Novel Methodological Tools for Behavioral Interventions: The Case of HRV-Biofeedback. Sham Control and Quantitative Physiology-Based Assessment of Training Quality and Fidelity
Abstract
1. Introduction
1.1. Heart Rate Variability (HRV)-Biofeedback
1.2. Methodological Issues in HRV-Biofeedback Research
1.3. Aims of the Study and Hypotheses
2. Method
2.1. Participants
2.2. HRV-Biofeedback Training Protocol
2.2.1. Real HRV-Biofeedback
2.2.2. Sham HRV-Biofeedback
2.3. Training Expectancy Questionnaire
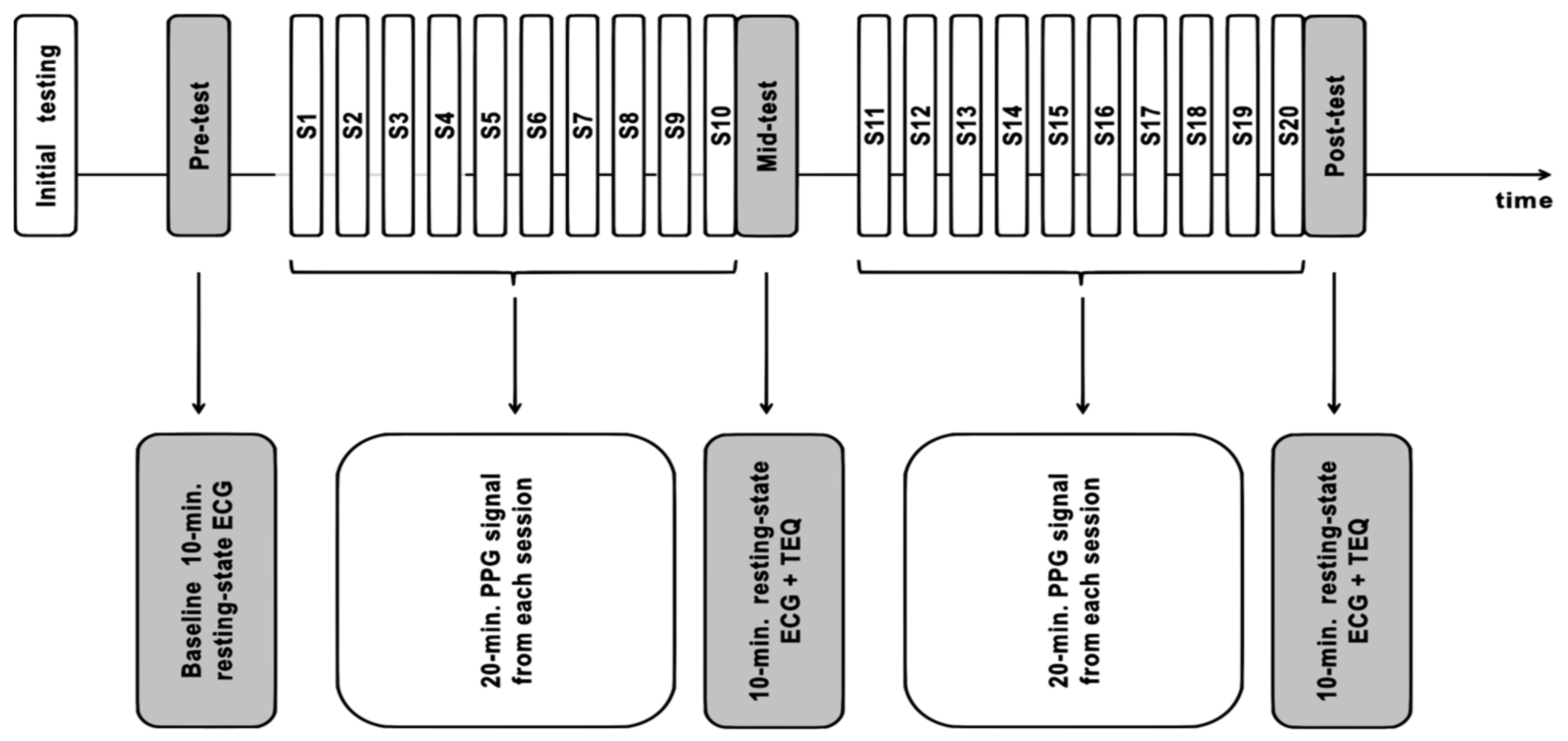
2.4. Study Design and Timeline
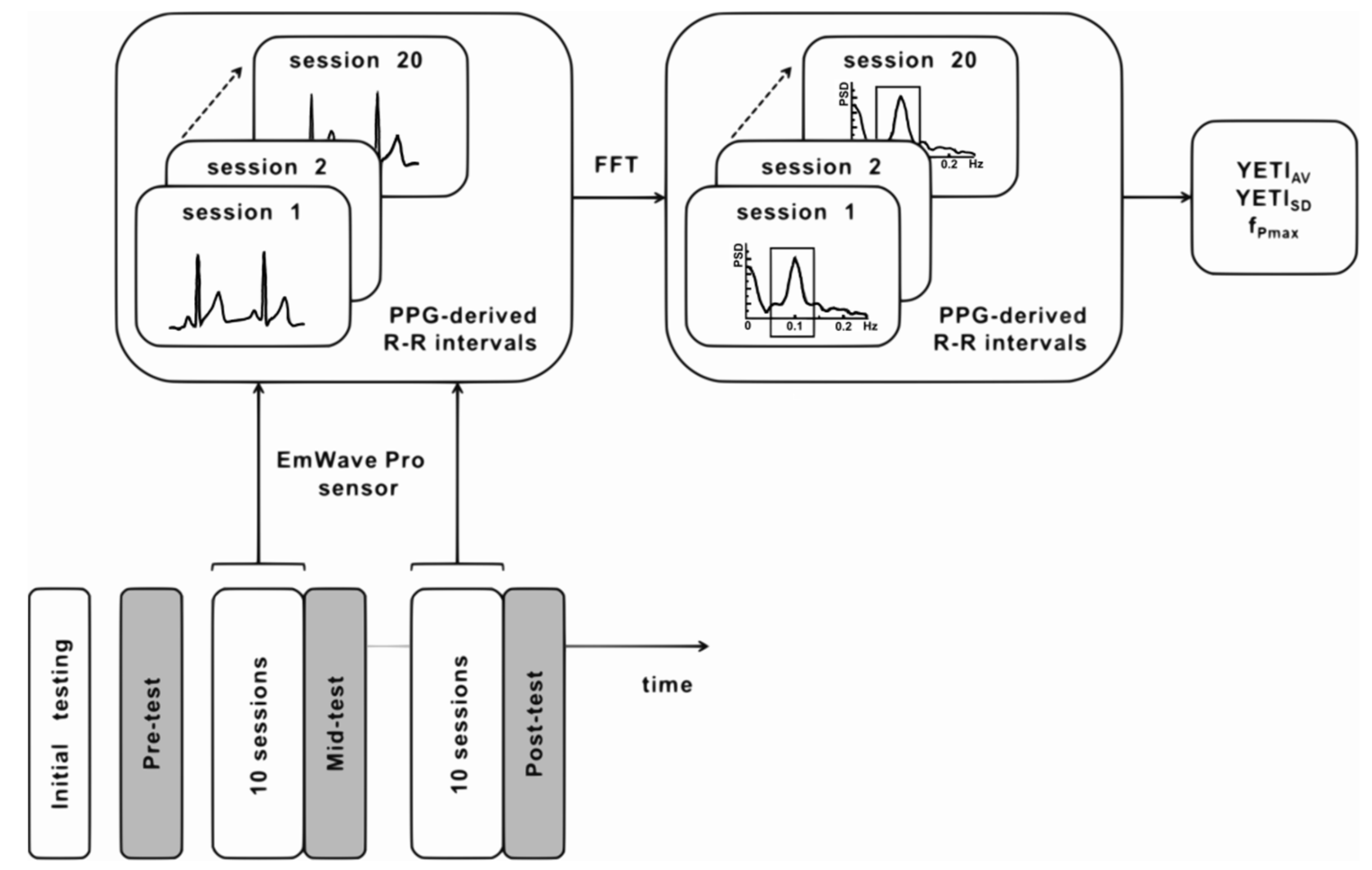
2.5. Quantitative Training Quality Assessment with Yield Efficiency of Training Index (YETI)
2.6. Physiological Data Analysis
2.6.1. HRV-Biofeedback Training Data
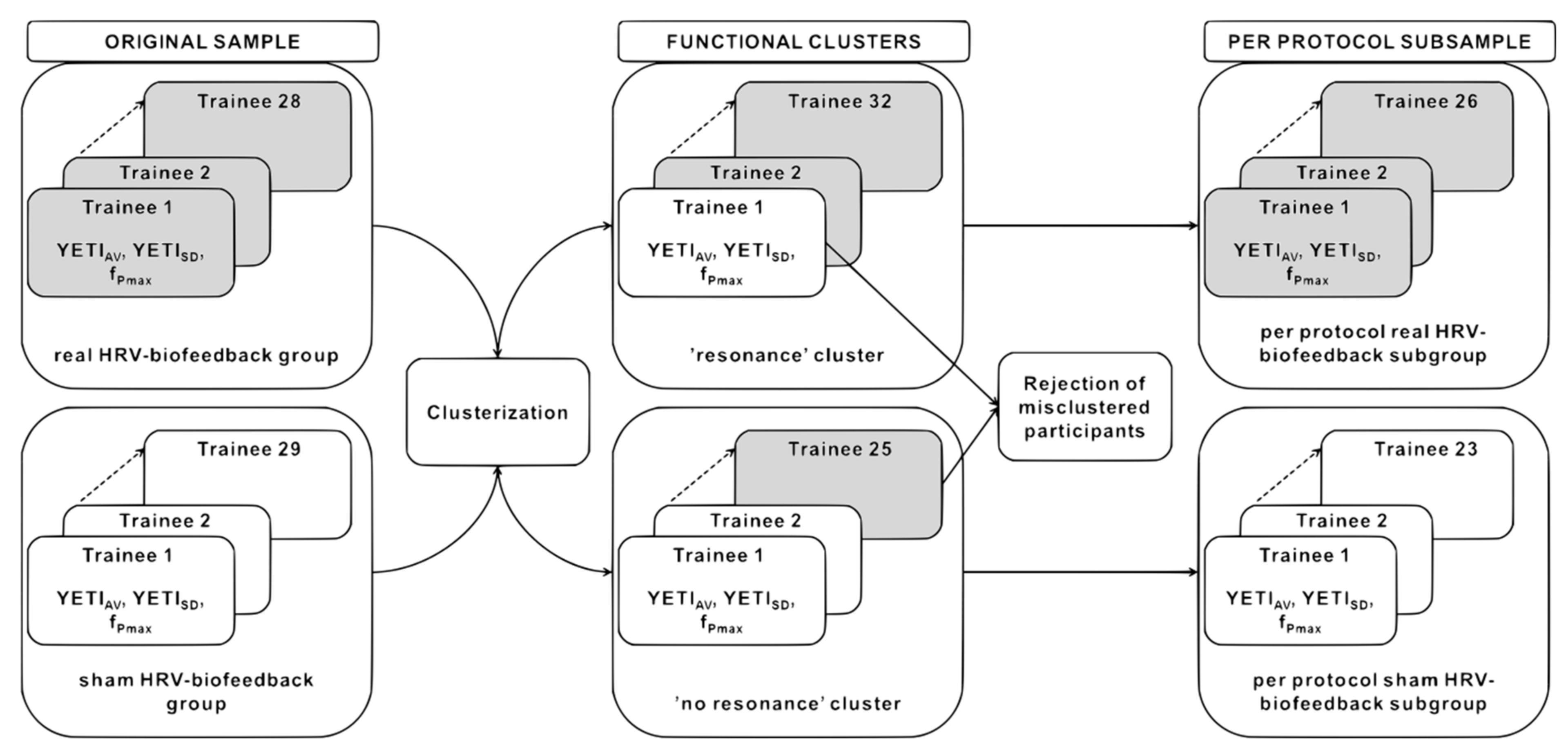
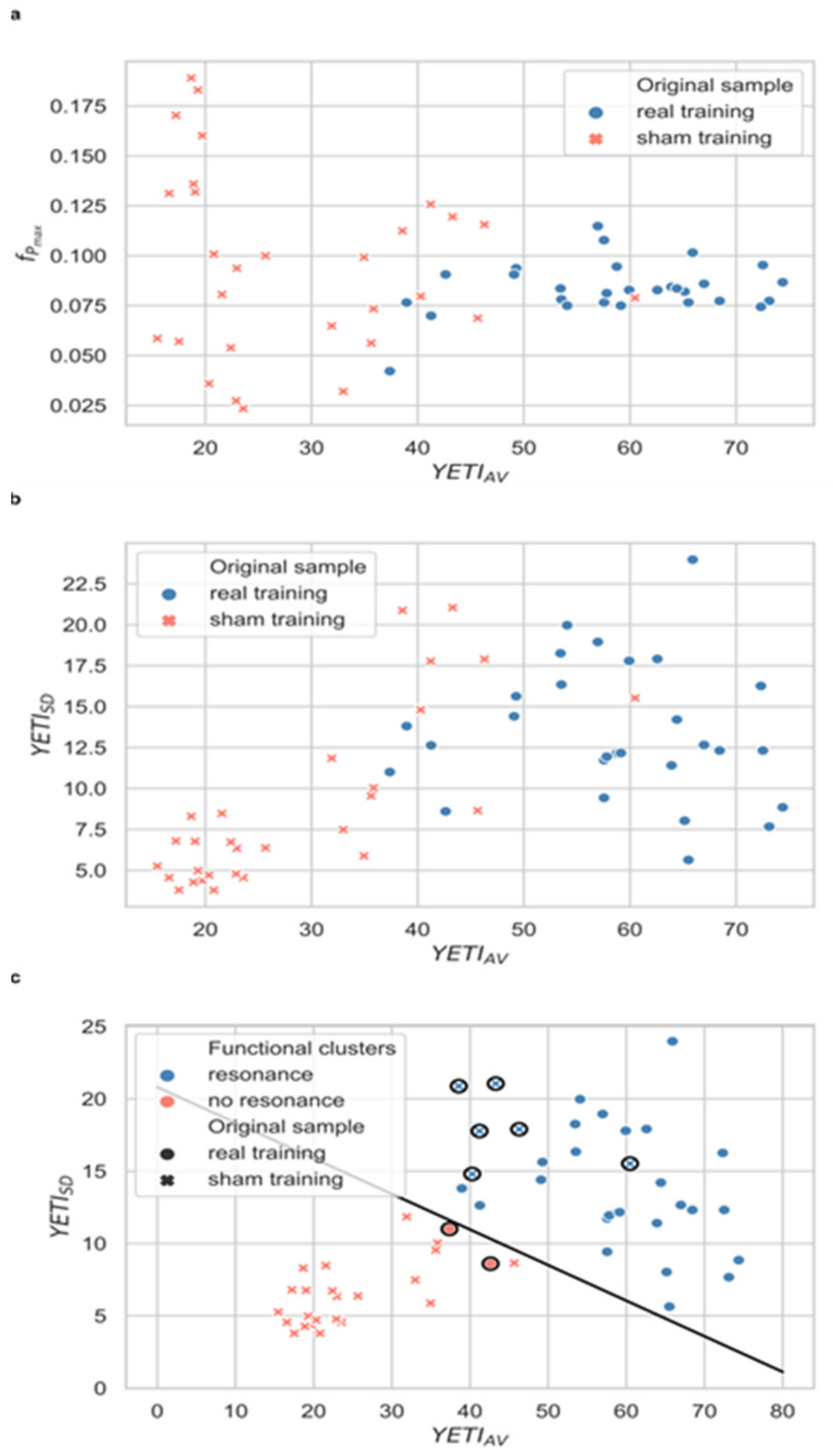
2.6.2. YETI-Based Clustering and the Fidelity Criterion
2.6.3. Resting-State HRV Data
2.7. Statistical Data Analysis
3. Results
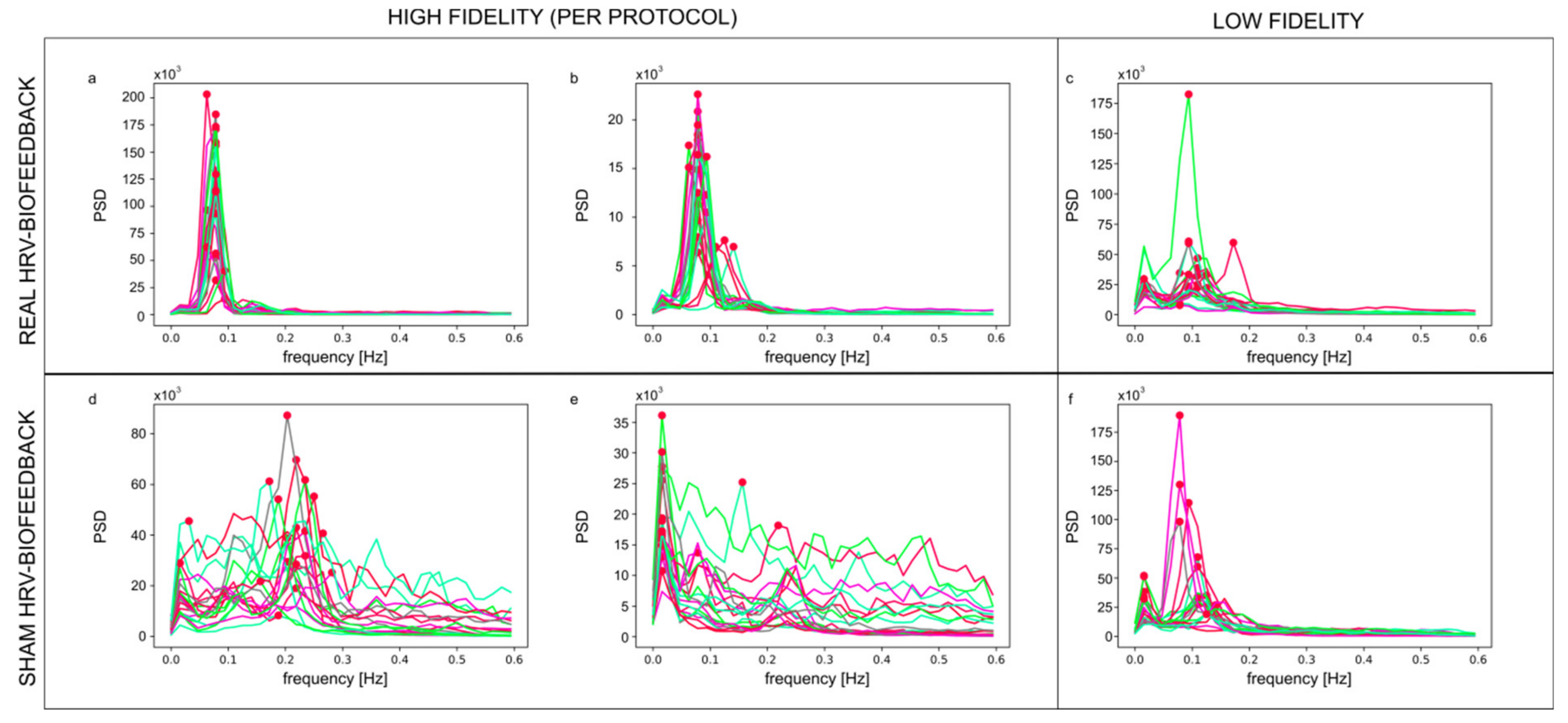
3.1. Clustering of Training Data and the Fidelity Criterion
3.2. Credibility of Sham HRV-Biofeedback
3.3. Quantitative Measures of Training
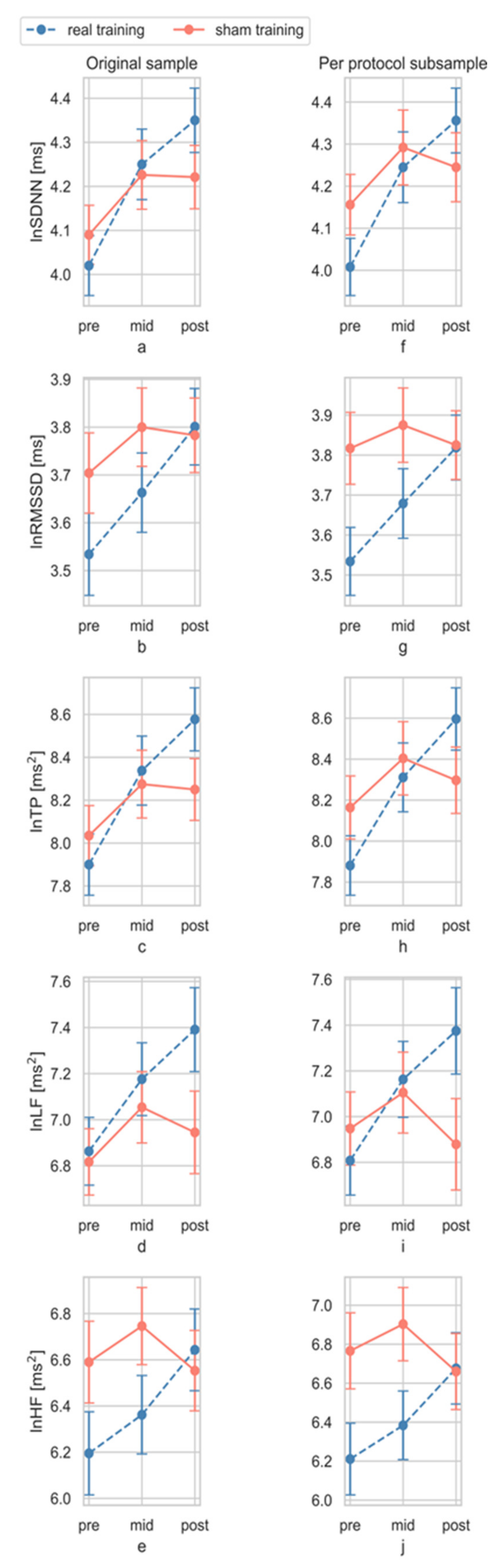
3.4. Effects of Training
3.4.1. Total Effect
3.4.2. Dose Effect
4. Discussion
4.1. Novel Sham HRV-Biofeedback Training
4.2. Training Quality and Applicability of the YETI Index
4.3. Quantitative Effects of Training
4.4. Beyond HRV-Biofeedback
4.5. Limitations and Further Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moss, D.; Shaffer, F. The application of heart rate variability biofeedback to medical and mental health disorders. Biofeedback 2017, 45, 2–8. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Gevirtz, R. Heart rate variability biofeedback: How and why does it work? Front. Psychol. 2014, 5, 756. [Google Scholar] [CrossRef] [PubMed]
- Yucha, C.B.; Montgomery, D. Evidence-Based Practice in Biofeedback and Neurofeedback; AAPB: Wheat Ridge, CO, USA, 2008. [Google Scholar]
- Thayer, J.F.; Ahs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Mccraty, R.; Atkinson, M.; Tomasino, D.; Bradley, R.T. The Coherent Heart Heart—Brain Interactions, Psychophysiological Coherence, and the Emergence of System-Wide Order. Integral Rev. Transdiscipl. Transcult. J. New Thought Res. Prax. 2009, 5, 10–115. Available online: https://www.heartmath.org/assets/uploads/2016/04/coherent-heart-integral-review-2009.pdf (accessed on 15 May 2021).
- Carr, O.; Andreotti, F.; Saunders, K.E.A.; Bilderbeck, A.C.; Goodwin, G.M.; De Vos, M. Linking changes in heart rate variability to mood changes in daily life. In Proceedings of the Computing in Cardiology, IEEE Computer Society, Rennes, France, 24–27 September 2017; Volume 44, pp. 1–4. [Google Scholar] [CrossRef]
- Seiler, S.; Haugen, O.; Kuffel, E. Autonomic recovery after exercise in trained athletes. Med. Sci. Sport. Exerc. 2007, 39, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Young, H.A.; Benton, D. Heart-rate variability: A biomarker to study the influence of nutrition on physiological and psychological health? Behav. Pharmacol. 2018, 29, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, J.; Tarumi, T.; Tanaka, H. Effect of mirthful laughter on vascular function. Am. J. Cardiol. 2010, 106, 856–859. [Google Scholar] [CrossRef]
- Trappe, H.-J. The effects of music on the cardiovascular system and cardiovascular health. Heart 2010, 96, 1868–1871. [Google Scholar] [CrossRef]
- Fiorentini, A.; Ora, J.; Tubani, L. Autonomic system modification in Zen practitioners. Indian J. Med. Sci. 2013, 67, 161–167. [Google Scholar] [CrossRef]
- Patra, S.; Telles, S. Heart rate variability during sleep following the practice of cyclic meditation and supine rest. Appl. Psychophysiol. Biofeedback 2010, 35, 135–140. [Google Scholar] [CrossRef]
- Lehrer, P.M. Heart rate variability biofeedback and other psychophysiological procedures as important elements in psychotherapy. Int. J. Psychophysiol. 2018, 131, 89–95. [Google Scholar] [CrossRef]
- Goessl, V.C.; Curtiss, J.E.; Hofmann, S.G. The effect of heart rate variability biofeedback training on stress and anxiety: A meta-analysis. Psychol. Med. 2017, 47, 2578–2586. [Google Scholar] [CrossRef] [PubMed]
- Wheat, A.L.; Larkin, K.T. Biofeedback of heart rate variability and related physiology: A critical review. Appl. Psychophysiol. Biofeedback 2010, 35, 229–242. [Google Scholar] [CrossRef]
- Gevirtz, R. The promise of heart rate variability biofeedback: Evidence-based applications. Biofeedback 2013, 41, 110–120. [Google Scholar] [CrossRef]
- Lehrer, P.M. heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosom. Med. 2003, 65, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Chalaye, P.; Goffaux, P.; Lafrenaye, S.; Marchand, S. Respiratory effects on experimental heat pain and cardiac activity. Pain Med. 2009, 10, 1334–1340. [Google Scholar] [CrossRef]
- Hassett, A.L.; Radvanski, D.C.; Vaschillo, E.G.; Vaschillo, B.; Sigal, L.H.; Karavidas, M.K.; Buyske, S.; Lehrer, P.M. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Appl. Psychophysiol. Biofeedback 2007, 32, 1–10. [Google Scholar] [CrossRef]
- Ginsberg, J.P.; Berry, M.E.; Powell, D.A. Cardiac coherence and posttraumatic stress disorder in combat veterans. Altern. Ther. Health Med. 2010, 16, 52–60. [Google Scholar]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Mather, M.; Thayer, J.F. How heart rate variability affects emotion regulation brain networks. Curr. Opin. Behav. Sci. 2018, 19, 98–104. [Google Scholar] [CrossRef]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Quintana, D.S.; Alvares, G.A.; Heathers, J.A.J. Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S. Statistical considerations for reporting and planning heart rate variability case-control studies. Psychophysiology 2017, 54, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research—Recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Mertgen, A. A unifying conceptual framework of factors associated to cardiac vagal control. Heliyon 2018, 4, e01002. [Google Scholar] [CrossRef]
- Bradley, R.T.; McCraty, R.; Atkinson, M.; Tomasino, D.; Daugherty, A.; Arguelles, L. Emotion self-regulation, psychophysiological coherence, and test anxiety: Results from an experiment using electrophysiological measures. Appl. Psychophysiol. Biofeedback 2010, 35, 261–283. [Google Scholar] [CrossRef]
- Dziembowska, I.; Izdebski, P.; Rasmus, A.; Brudny, J.; Grzelczak, M.; Cysewski, P. Effects of heart rate variability biofeedback on eeg alpha asymmetry and anxiety symptoms in male athletes: A pilot study. Appl. Psychophysiol. Biofeedback 2016, 41, 141–150. [Google Scholar] [CrossRef]
- Lin, I.-M.; Fan, S.-Y.; Lu, H.-C.; Lin, T.-H.; Chu, C.-S.; Kuo, H.-F.; Lee, C.-S.; Lu, Y.-H. Randomized controlled trial of heart rate variability biofeedback in cardiac autonomic and hostility among patients with coronary artery disease. Behav. Res. Ther. 2015, 70, 38–46. [Google Scholar] [CrossRef]
- McCraty, R.; Atkinson, M.; Tomasino, D.; Goelitz, J.; Mayrovitz, H.N. The impact of an emotional self-management skills course on psychosocial functioning and autonomic recovery to stress in middle school children. Integr. Physiol. Behav. Sci. 1999, 34, 246–268. [Google Scholar] [CrossRef]
- Prinsloo, G.E.; Derman, W.E.; Lambert, M.I.; Laurie Rauch, H.G. The effect of a single session of short duration biofeedback-induced deep breathing on measures of heart rate variability during laboratory-induced cognitive stress: A pilot study. Appl. Psychophysiol. Biofeedback 2013, 38, 81–90. [Google Scholar] [CrossRef]
- Whited, A.; Larkin, K.T.; Whited, M. Effectiveness of emWave biofeedback in improving heart rate variability reactivity to and recovery from stress. Appl. Psychophysiol. Biofeedback 2014, 39, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Sherlin, L.; Gevirtz, R.; Wyckoff, S.; Muench, F. Effects of respiratory sinus arrhythmia biofeedback versus passive biofeedback control. Int. J. Stress Manag. 2009, 16, 233–248. [Google Scholar] [CrossRef]
- Sutarto, A.P.; Abdul Wahab, M.N.; Mat Zin, N. Heart rate variability (HRV) biofeedback: A new training approach for operator’s performance enhancement. J. Ind. Eng. Manag. 2010, 3, 176–198. [Google Scholar] [CrossRef]
- Gruzelier, J.H.; Thompson, T.; Redding, E.; Brandt, R.; Steffert, T. Application of alpha/theta neurofeedback and heart rate variability training to young contemporary dancers: State anxiety and creativity. Int. J. Psychophysiol. 2014, 93, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.; Varney, C.; Parkinson, L.A.; Gruzelier, J.H. The effects of alpha/theta neurofeedback on personality and mood. Brain Res. Cogn. Brain Res. 2005, 23, 287–292. [Google Scholar] [CrossRef]
- Paul, M.; Garg, K.; Singh Sandhu, J. Role of biofeedback in optimizing psychomotor performance in sports. Asian J. Sports Med. 2012, 3, 29–40. [Google Scholar] [CrossRef]
- Paul, M.; Garg, K. The effect of heart rate variability biofeedback on performance psychology of basketball players. Appl. Psychophysiol. Biofeedback 2012, 37, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, M.; Aykac, V.; Unterdörfer, J.; Petrowski, K.; Mueck-Weymann, M. A pilot study on the effects of heart rate variability biofeedback in patients with depression and in healthy subjects. Appl. Psychophysiol. Biofeedback 2008, 33, 195–201. [Google Scholar] [CrossRef]
- Wells, R.; Outhred, T.; Heathers, J.A.J.; Quintana, D.S.; Kemp, A.H. Matter over mind: A randomised-controlled trial of single-session biofeedback training on performance anxiety and heart rate variability in musicians. PLoS ONE 2012, 7, e46597. [Google Scholar] [CrossRef]
- Sherlin, L.; Muench, F.; Wyckoff, S. Respiratory sinus arrhythmia feedback in a stressed population exposed to a brief stressor demonstrated by quantitative EEG and sLORETA. Appl. Psychophysiol. Biofeedback 2010, 35, 219–228. [Google Scholar] [CrossRef]
- Arnold, L.E.; Lofthouse, N.; Hersch, S.; Pan, X.; Hurt, E.; Bates, B.; Kassouf, K.; Moone, S.; Grantier, C. EEG neurofeedback for ADHD: Double-blind sham-controlled randomized pilot feasibility trial. J. Atten. Disord. 2013, 17, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Lansbergen, M.M.; Van Dongen-Boomsma, M.; Buitelaar, J.K.; Slaats-Willemse, D. ADHD and EEG-neurofeedback: A double-blind randomized placebo-controlled feasibility study. J. Neural Transm. 2011, 118, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Logemann, H.N.A.; Lansbergen, M.M.; Van Os, T.W.D.P.; Böcker, K.B.E.; Kenemans, J.L. The effectiveness of EEG-feedback on attention, impulsivity and EEG: A sham feedback controlled study. Neurosci. Lett. 2010, 479, 49–53. [Google Scholar] [CrossRef]
- Mehler, D.M.A.; Sokunbi, M.O.; Habes, I.; Barawi, K.; Subramanian, L.; Range, M.; Evans, J.; Hood, K.; Lührs, M.; Keedwell, P.; et al. Targeting the affective brain—A randomized controlled trial of real-time fMRI neurofeedback in patients with depression. Neuropsychopharmacology 2018, 43, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Meir-Hasson, Y.; Keynan, J.N.; Kinreich, S.; Jackont, G.; Cohen, A.; Podlipsky-Klovatch, I.; Hendler, T.; Intrator, N. One-class fMRI-inspired EEG model for self-regulation training. PLoS ONE 2016, 11, e0154968. [Google Scholar] [CrossRef] [PubMed]
- Perreau-Linck, E.; Lessard, N.; Lévesque, J.; Beauregard, M. Effects of neurofeedback training on inhibitory capacities in ADHD children: A single-blind, randomized, placebo-controlled study. J. Neurother. 2010, 14, 229–242. [Google Scholar] [CrossRef]
- Breach, B. Heart Rate Variability Biofeedback in the Treatment of Major Depression. Ph.D. Thesis, Graduate School of Applied and Professional Psychology, Rutgers University, New Brunswick, NJ, USA, 2012. Available online: https://rucore.libraries.rutgers.edu/rutgers-lib/39081/pdf/1/ (accessed on 15 May 2021).
- Perepletchikova, F.; Treat, T.A.; Kazdin, A.E. Treatment integrity in psychotherapy research: Analysis of the studies and examination of the associated factors. J. Consult. Clin. Psychol. 2007, 75, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Kulik, O.L.; Schmidt, O.J.; Belal, S.A.S.; Rank, I.A. Implementation of Biofeedback in a Closed Loop of Heart Rate Variability and Paced Breathing in Patients with Arterial Hypertension; V. N. Karazin Kharkiv National University: Kharkiv, Ukraine, 2014; Available online: https://cyberleninka.ru/article/n/implementation-of-biofeedback-in-a-closed-loop-of-heart-rate-variability-and-paced-breathing-in-patients-with-arterial-hypertension/viewer (accessed on 15 May 2021).
- Kulik, A.L.; Martynenko, A.V.; Yabluchansky, N.I. Biofeedback quality in healthy volunteers in paced breathing algorithm starting from the age physiological norm. In IFMBE Proceedings; Springer: Berlin/Heidelberg, Germany, 2011; Volume 37, pp. 408–411. [Google Scholar] [CrossRef]
- Clamor, A.; Koenig, J.; Thayer, J.F.; Lincoln, T.M. A randomized-controlled trial of heart rate variability biofeedback for psychotic symptoms. Behav. Res. Ther. 2016, 87, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Resnick, B.; Inguito, P.; Orwig, D.; Yahiro, J.Y.; Hawkes, W.; Werner, M.; Zimmerman, S.; Magaziner, J. Treatment fidelity in behavior change research: A case example. Nurs. Res. 2005, 54, 139–143. [Google Scholar] [CrossRef]
- Lehrer, P.M.; Vaschillo, E.; Vaschillo, B. Resonant frequency biofeedback training to increase cardiac variability: Rationale and manual for training. Appl. Psychophysiol. Biofeedback 2000, 25, 177–191. [Google Scholar] [CrossRef]
- Mccraty, R. From depletion to renewal: Positive emotions and heart rhythm coherence feedback. Biofeedback 2008, 36, 30–34. Available online: https://www.aapb.org/files/publications/biofeedback/2008/biof_depletion_renewal.pdf (accessed on 15 May 2021).
- McCraty, R.; Atkinson, M.; Tiller, W.A.; Rein, G.; Watkins, A.D. The effects of emotions on short-term power spectrum analysis of heart rate variability. Am. J. Cardiol. 1995, 76, 1089–1093. [Google Scholar] [CrossRef]
- McCraty, R.; Zayas, M.A. Cardiac coherence, self-regulation, autonomic stability and psychosocial well-being. Front. Psychol. 2014, 5, 1090. [Google Scholar] [CrossRef]
- Schwerdtfeger, A.R.; Friedrich-Mai, P.; Gerteis, A.K.S. Daily Positive Affect and Nocturnal Cardiac Activation. Int. J. Behav. Med. 2015, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Schwerdtfeger, A.R.; Gerteis, A.K.S. The manifold effects of positive affect on heart rate variability in everyday life: Distinguishing within-person and between-person associations. Health Psychol. 2014, 33, 1065–1073. [Google Scholar] [CrossRef]
- Devilly, G.J.; Borkovec, T.D. Psychometric properties of the credibility/expectancy questionnaire. J. Behav. Ther. Exp. Psychiatry 2000, 31, 73–86. [Google Scholar] [CrossRef]
- Benassi, M.; Garofalo, S.; Ambrosini, F.; Sant’Angelo, R.P.; Raggini, R.; De Paoli, G.; Ravani, C.; Giovagnoli, S.; Orsoni, M.; Piraccini, G. Using two-step cluster analysis and latent class cluster analysis to classify the cognitive heterogeneity of cross-diagnostic psychiatric inpatients. Front. Psychol. 2020, 11, 1085. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. When to use the Bonferroni correction. Ophthalmic Physiol. Opt. 2014, 34, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Halsey, L.G. The reign of the p-value is over: What alternative analyses could we employ to fill the power vacuum? Biol. Lett. 2019, 15. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.H. Handbook of Biological Statistics, 2nd ed.; Sparky House Publishing: Baltimore, MD, USA, 2008; Available online: http://www.uni-koeln.de/math-nat-fak/genetik/groups/Langer/HandbookBioStatSecond.pdf (accessed on 15 May 2021).
- Rollo, A. Effects of a Heart Rate Variability Biofeedback Intervention on Athletes’ Psychological Response Following Injury. Master’s Thesis, Wilfrid Laurier University, Waterloo, ON, Canada, 2014. Available online: http://scholars.wlu.ca/etd/1658 (accessed on 15 May 2021).
- Reynard, A.; Gevirtz, R.; Berlow, R.; Brown, M.; Boutelle, K. Heart rate variability as a marker of self-regulation. Appl. Psychophysiol. Biofeedback 2011, 36, 209–215. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, I.A.; O’Hare, P.; Corrall, R.J.M. Heart rate variability in healthy subjects: Effect of age and the derivation of normal ranges for tests of autonomic function. Br. Heart J. 1986, 55, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.; Barnes, R.W.; Chambless, L.E.; Simpson, R.J.; Sorlie, P.; Heiss, G. The ARIC Investigators Age, race, and sex differences in autonomic cardiac function measured by spectral analysis of heart rate variability—The ARIC study. Am. J. Cardiol. 1995, 76, 906–912. [Google Scholar] [CrossRef]
- Lehrer, P.M. Biofeedback training to increase heart rate variability. In Principles and Practice of Stress Management, 3rd ed.; Paul, M., Lehrer, R.L., Woolfolk, W.E.S., Eds.; Guilford Press: New York, NY, USA, 2007; pp. 227–248. ISBN 9781606238288. [Google Scholar]
- Krygier, J.R.; Heathers, J.A.J.; Shahrestani, S.; Abbott, M.; Gross, J.J.; Kemp, A.H. Mindfulness meditation, well-being, and heart rate variability: A preliminary investigation into the impact of intensive Vipassana meditation. Int. J. Psychophysiol. 2013, 89, 305–313. [Google Scholar] [CrossRef]
- Wu, S.-D.; Lo, P.-C. Inward-attention meditation increases parasympathetic activity: A study based on heart rate variability. Biomed. Res. 2008, 29, 245–250. [Google Scholar] [CrossRef] [PubMed]
- van der Zwan, J.E.; de Vente, W.; Huizink, A.C.; Bögels, S.M.; de Bruin, E.I. Physical activity, mindfulness meditation, or heart rate variability biofeedback for stress reduction: A randomized controlled trial. Appl. Psychophysiol. Biofeedback 2015, 40, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Darragh, M.; Vanderboor, T.; Booth, R.J.; Sollers, J.J.; Consedine, N.S. Placebo ‘serotonin’ increases heart rate variability in recovery from psychosocial stress. Physiol. Behav. 2015, 145, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Vaschillo, E.G.; Bates, M.E.; Vaschillo, B.; Lehrer, P.; Udo, T.; Mun, E.Y.; Ray, S. Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: Effects of 0.1-Hz stimulation. Psychophysiology 2008, 45, 847–858. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Seaton, K.; Miller, M.; Brown, K.; Nygaard, I.; Stumbo, P.; Zimmerman, B.; Schulze, K. Randomized controlled trial of biofeedback, sham feedback, and standard therapy for dyssynergic defecation. Clin. Gastroenterol. Hepatol. 2007, 5, 331–338. [Google Scholar] [CrossRef]
- Woodward, S.; Norton, C.; Chiarelli, P. Biofeedback for treatment of chronic idiopathic constipation in adults. Cochrane Database Syst. Rev. 2014, 2014. [Google Scholar] [CrossRef]
- Sheffer, C.E.; Mennemeier, M.S.; Landes, R.D.; Dornhoffer, J.; Kimbrell, T.; Bickel, W.K.; Brackman, S.; Chelette, K.C.; Brown, G.; Vuong, M. Focal electrical stimulation as an effective sham control for active rTMS and biofeedback treatments. Appl. Psychophysiol. Biofeedback 2013, 38, 171–176. [Google Scholar] [CrossRef]
- Noone, C.; Hogan, M.J. A randomised active-controlled trial to examine the effects of an online mindfulness intervention on executive control, critical thinking and key thinking dispositions in a university student sample. BMC Psychol. 2018, 6, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, F.; Emerson, N.M.; Farris, S.R.; Ray, J.N.; Jung, Y.; McHaffie, J.G.; Coghill, R.C. Mindfulness meditation-based pain relief employs different neural mechanisms than placebo and sham mindfulness meditation-induced analgesia. J. Neurosci. 2015, 35, 15307–15325. [Google Scholar] [CrossRef] [PubMed]
- Zeidan, F.; Johnson, S.K.; Gordon, N.S.; Goolkasian, P. Effects of brief and sham mindfulness meditation on mood and cardiovascular variables. J. Altern. Complement. Med. 2010, 16, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Léonard, A.; Clément, S.; Kuo, C.D.; Manto, M. Changes in heart rate variability during heartfulness meditation: A power spectral analysis including the residual spectrum. Front. Cardiovasc. Med. 2019, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.M.; Chueh, M.T.W.; Lai, Y.J. Meditation practice improves short-term changes in heart rate variability. Int. J. Environ. Res. Public Health 2020, 17, 2128. [Google Scholar] [CrossRef] [PubMed]
- Phongsuphap, S.; Pongsupap, Y.; Chandanamattha, P.; Lursinsap, C. Changes in heart rate variability during concentration meditation. Int. J. Cardiol. 2008, 130, 481–484. [Google Scholar] [CrossRef]
- Lambert, J.D.; Greaves, C.J.; Farrand, P.; Cross, R.; Haase, A.M.; Taylor, A.H. Assessment of fidelity in individual level behaviour change interventions promoting physical activity among adults: A systematic review. BMC Public Health 2017, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mars, T.; Ellard, D.; Carnes, D.; Homer, K.; Underwood, M.; Taylor, S.J.C. Fidelity in complex behaviour change interventions: A standardised approach to evaluate intervention integrity. BMJ Open 2013, 3, 3555. [Google Scholar] [CrossRef]
- Bellg, A.J.; Resnick, B.; Minicucci, D.S.; Ogedegbe, G.; Ernst, D.; Borrelli, B.; Hecht, J.; Ory, M.; Orwig, D.; Czajkowski, S. Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004, 23, 443–451. [Google Scholar] [CrossRef]

| Original Sample | Per Protocol Subsample | |||||||
|---|---|---|---|---|---|---|---|---|
| Mreal (sd) | Msham (sd) | U | p | Mreal (sd) | Msham (sd) | U | p | |
| YETIAV | 58.67 (1.33) | 28.6 (11.55) | 33.0 | <0.001 | 6.10 (9.20) | 24.33 (7.91) | 2.0 | <0.001 |
| YETI10 | 57.93 (11.54) | 28.02 (12.19) | 44.0 | <0.001 | 59.41 (1.52) | 24.35 (8.38) | 7.0 | <0.001 |
| YETI20 | 59.17 (11.51) | 28.86 (12.09) | 37.0 | <0.001 | 6.54 (1.75) | 24.45 (7.92) | 2.0 | <0.001 |
| ΔYETI | 1.23 (9.18) | 0.84 (8.59) | 385.0 | 0.737 | 1.12 (9.51) | 0.09 (3.13) | 279.0 | 0.689 |
| HRV Index | Inter-Action Effects | Original Sample | Functional Cluster | Per Protocol Subsample | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F(dfs) | p | ηp2 | pow | F(dfs) | p | ηp2 | pow | F(dfpp) | p | ηp2 | pow | ||
| ln SDNN | pre-post | 4.50 | 0.038 * | 0.08 | 0.55 | 7.10 | 0.010 ** | 0.11 | 0.74 | 6.56 | 0.014 ** | 0.12 | 0.71 |
| pre-mid-post | 2.05 | 0.133 | 0.04 | 0.42 | 3.29 | 0.041 * | 0.06 | 0.61 | 2.79 | 0.066 * | 0.06 | 0.54 | |
| ln RMSSD | pre-post | 2.79 | 0.101 | 0.05 | 0.38 | 6.88 | 0.011 ** | 0.11 | 0.73 | 5.67 | 0.021 ** | 0.11 | 0.65 |
| pre-mid-post | 1.61 | 0.205 | 0.03 | 0.33 | 3.39 | 0.037 * | 0.06 | 0.63 | 2.70 | 0.072 * | 0.05 | 0.52 | |
| ln TP | pre-post | 5.64 | 0.021 * | 0.09 | 0.65 | 8.01 | 0.006 ** | 0.13 | 0.79 | 7.75 | 0.008 ** | 0.14 | 0.78 |
| pre-mid-post | 2.76 | 0.068 | 0.05 | 0.54 | 4.19 | 0.018 ** | 0.07 | 0.73 | 3.68 | 0.029 * | 0.07 | 0.66 | |
| ln LF | pre-post | 2.45 | 0.123 | 0.04 | 0.34 | 7.78 | 0.007 ** | 0.12 | 0.78 | 5.74 | 0.021 ** | 0.11 | 0.65 |
| pre-mid-post | 1.70 | 0.188 | 0.03 | 0.35 | 4.68 | 0.011 ** | 0.08 | 0.78 | 3.48 | 0.035 * | 0.07 | 0.64 | |
| ln HF | pre-post | 4.75 | 0.034 * | 0.08 | 0.57 | 5.03 | 0.029 ** | 0.08 | 0.60 | 6.07 | 0.017 ** | 0.11 | 0.68 |
| pre-mid-post | 2.81 | 0.065 | 0.05 | 0.54 | 2.74 | 0.069 * | 0.05 | 0.53 | 3.14 | 0.048 * | 0.06 | 0.59 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratajczak, E.; Hajnowski, M.; Stawicki, M.; Duch, W. Novel Methodological Tools for Behavioral Interventions: The Case of HRV-Biofeedback. Sham Control and Quantitative Physiology-Based Assessment of Training Quality and Fidelity. Sensors 2021, 21, 3670. https://doi.org/10.3390/s21113670
Ratajczak E, Hajnowski M, Stawicki M, Duch W. Novel Methodological Tools for Behavioral Interventions: The Case of HRV-Biofeedback. Sham Control and Quantitative Physiology-Based Assessment of Training Quality and Fidelity. Sensors. 2021; 21(11):3670. https://doi.org/10.3390/s21113670
Chicago/Turabian StyleRatajczak, Ewa, Marcin Hajnowski, Mateusz Stawicki, and Włodzisław Duch. 2021. "Novel Methodological Tools for Behavioral Interventions: The Case of HRV-Biofeedback. Sham Control and Quantitative Physiology-Based Assessment of Training Quality and Fidelity" Sensors 21, no. 11: 3670. https://doi.org/10.3390/s21113670
APA StyleRatajczak, E., Hajnowski, M., Stawicki, M., & Duch, W. (2021). Novel Methodological Tools for Behavioral Interventions: The Case of HRV-Biofeedback. Sham Control and Quantitative Physiology-Based Assessment of Training Quality and Fidelity. Sensors, 21(11), 3670. https://doi.org/10.3390/s21113670






