counterfeit antimalarial tablets by Raman spectroscopy. J. Raman Spectrosc. 2007, 38, 181–187. [Google Scholar] [CrossRef]
- De Veij, M.; Deneckere, A.; Vandenabeele, P.; de Kaste, D.; Moens, L. Detection of counterfeit Viagra® with Raman spectroscopy. J. Pharm. Biomed. Anal. 2008, 46, 303–309. [Google Scholar] [CrossRef]
- Sherma, J. Analysis of counterfeit drugs by thin layer chromatography. Acta Chromatogr. 2007, 19, 5. [Google Scholar]
- Custers, D.; Canfyn, M.; Courselle, P.; De Beer, J.; Apers, S.; Deconinck, E. Headspace–gas chromatographic fingerprints to discriminate and classify counterfeit medicines. Talanta 2014, 123, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Lebel, P.; Gagnon, J.; Furtos, A.; Waldron, K. A rapid, quantitative liquid chromatography-mass spectrometry screening method for 71 active and 11 natural erectile dysfunction ingredients present in potentially adulterated or counterfeit products. J. Chromatogr. A 2014, 1343, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Mariotti, K.; Schwab, N.; Sabin, G.; Rocha, W.; de Castro, E.; Limberger, R.; Mayorga, P.; Bueno, M.; Romão, W. Fingerprinting of sildenafil citrate and tadalafil tablets in pharmaceutical formulations via X-ray fluorescence (XRF) spectrometry. J. Pharm. Biomed. Anal. 2012, 58, 7–11. [Google Scholar] [CrossRef]
- Yakhno, T.A.; Yakhno, V.; Sanin, A.; Sanina, O.; Pelyushenko, A.; Egorova, N.; Terentiev, I.; Smetanina, S.; Korochkina, O.; Yashukova, E. The informative-capacity phenomenon of drying drops. IEEE Eng. Med. Biol. 2005, 24, 96–104. [Google Scholar] [CrossRef]
- Rapis, E. A change in the physical state of a nonequilibrium blood plasma protein film in patients with carcinoma. Tech. Phys. 2002, 47, 510–512. [Google Scholar] [CrossRef]
- Yakhno, T.; Sedova, O.; Sanin, A.; Pelyushenko, A. On the existence of regular structures in liquid human blood serum (plasma) and phase transitions in the course of its drying. Tech. Phys. 2003, 48, 399–403. [Google Scholar] [CrossRef]
- Kasyap, T.; Koch, D.; Wu, M. Bacterial collective motion near the contact line of an evaporating sessile drop. Phys. Fluids 2014, 26, 111703. [Google Scholar] [CrossRef]
- Thokchom, A.; Swaminathan, R.; Singh, A. Fluid flow and particle dynamics inside an evaporating droplet containing live bacteria displaying chemotaxis. Langmuir 2014, 30, 12144–12153. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Ramírez, M.; Reyes-Figueroa, A.; Ruiz-Suárez, J.; González-Gutiérrez, J. Pattern formation of stains from dried drops to identify spermatozoa motility. Colloids Surf. B 2018, 169, 486–493. [Google Scholar] [CrossRef] [PubMed]
- González-Gutiérrez, J.; Pérez-Isidoro, R.; Pérez-Camacho, M.; Ruiz-Suárez, J. The calorimetric properties of liposomes determine the morphology of dried droplets. Colloids Surf. B 2017, 155, 215–222. [Google Scholar] [CrossRef]
- Andersen, J.; Henriksen, C.; Laursen, J.; Nielsen, A. Computerised image analysis of biocrystallograms originating from agricultural products. Comput. Electron. Agric. 1999, 22, 51–69. [Google Scholar] [CrossRef]
- Busscher, N.; Kahl, J.; Andersen, J.; Huber, M.; Mergardt, G.; Doesburg, P.; Paulsen, M.; Ploeger, A. Standardization of the biocrystallization method for carrot samples. Biol. Agric. Hortic. 2010, 27, 1–23. [Google Scholar] [CrossRef]
- Kokornaczyk, M.; Dinelli, G.; Marotti, I.; Benedettelli, S.; Nani, D.; Betti, L. Self-organized crystallization patterns from evaporating droplets of common wheat grain leakages as a potential tool for quality analysis. Sci. World J. 2011, 11, 1712–1725. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Iannacchione, G.S. A comparative study of the phase separation of a nematic liquid crystal in the self-assembling drying protein drops. MRS Adv. 2019, 4, 1309–1314. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Athair, A.S.; Iannacchione, G.S. A comparative study of the drying evolution and dried morphology of two globular proteins in de-ionized water solutions. RSC Adv. 2020, 10, 16906–16916. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Obayemi, J.D.; Iannacchione, G.S. Concentration-driven phase transition and self-assembly in drying droplets of diluting whole blood. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Hamadeh, L.; Imran, S.; Bencsik, M.; Sharpe, G.R.; Johnson, M.A.; Fairhurst, D.J. Machine learning analysis for quantitative discrimination of dried blood droplets. Sci. Rep. 2020, 10, 1–13. [Google Scholar]
- Li, X.; Sanderson, A.R.; Allen, S.S.; Lahr, R.H. Tap water fingerprinting using a convolutional neural network built from images of the coffee-ring effect. Analyst 2020, 145, 1511–1523. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Iannacchione, G.S. Statistical Image Analysis of Drying Bovine Serum Albumin Droplets in Phosphate Buffered Saline. arXiv 2021, arXiv:2105.05922. [Google Scholar]
- Harindran, A.; Hashmi, S.; Madhurima, V. Pattern formation of dried droplets of milk during different processes and classifying them using artificial neural networks. J. Dispers. Sci. Technol. 2021, 1880927. [Google Scholar] [CrossRef]
- Carreón, Y.; González-Gutiérrez, J.; Pérez-Camacho, M.; Mercado-Uribe, H. Patterns produced by dried droplets of protein binary mixtures suspended in water. Colloids Surf. B 2018, 161, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Devineau, S.; Anyfantakis, M.; Marichal, L.; Kiger, L.; Morel, M.; Rudiuk, S.; Baigl, D. Protein adsorption and reorganization on nanoparticles probed by the coffee-ring effect: Application to single point mutation detection. J. Am. Chem. Soc. 2016, 138, 11623–11632. [Google Scholar] [CrossRef]
- Sett, A.; Dasgupta, S.; DasGupta, S. Rapid estimation of the β-sheet content of Human Serum Albumin from the drying patterns of HSA-nanoparticle droplets. Colloids Surf. A Physicochem. Eng. Asp. 2018, 540, 177–185. [Google Scholar] [CrossRef]
- Choi, S.; Moon, S.; Shin, J.; Park, H.; Jin, K. Label-free biochemical analytic method for the early detection of adenoviral conjunctivitis using human tear biofluids. Anal. Chem. 2014, 86, 11093–11099. [Google Scholar] [CrossRef]
- Trantum, J.; Wright, D.; Haselton, F. Biomarker-mediated disruption of coffee-ring formation as a low resource diagnostic indicator. Langmuir 2012, 28, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Gulka, C.; Swartz, J.; Trantum, J.; Davis, K.; Peak, C.; Denton, A.; Haselton, F.; Wright, D. Coffee rings as low-resource diagnostics: Detection of the malaria biomarker plasmodium falciparum histidine-rich protein-ii using a surface-coupled ring of ni (ii) nta gold-plated polystyrene particles. ACS Appl. Mater. Interfaces 2014, 6, 6257–6263. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Huang, C. Fluorimetric determination of fluorescein at the femtomole level with a self-ordered ring of a sessile droplet on glass slide support. J. Anal. Chem. 2006, 61, 647–653. [Google Scholar] [CrossRef]
- Huang, C.; Liu, Y.; Li, Y. Microscopic determination of tetracycline based on aluminum-sensitized fluorescence of a self-ordered ring formed by a sessile droplet on glass slide support. J. Pharm. Biomed. 2004, 34, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Giri, A.; Choudhury, M.; Tarafdar, S. Experiment and simulation of multifractal growth of crystalline NaCl aggregates in aqueous gelatin medium. Colloids Surf. A Physicochem. Eng. Asp. 2013, 432, 127–131. [Google Scholar] [CrossRef]
- Pérez-Isidoro, R.; Reyes-Figueroa, A.; Ruiz-Suárez, J. Ionic interactions determine the morphology of dried alkali/liposome suspension droplets. Colloids Surf. B 2017, 160, 473–482. [Google Scholar] [CrossRef]
- Gorr, H.; Zueger, J.; McAdams, D.; Barnard, J. Salt-induced pattern formation in evaporating droplets of lysozyme solutions. Colloids Surf. B 2013, 103, 59–66. [Google Scholar] [CrossRef]
- Chen, G.; Mohamed, G. Complex protein patterns formation via salt-induced self-assembly and droplet evaporation. Eur. Phys. J. E 2010, 33, 19–26. [Google Scholar] [CrossRef]
- Glibitskiy, G.; Glibitskiy, D.; Gorobchenko, O.; Nikolov, O.; Roshal, A.; Semenov, M.; Gasan, A. Textures on the surface of BSA films with different concentrations of sodium halides and water state in solution. Nanoscale Res. Lett. 2015, 10, 155. [Google Scholar] [CrossRef][Green Version]
- González-Gutiérrez, J.; Pérez-Isidoro, R.; Ruiz-Suárez, J. A technique based on droplet evaporation to recognize alcoholic drinks. Rev. Sci. Instrum. 2017, 88, 074101. [Google Scholar] [CrossRef]
- Hosseini, M.; Nahidi, F.; Majdfar, Z. Comparison of fern and evaporation tests for detection of ruptured fetal membranes. East. Mediterr. Health J. 2007, 13, 197–200. [Google Scholar]
- Kimovich, M.; Zimin, Y.; Bochkareva, A. Morphology of dried blood serum specimens of viral hepatitis. Hepat. Mon. 2007, 7, 207–210. [Google Scholar]
- Choi, Y.; Han, J.; Kim, C. Pattern formation in drying of particle-laden sessile drops of polymer solutions on solid substrates. Korean J. Chem. Eng. 2011, 28, 2130–2136. [Google Scholar] [CrossRef]
- Yunker, P. Coffee-Rings and Glasses: Colloids Out of Equilibrium. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2012. [Google Scholar]
- Deegan, R.; Bakajin, O.; Dupont, T.; Huber, G.; Nagel, S.; Witten, T. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R. Marangoni effect reverses coffee-ring depositions. J. Phys. Chem. B 2006, 110, 7090–7094. [Google Scholar] [CrossRef]
- Sempels, W.; De Dier, R.; Mizuno, H.; Hofkens, J.; Vermant, J. Auto-production of biosurfactants reverses the coffee ring effect in a bacterial system. Nat. Commun. 2013, 4, 1757. [Google Scholar] [CrossRef] [PubMed]
- Still, T.; Yunker, P.; Yodh, A. Surfactant-induced Marangoni eddies alter the coffee-rings of evaporating colloidal drops. Langmuir 2012, 28, 4984–4988. [Google Scholar] [CrossRef]
- Kajiya, T.; Kobayashi, W.; Okuzono, T.; Doi, M. Controlling the drying and film formation processes of polymer solution droplets with addition of small amount of surfactants. J. Phys. Chem. B. 2009, 113, 15460–15466. [Google Scholar] [CrossRef]
- Rossi, M.; Marin, A.; Kähler, C. Interfacial flows in sessile evaporating droplets of mineral water. Phys. Rev. E 2019, 100, 033103. [Google Scholar] [CrossRef]
- Deegan, R.; Bakajin, O.; Dupont, T.; Huber, G.; Nagel, S.; Witten, T. Contact line deposits in an evaporating drop. Phys. Rev. E 2000, 62, 756. [Google Scholar] [CrossRef]
- Carreón, Y.J.; Ríos-Ramírez, M.; Vázquez-Vergara, P.; Salinas-Almaguer, S.; Cipriano-Urbano, I.; Briones-Aranda, A.; Díaz-Hernández, O.; Santos, G.J.E.; González-Gutiérrez, J. Effects of substrate temperature on patterns produced by dried droplets of proteins. Colloids Surfaces Biointerfaces 2021, 203, 111763. [Google Scholar] [CrossRef]
- Carreón, Y.; Ríos-Ramírez, M.; Moctezuma, R.; González-Gutiérrez, J. Texture analysis of protein deposits produced by droplet evaporation. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gope, A.; Kafle, R.; Iannacchione, G. Phase separation of a nematic liquid crystal in the self-assembly of lysozyme in a drying aqueous solution drop. MRS Commun. 2019, 9, 150–158. [Google Scholar] [CrossRef]
- Shultz, E.K. Multivariate receiver-operating characteristic curve analysis: Prostate cancer screening as an example. Clin. Chem. 1995, 41, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Li, W.H.; Song, Y.C.; Zhang, H.; Zhou, Z.J.; Xie, X.; Zeng, Q.N.; Guo, K.; Wang, T.; Xia, P.; Chang, D.M. Decreased expression of Hsa_circ_00001649 in gastric cancer and its clinical significance. Dis. Markers 2017, 4587698. [Google Scholar] [CrossRef] [PubMed]
- Munir, K.; Elahi, H.; Ayub, A.; Frezza, F.; Rizzi, A. Cancer diagnosis using deep learning: A bibliographic review. Cancers 2019, 11, 1235. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics; Cengage Learning: Belmont, CA, USA, 2015. [Google Scholar]
- Bin, L.; Bennacer, R.; Bouvet, A. Evaporation of methanol droplet on the Teflon surface under different air velocities. Appl. Therm. Eng. 2011, 31, 3792–3798. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, F.; Han, K.; Yang, B.; Song, G. Evaporation characteristics of acetone–butanol–ethanol and diesel blends droplets at high ambient temperatures. Fuel 2015, 160, 43–49. [Google Scholar] [CrossRef]
- Marin, A.; Liepelt, R.; Rossi, M.; Kähler, C. Surfactant-driven flow transitions in evaporating droplets. Soft Matter 2016, 12, 1593–1600. [Google Scholar] [CrossRef]
- Goldman, I.; Lichtenstein, N.; Oliverio, V. Carrier-mediated transport of the folic acid analogue, methotrexate, in the L1210 leukemia cell. J. Biol. Chem. 1968, 243, 5007–5017. [Google Scholar] [CrossRef]
- Tian, H.; Cronstein, B. Understanding the mechanisms of action of methotrexate. Bull. NYU Hosp. Jt. Dis. 2007, 65, 168–173. [Google Scholar]
- Lomesh, S.; Bala, M.; Kumar, D.; Kumar, I. Investigation of molecular interactions of the drug Diclofenac Sodium Salt in water and aqueous sorbitol systems at different temperatures (305.15 K–315.15 K). J. Mol. Liq. 2019, 289, 109479. [Google Scholar] [CrossRef]
- Islam, M.; Hossain, M.; Ahmed, T.; Bhusal, P.; Rana, M.; Khan, T. The effect of hydrophilic and hydrophobic polymers on release profiles of diclofenac sodium from matrix tablets. Arch. Pharm. Pract. 2013, 4, 119064. [Google Scholar]
- Candido, K.D.; Perozo, O.; Knezevic, N. Pharmacology of acetaminophen, nonsteroidal antiinflammatory drugs, and steroid medications: Implications for anesthesia or unique associated risks. Anesthesiol. Clin. 2017, 35, e145–e162. [Google Scholar] [CrossRef]
- Lock, L.; LaComb, M.; Schwarz, K.; Cheetham, A.; Lin, Y.; Zhang, P.; Cui, H. Self-assembly of natural and synthetic drug amphiphiles into discrete supramolecular nanostructures. Faraday Discuss. 2013, 166, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Chaudhry, M. Thermodynamic study of three pharmacologically significant drugs: Density, viscosity, and refractive index measurements at different temperatures. J. Chem. Thermodyn. 2009, 41, 221–226. [Google Scholar] [CrossRef]
- Wu, M.; Di, Y.; Man, X.; Doi, M. Drying Droplets with Soluble Surfactants. Langmuir 2019, 35, 14734–14741. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R. Analysis of the microfluid flow in an evaporating sessile droplet. Langmuir 2005, 21, 3963–3971. [Google Scholar] [CrossRef]
- Nguyen, V.; Stebe, K. Patterning of small particles by a surfactant-enhanced Marangoni-Bénard instability. Phys. Rev. Lett. 2002, 88, 164501. [Google Scholar] [CrossRef]
- Truskett, V.; Stebe, K. Influence of surfactants on an evaporating drop: Fluorescence images and particle deposition patterns. Langmuir 2003, 19, 8271–8279. [Google Scholar] [CrossRef]
- Roy, B.; Karmakar, S.; Tarafdar, S. Self assembled transparent conducting network of multi-walled carbon nanotubes formed by evaporation. Mater. Lett. 2017, 207, 86–88. [Google Scholar] [CrossRef]
- Yan, L.; Li, Y.; Deng, Z.; Zhuang, J.; Sun, X. Surfactant-assisted hydrothermal synthesis of hydroxyapatite nanorods. Int. J. Inorg. Mater. 2001, 3, 633–637. [Google Scholar] [CrossRef]
- Talmon, Y. Staining and drying-induced artifacts in electron microscopy of surfactant dispersions. J. Colloid Interface Sci. 1983, 93, 366–382. [Google Scholar] [CrossRef]
- Anyfantakis, M.; Geng, Z.; Morel, M.; Rudiuk, S.; Baigl, D. Modulation of the coffee-ring effect in particle/surfactant mixtures: The importance of particle–interface interactions. Langmuir 2015, 31, 4113–4120. [Google Scholar] [CrossRef]
- Noh, H.; Vogler, E. Volumetric interpretation of protein adsorption: Competition from mixtures and the Vroman effect. Biomaterials 2007, 28, 405–422. [Google Scholar] [CrossRef] [PubMed]
- Vehring, R. Pharmaceutical particle engineering via spray drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Sievers, R.; Huang, E.; Villa, J.; Engling, G.; Brauer, P. Micronization of water-soluble or alcohol-soluble pharmaceuticals and model compounds with a low-temperature Bubble Dryer®. J. Supercrit. Fluids 2003, 26, 9–16. [Google Scholar] [CrossRef]
- Takhistov, P.; Chang, H. Complex stain morphologies. Ind. Eng. Chem. Res. 2002, 41, 6256–6269. [Google Scholar] [CrossRef]
- Yakhno, T. Sodium chloride crystallization from drying drops of albumin–salt solutions with different albumin concentrations. Tech. Phys. 2015, 60, 1601–1608. [Google Scholar] [CrossRef]
- Old, O.; Fullwood, L.; Scott, R.; Lloyd, G.; Almond, L.; Shepherd, N.; Stone, N.; Barr, H.; Kendall, C. Vibrational spectroscopy for cancer diagnostics. Anal. Methods 2014, 6, 3901–3917. [Google Scholar] [CrossRef]
- Griffiths, P.; De Haseth, J. Fourier Transform Infrared Spectrometry; John Wiley & Sons: Hoboken, NJ, USA, 2007; Volume 171. [Google Scholar]
- Smith, B. Fundamentals of Fourier Transform Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Witkowski, M. The use of Raman spectroscopy in the detection of counterfeit and adulterated pharmaceutical products. Am. Pharm. Rev. 2005, 8, 56. [Google Scholar]
- Baeten, V.; Meurens, M.; Morales, M.; Aparicio, R. Detection of virgin olive oil adulteration by Fourier transform Raman spectroscopy. J. Agric. Food Chem. 1996, 44, 2225–2230. [Google Scholar] [CrossRef]
- Oliveira, F.; Brandao, C.; Ramalho, H.; da Costa, L.; Suarez, P.; Rubim, J. Adulteration of diesel/biodiesel blends by vegetable oil as determined by Fourier transform (FT) near infrared spectrometry and FT-Raman spectroscopy. Anal. Chim. Acta 2007, 587, 194–199. [Google Scholar] [CrossRef] [PubMed]





| CROWN | ||||||||||
| Energy | ||||||||||
| P.S.() % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.77556 | 0.05941 | 2.46× 10 | 0.65912 | 0.89199 | 0.60444 | 0.10853 | 0.32969 | 0.39174 | 0.81715 |
| 20 | 0.91667 | 0.03496 | 9.33× 10 | 0.84815 | 0.98518 | 0.91282 | 0.05388 | 2.09× 10 | 0.80723 | 1.01841 |
| 30 | 0.96944 | 0.01734 | 4.20× 10 | 0.93547 | 1.00342 | 0.97778 | 0.02099 | 8.24× 10 | 0.93663 | 1.01892 |
| 40 | 0.99111 | 0.00874 | 6.37× 10 | 0.97399 | 1.00823 | 0.97333 | 0.02408 | 9.99× 10 | 0.92613 | 1.02053 |
| 50 | 0.99333 | 0.00647 | 5.23× 10 | 0.98065 | 1.00602 | 1 | 0 | 4.59× 10 | 1 | 1 |
| 60 | 1 | 0 | 2.87× 10 | 1 | 1 | 1 | 0 | 3.07× 10 | 1 | 1 |
| 70 | 1 | 0 | 6.34× 10 | 1 | 1 | 1 | 0 | 4.59× 10 | 1 | 1 |
| 80 | 1 | 0 | 3.69× 10 | 1 | 1 | 1 | 0 | 1.13× 10 | 1 | 1 |
| 90 | 1 | 0 | 2.87× 10 | 1 | 1 | 1 | 0 | 4.59× 10 | 1 | 1 |
| Inertia | Correlation | |||||||||
| P.S.() % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.65778 | 0.10158 | 0.14089 | 0.45868 | 0.85688 | 0.87556 | 0.06812 | 4.57× 10 | 0.74203 | 1.00908 |
| 20 | 0.64103 | 0.11708 | 0.20523 | 0.41156 | 0.87049 | 0.85641 | 0.08099 | 0.00137 | 0.69767 | 1.01515 |
| 30 | 0.52889 | 0.1193 | 0.78746 | 0.29507 | 0.7627 | 0.73111 | 0.09476 | 0.03102 | 0.54539 | 0.91683 |
| 40 | 0.62222 | 0.1084 | 0.25402 | 0.40977 | 0.83468 | 0.74 | 0.09276 | 0.0251 | 0.55819 | 0.92181 |
| 50 | 0.79048 | 0.08772 | 0.00776 | 0.61855 | 0.9624 | 0.52143 | 0.11191 | 0.8443 | 0.30209 | 0.74077 |
| 60 | 0.84889 | 0.08019 | 0.00113 | 0.69172 | 1.00606 | 0.87556 | 0.06508 | 4.57× 10 | 0.748 | 1.00311 |
| 70 | 0.94286 | 0.05003 | 4.93× 10 | 0.84479 | 1.04092 | 0.91429 | 0.0546 | 1.46× 10 | 0.80727 | 1.0213 |
| 80 | 0.96111 | 0.03916 | 5.12× 10 | 0.88436 | 1.03787 | 0.96111 | 0.03686 | 5.12× 10 | 0.88887 | 1.03336 |
| 90 | 0.95238 | 0.04887 | 3.38× 10 | 0.85659 | 1.04817 | 1 | 0 | 4.59× 10 | 1 | 1 |
| IDM | Entropy | |||||||||
| P.S.() % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.61111 | 0.1088 | 0.29976 | 0.39786 | 0.82436 | 0.62444 | 0.1058 | 0.24549 | 0.41708 | 0.83181 |
| 20 | 0.91282 | 0.054 | 2.09× 10 | 0.80699 | 1.01865 | 0.92821 | 0.0482 | 1.20× 10 | 0.83374 | 1.02267 |
| 30 | 0.97556 | 0.02247 | 9.07× 10 | 0.93152 | 1.01959 | 0.99111 | 0.01183 | 4.58× 10 | 0.96793 | 1.0143 |
| 40 | 0.97556 | 0.02247 | 9.07× 10 | 0.93152 | 1.01959 | 0.99111 | 0.01183 | 4.58× 10 | 0.96793 | 1.0143 |
| 50 | 1 | 0 | 4.59× 10 | 1 | 1 | 1 | 0 | 4.59× 10 | 1 | 1 |
| 60 | 1 | 0 | 3.07× 10 | 1 | 1 | 1 | 0 | 3.07× 10 | 1 | 1 |
| 70 | 1 | 0 | 4.59× 10−6 | 1 | 1 | 1 | 0 | 4.59× 10−6 | 1 | 1 |
| 80 | 1 | 0 | 1.13× 10 | 1 | 1 | 1 | 0 | 1.13× 10 | 1 | 1 |
| 90 | 1 | 0 | 4.59× 10 | 1 | 1 | 1 | 0 | 4.59× 10 | 1 | 1 |
| CENTER | ||||||||||
| Area | Energy | |||||||||
| P.S.() % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.89778 | 0.06096 | 2.05× 10 | 0.77829 | 1.01726 | 0.50222 | 0.10877 | 0.98345 | 0.28903 | 0.71541 |
| 20 | 0.54872 | 0.11511 | 0.66166 | 0.3231 | 0.77434 | 0.62051 | 0.11223 | 0.27901 | 0.40054 | 0.84049 |
| 30 | 0.77333 | 0.08836 | 0.01074 | 0.60016 | 0.94651 | 0.76444 | 0.0883 | 0.01359 | 0.59139 | 0.9375 |
| 40 | 0.88889 | 0.06519 | 2.84× 10 | 0.76113 | 1.01665 | 0.95111 | 0.0372 | 2.55× 10 | 0.87819 | 1.02403 |
| 50 | 0.89778 | 0.06478 | 2.05× 10 | 0.77082 | 1.02474 | 0.99111 | 0.01183 | 4.58× 10 | 0.96793 | 1.0143 |
| 60 | 0.96444 | 0.03088 | 1.46× 10 | 0.90391 | 1.02497 | 1 | 0 | 3.07× 10 | 1 | 1 |
| 70 | 0.99524 | 0.00787 | 5.65× 10 | 0.97981 | 1.01067 | 1 | 0 | 4.59× 10 | 1 | 1 |
| 80 | 1 | 0 | 1.13× 10 | 1 | 1 | 1 | 0 | 1.13× 10 | 1 | 1 |
| 90 | 1 | 0 | 1.13× 10 | 1 | 1 | 1 | 0 | 3.07× 10 | 1 | 1 |
| Inertia | Correlation | |||||||||
| P.S.() % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.69333 | 0.09833 | 0.07118 | 0.50061 | 0.88606 | 0.33778 | 0.10267 | 0.13004 | 0.13654 | 0.53901 |
| 20 | 0.51282 | 0.11935 | 0.90832 | 0.2789 | 0.74675 | 0.43846 | 0.11819 | 0.58041 | 0.20681 | 0.67011 |
| 30 | 0.6 | 0.10963 | 0.35069 | 0.38514 | 0.81486 | 0.82667 | 0.07914 | 0.0023 | 0.67155 | 0.98178 |
| 40 | 0.54222 | 0.10975 | 0.69355 | 0.32712 | 0.75732 | 0.83333 | 0.07762 | 0.00225 | 0.68121 | 0.98546 |
| 50 | 0.8 | 0.0797 | 0.00511 | 0.64378 | 0.95622 | 0.62667 | 0.10584 | 0.23716 | 0.41923 | 0.83411 |
| 60 | 0.79111 | 0.09878 | 0.00659 | 0.5975 | 0.98473 | 0.57778 | 0.10858 | 0.46792 | 0.36497 | 0.79059 |
| 70 | 0.88571 | 0.06707 | 4.08× 10 | 0.75425 | 1.01718 | 0.52381 | 0.11333 | 0.82726 | 0.30169 | 0.74593 |
| 80 | 0.81667 | 0.1075 | 0.00541 | 0.60598 | 1.02736 | 0.89722 | 0.07095 | 4.85× 10 | 0.75816 | 1.03628 |
| 90 | 0.76 | 0.10475 | 0.01525 | 0.55469 | 0.96531 | 1 | 0 | 3.07× 10 | 1 | 1 |
| IDM | Entropy | |||||||||
| P.S.() % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.53556 | 0.11144 | 0.74002 | 0.31714 | 0.75397 | 0.52444 | 0.11011 | 0.81955 | 0.30864 | 0.74025 |
| 20 | 0.63333 | 0.11334 | 0.23103 | 0.4112 | 0.85547 | 0.63333 | 0.11357 | 0.23103 | 0.41074 | 0.85593 |
| 30 | 0.77778 | 0.09229 | 0.00953 | 0.59689 | 0.95866 | 0.62889 | 0.10737 | 0.22903 | 0.41845 | 0.83933 |
| 40 | 0.91111 | 0.05535 | 1.25× 10 | 0.80262 | 1.0196 | 0.79778 | 0.0831 | 0.00545 | 0.63491 | 0.96064 |
| 50 | 0.97778 | 0.02425 | 8.24× 10 | 0.93024 | 1.02531 | 0.92444 | 0.04749 | 7.46× 10 | 0.83138 | 1.01751 |
| 60 | 1 | 0 | 3.07× 10 | 1 | 1 | 0.94 | 0.04441 | 4.02× 10 | 0.85296 | 1.02704 |
| 70 | 1 | 0 | 4.59× 10 | 1 | 1 | 0.96667 | 0.02964 | 1.89× 10 | 0.90858 | 1.02475 |
| 80 | 1 | 0 | 1.13× 10 | 1 | 1 | 1 | 0 | 1.13× 10 | 1 | 1 |
| 90 | 1 | 0 | 3.07× 10 | 1 | 1 | 1 | 0 | 3.07× 10 | 1 | 1 |
| COMPLETE | ||||||||||
| Mean Pixel I. | Energy | |||||||||
| P.S. % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.82 | 0.08209 | 0.00282 | 0.6591 | 0.9809 | 0.49556 | 0.10757 | 0.96691 | 0.28472 | 0.70639 |
| 20 | 0.72821 | 0.10339 | 0.04037 | 0.52557 | 0.93084 | 0.79231 | 0.08537 | 0.00865 | 0.62499 | 0.95963 |
| 30 | 0.57778 | 0.10681 | 0.46792 | 0.36843 | 0.78712 | 0.58889 | 0.10693 | 0.40679 | 0.37931 | 0.79847 |
| 40 | 0.72 | 0.09515 | 0.04006 | 0.53351 | 0.90649 | 0.74 | 0.08984 | 0.0251 | 0.56393 | 0.91607 |
| 50 | 0.92222 | 0.05083 | 8.13× 10 | 0.8226 | 1.02184 | 0.84 | 0.07163 | 0.00151 | 0.69962 | 0.98038 |
| 60 | 0.96667 | 0.03433 | 1.33× 10 | 0.89938 | 1.03396 | 0.56444 | 0.1095 | 0.54755 | 0.34982 | 0.77907 |
| 70 | 0.99048 | 0.01254 | 6.95× 10 | 0.9659 | 1.01505 | 0.7119 | 0.09701 | 0.05212 | 0.52177 | 0.90204 |
| 80 | 1 | 0 | 1.13× 10 | 1 | 1 | 0.56944 | 0.11426 | 0.5419 | 0.3455 | 0.79339 |
| 90 | 1 | 0 | 3.07× 10 | 1 | 1 | 0.68444 | 0.10063 | 0.08519 | 0.48721 | 0.88168 |
| Inertia | Correlation | |||||||||
| P.S.() % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.59556 | 0.1093 | 0.37251 | 0.38134 | 0.80977 | 0.70667 | 0.0984 | 0.05376 | 0.51381 | 0.89952 |
| 20 | 0.5641 | 0.12243 | 0.56474 | 0.32414 | 0.80407 | 0.56923 | 0.11945 | 0.53402 | 0.33512 | 0.80334 |
| 30 | 0.60889 | 0.10971 | 0.30953 | 0.39386 | 0.82392 | 0.62667 | 0.10653 | 0.23716 | 0.41787 | 0.83547 |
| 40 | 0.52444 | 0.10859 | 0.81955 | 0.31161 | 0.73728 | 0.82222 | 0.07846 | 0.00264 | 0.66844 | 0.976 |
| 50 | 0.74667 | 0.08949 | 0.02133 | 0.57127 | 0.92207 | 0.83333 | 0.0747 | 0.00187 | 0.68693 | 0.97974 |
| 60 | 0.75556 | 0.10351 | 0.01708 | 0.55267 | 0.95844 | 0.81333 | 0.0791 | 0.00345 | 0.65829 | 0.96837 |
| 70 | 0.84762 | 0.07623 | 0.00144 | 0.69821 | 0.99703 | 0.84048 | 0.07949 | 0.00181 | 0.68469 | 0.99627 |
| 80 | 0.73889 | 0.11734 | 0.03589 | 0.50891 | 0.96887 | 0.99444 | 0.00922 | 1.41× 10 | 0.97637 | 1.01252 |
| 90 | 0.68 | 0.11305 | 0.09298 | 0.45842 | 0.90158 | 1 | 0 | 3.07× 10 | 1 | 1 |
| IDM | Entropy | |||||||||
| P.S.() % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.50444 | 0.10811 | 0.96691 | 0.29255 | 0.71634 | 0.55333 | 0.11077 | 0.61867 | 0.33623 | 0.77044 |
| 20 | 0.76154 | 0.09494 | 0.01881 | 0.57545 | 0.94762 | 0.72821 | 0.10749 | 0.04037 | 0.51753 | 0.93888 |
| 30 | 0.50667 | 0.11263 | 0.95039 | 0.28591 | 0.72743 | 0.53778 | 0.11455 | 0.72442 | 0.31326 | 0.7623 |
| 40 | 0.68222 | 0.09928 | 0.08902 | 0.48764 | 0.87681 | 0.71111 | 0.09812 | 0.04881 | 0.5188 | 0.90342 |
| 50 | 0.86444 | 0.06482 | 6.71× 10 | 0.7374 | 0.99148 | 0.84889 | 0.07013 | 0.00113 | 0.71143 | 0.98635 |
| 60 | 0.79111 | 0.08757 | 0.00659 | 0.61947 | 0.96275 | 0.81556 | 0.09132 | 0.00323 | 0.63658 | 0.99453 |
| 70 | 0.92143 | 0.04884 | 1.12× 10 | 0.8257 | 1.01716 | 0.88571 | 0.06712 | 4.08× 10 | 0.75416 | 1.01726 |
| 80 | 0.86667 | 0.07464 | 0.00128 | 0.72038 | 1.01296 | 0.9 | 0.07463 | 4.43× 10 | 0.75372 | 1.04628 |
| 90 | 0.90444 | 0.05854 | 1.60× 10 | 0.78971 | 1.01918 | 0.85111 | 0.0797 | 0.00105 | 0.69489 | 1.00733 |
| Clonazepam | |||||
| Entropy | |||||
| P.S. () % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.73016 | 0.13326 | 0.06773 | 0.46897 | 0.99135 |
| 20 | 0.74107 | 0.11309 | 0.06536 | 0.51941 | 0.96273 |
| 30 | 0.9881 | 0.01542 | 2.47 × 10 | 0.95787 | 1.01833 |
| 40 | 0.86364 | 0.07332 | 0.00217 | 0.71993 | 1.00734 |
| 50 | 0.91837 | 0.04996 | 1.65 × 10 | 0.82045 | 1.01628 |
| Ciprofloxacin | |||||
| Entropy | |||||
| P.S.() % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.99111 | 0.01183 | 4.58 × 10 | 0.96793 | 1.0143 |
| 20 | 1 | 0 | 7.08 × 10 | 1 | 1 |
| 30 | 1 | 0 | 1.13 × 10 | 1 | 1 |
| 40 | 1 | 0 | 7.08 × 10 | 1 | 1 |
| 50 | 1 | 0 | 2.10 × 10 | 1 | 1 |
| Budesonide | |||||
| Aggregate size | |||||
| P.S.() % | AUC | Std. Error | Asymptotic Prob | 95% LCL | 95% UCL |
| 10 | 0.72444 | 0.10247 | 0.0362 | 0.5236 | 0.92529 |
| 20 | 0.77778 | 0.09272 | 0.00953 | 0.59605 | 0.95951 |
| 30 | 0.95556 | 0.03616 | 2.12 × 10 | 0.88469 | 1.02642 |
| 40 | 1 | 0 | 3.07 × 10 | 1 | 1 |
| 50 | 1 | 0 | 3.07 × 10 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carreón, Y.J.P.; Díaz-Hernández, O.; Escalera Santos, G.J.; Cipriano-Urbano, I.; Solorio-Ordaz, F.J.; González-Gutiérrez, J.; Zenit, R. Texture Analysis of Dried Droplets for the Quality Control of Medicines. Sensors 2021, 21, 4048. https://doi.org/10.3390/s21124048
Carreón YJP, Díaz-Hernández O, Escalera Santos GJ, Cipriano-Urbano I, Solorio-Ordaz FJ, González-Gutiérrez J, Zenit R. Texture Analysis of Dried Droplets for the Quality Control of Medicines. Sensors. 2021; 21(12):4048. https://doi.org/10.3390/s21124048
Chicago/Turabian StyleCarreón, Yojana J. P., Orlando Díaz-Hernández, Gerardo J. Escalera Santos, Ivan Cipriano-Urbano, Francisco J. Solorio-Ordaz, Jorge González-Gutiérrez, and Roberto Zenit. 2021. "Texture Analysis of Dried Droplets for the Quality Control of Medicines" Sensors 21, no. 12: 4048. https://doi.org/10.3390/s21124048
APA StyleCarreón, Y. J. P., Díaz-Hernández, O., Escalera Santos, G. J., Cipriano-Urbano, I., Solorio-Ordaz, F. J., González-Gutiérrez, J., & Zenit, R. (2021). Texture Analysis of Dried Droplets for the Quality Control of Medicines. Sensors, 21(12), 4048. https://doi.org/10.3390/s21124048







