Titanium Nitride Thin Film Based Low-Redox-Interference Potentiometric pH Sensing Electrodes
Abstract
1. Introduction
2. Materials and Methods
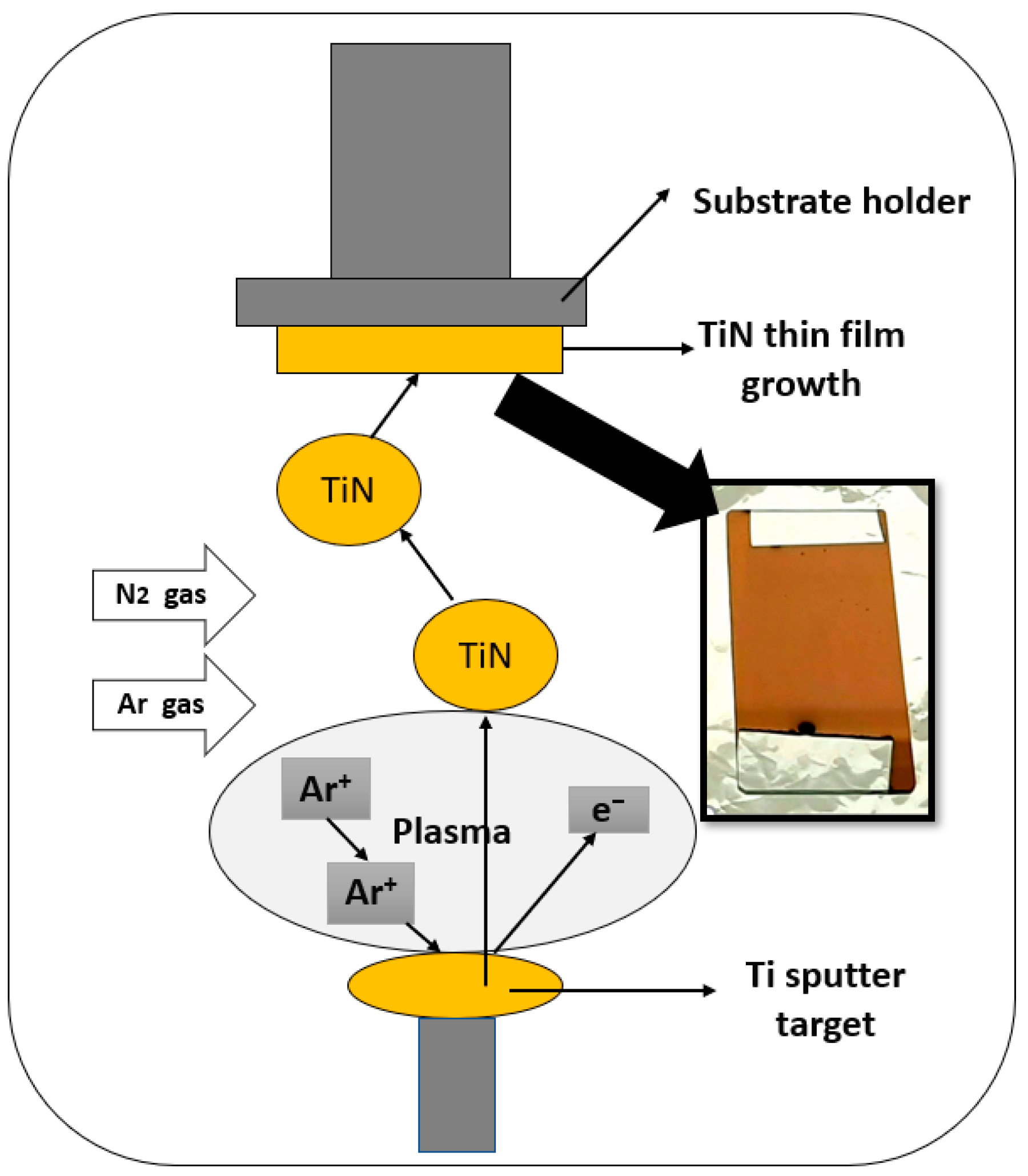
2.1. Working Electrodes Fabrication
2.2. pH Characteristics by Potentiometric Method
3. Results and Discussion
3.1. Characteristics Results of Sputtered TiN Films
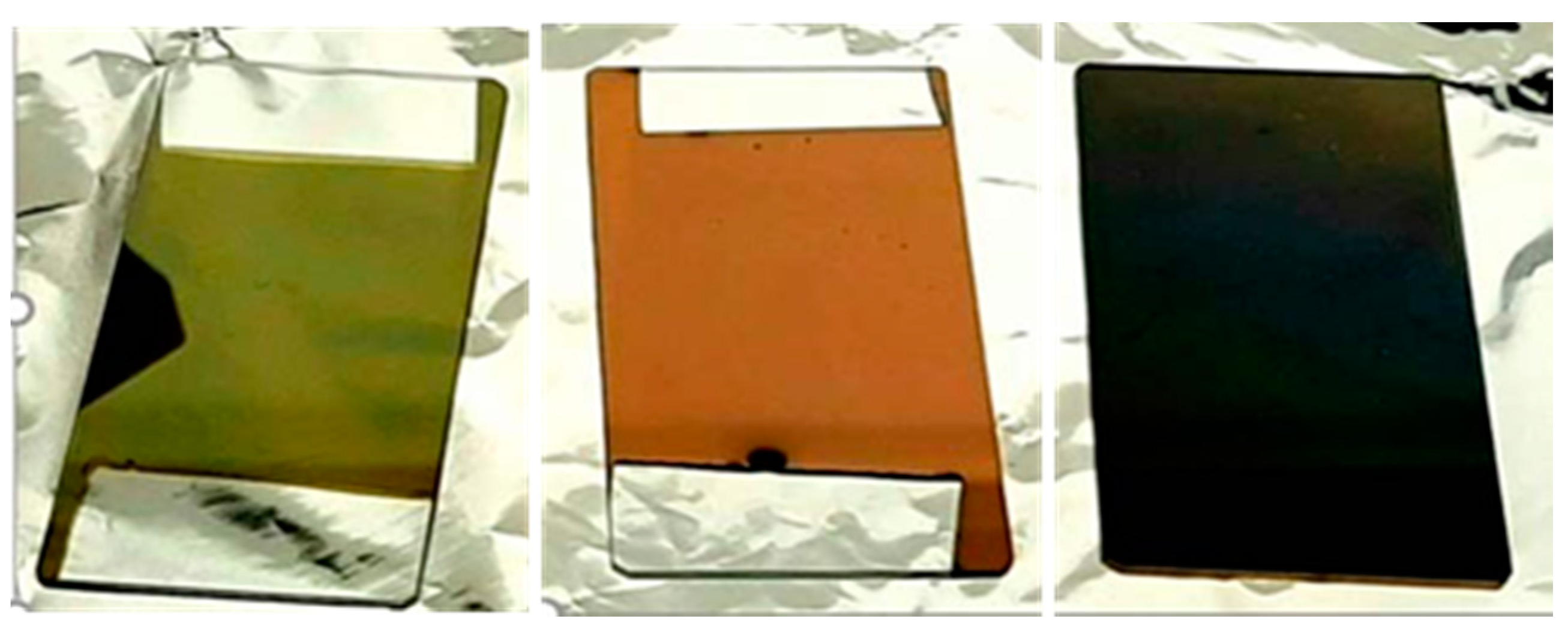
Color Chromaticity Properties of As-Deposited TiN Films
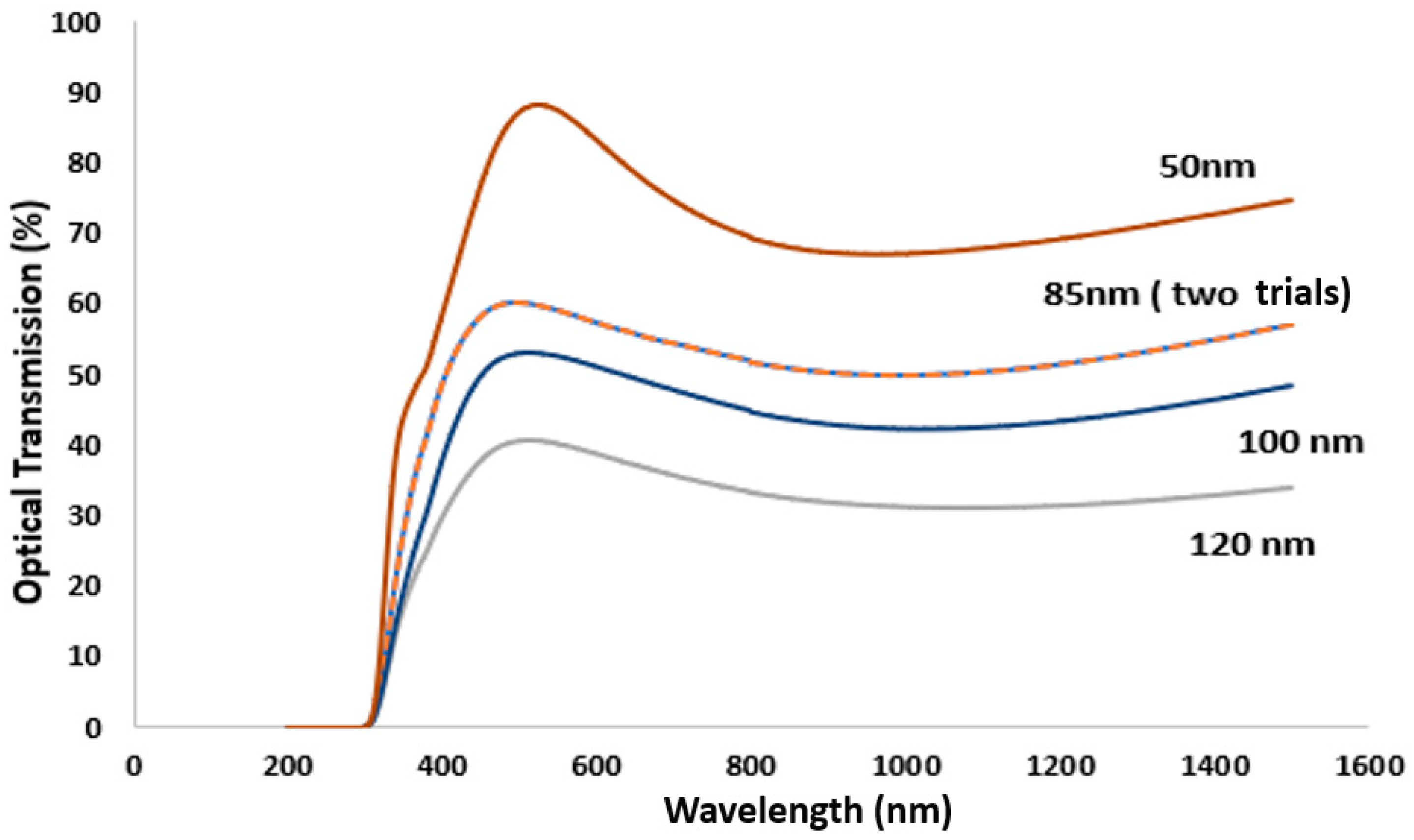
3.2. Effect of TiN Film Thickness
Measurement of the TiN Film Thickness
3.3. Microstructure Characterization of the Sputtered TiN Film
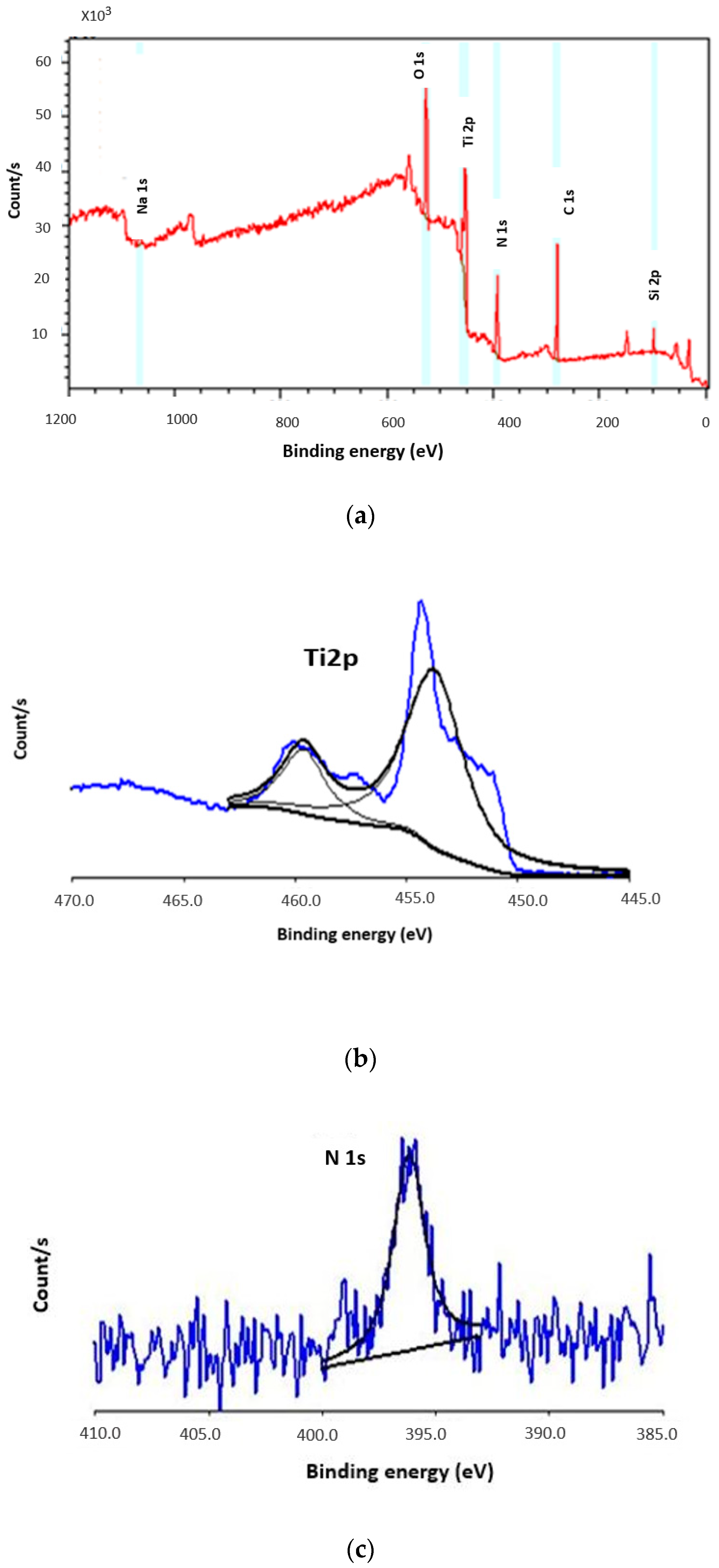
3.4. X-ray Photoelectron Spectroscopy Analysis
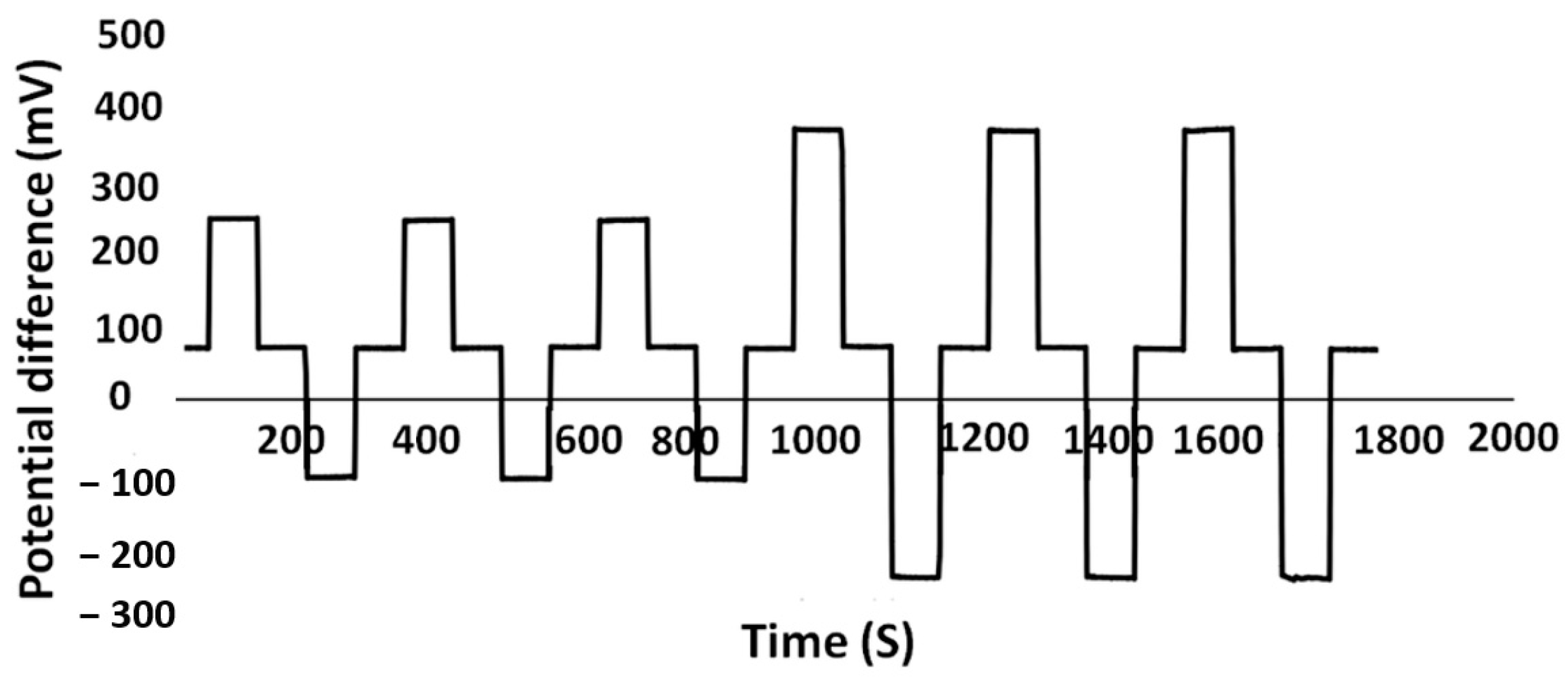
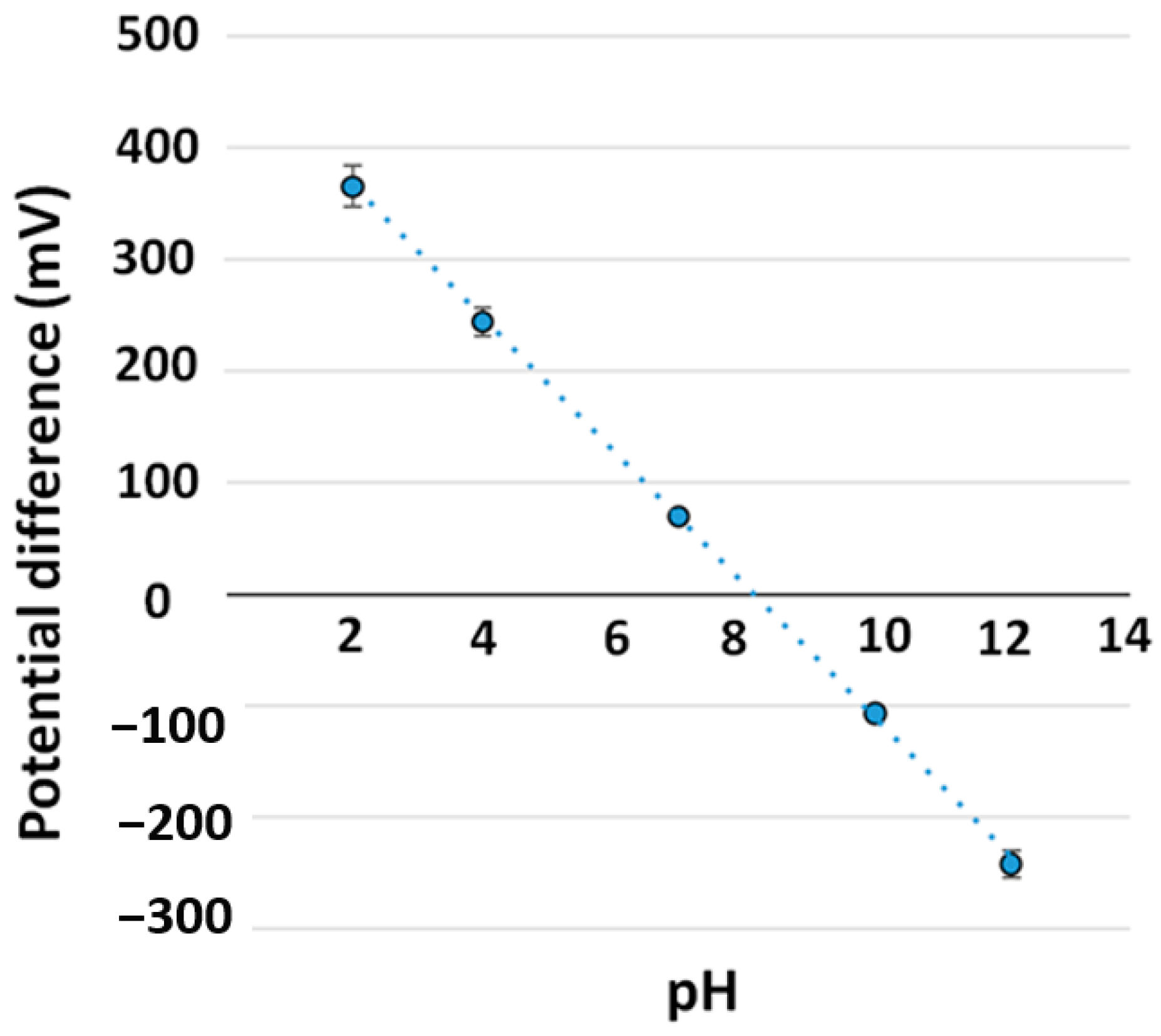
3.5. Sensor Performance
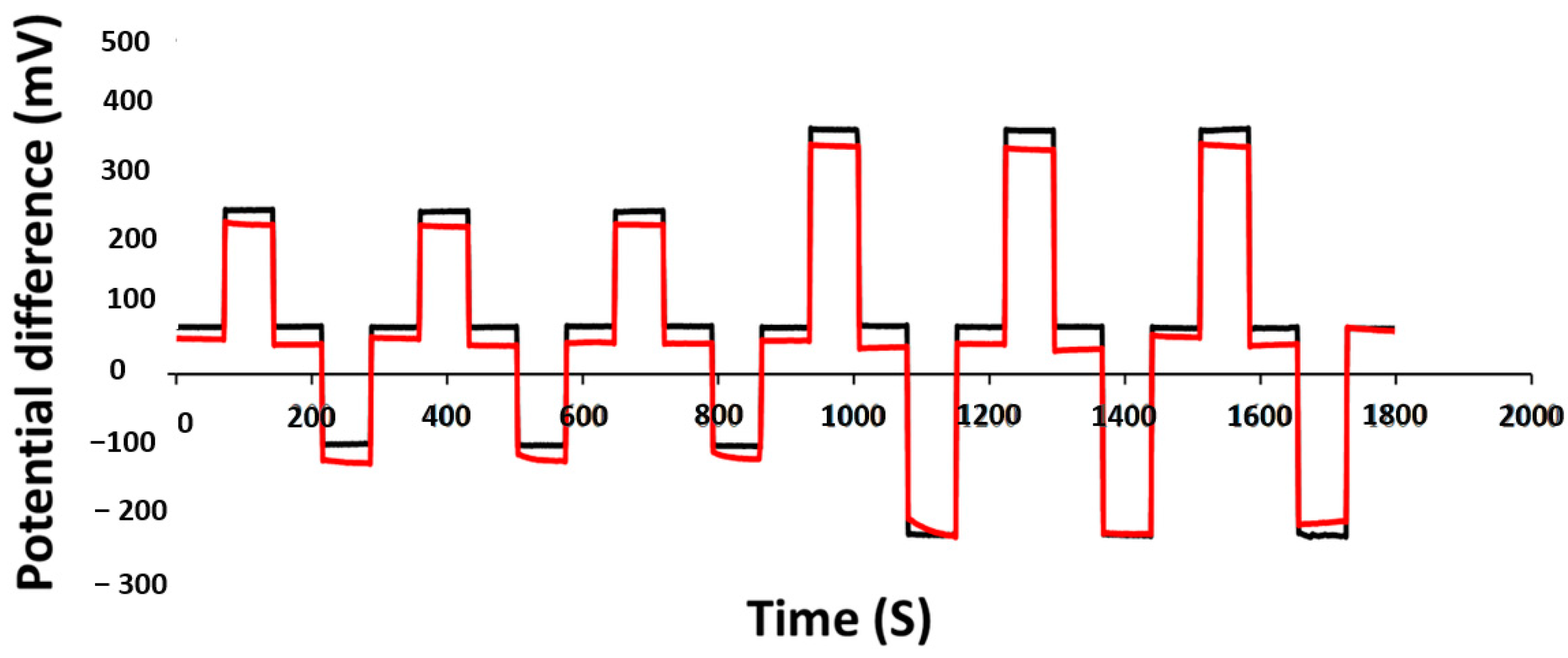
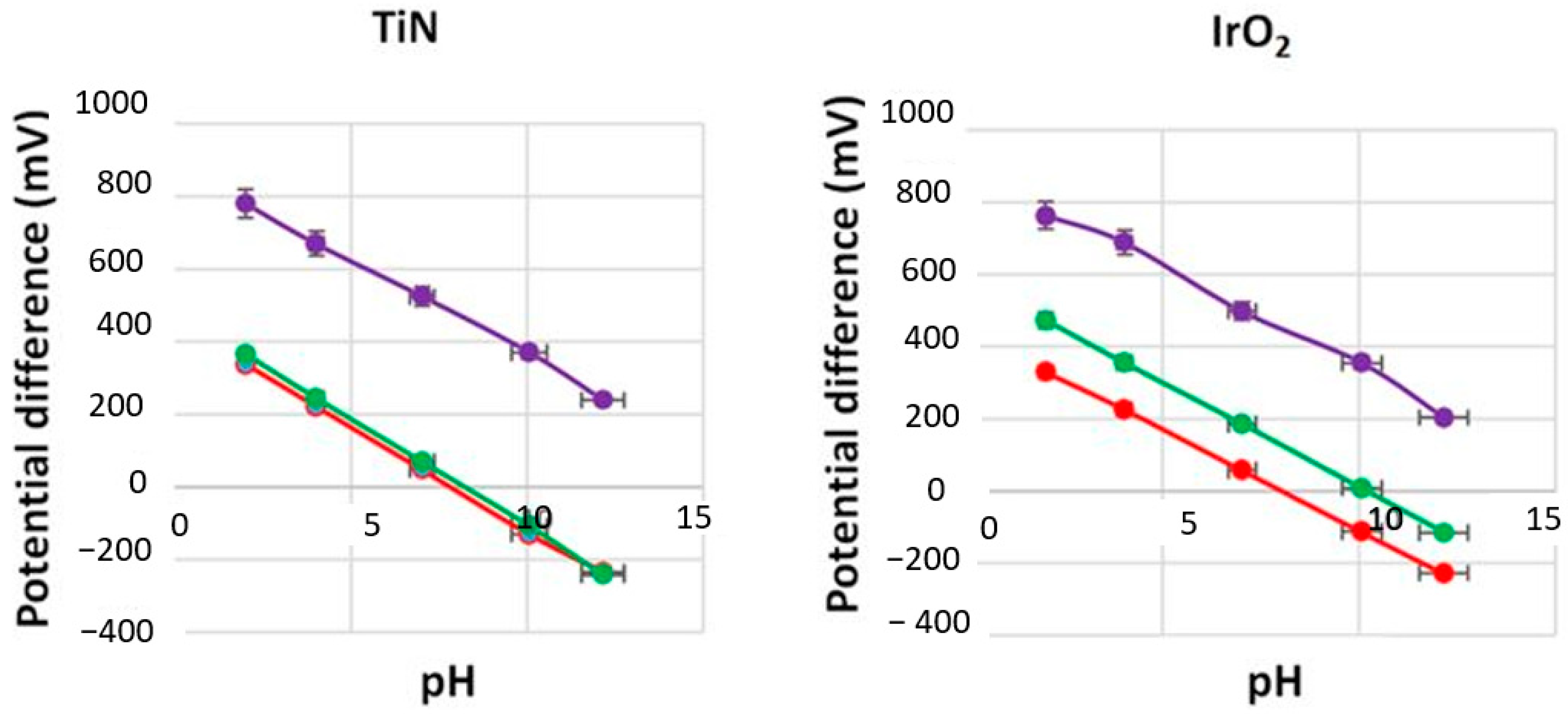
3.6. Redox Effects
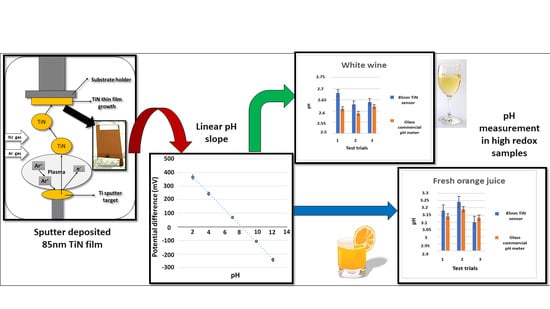
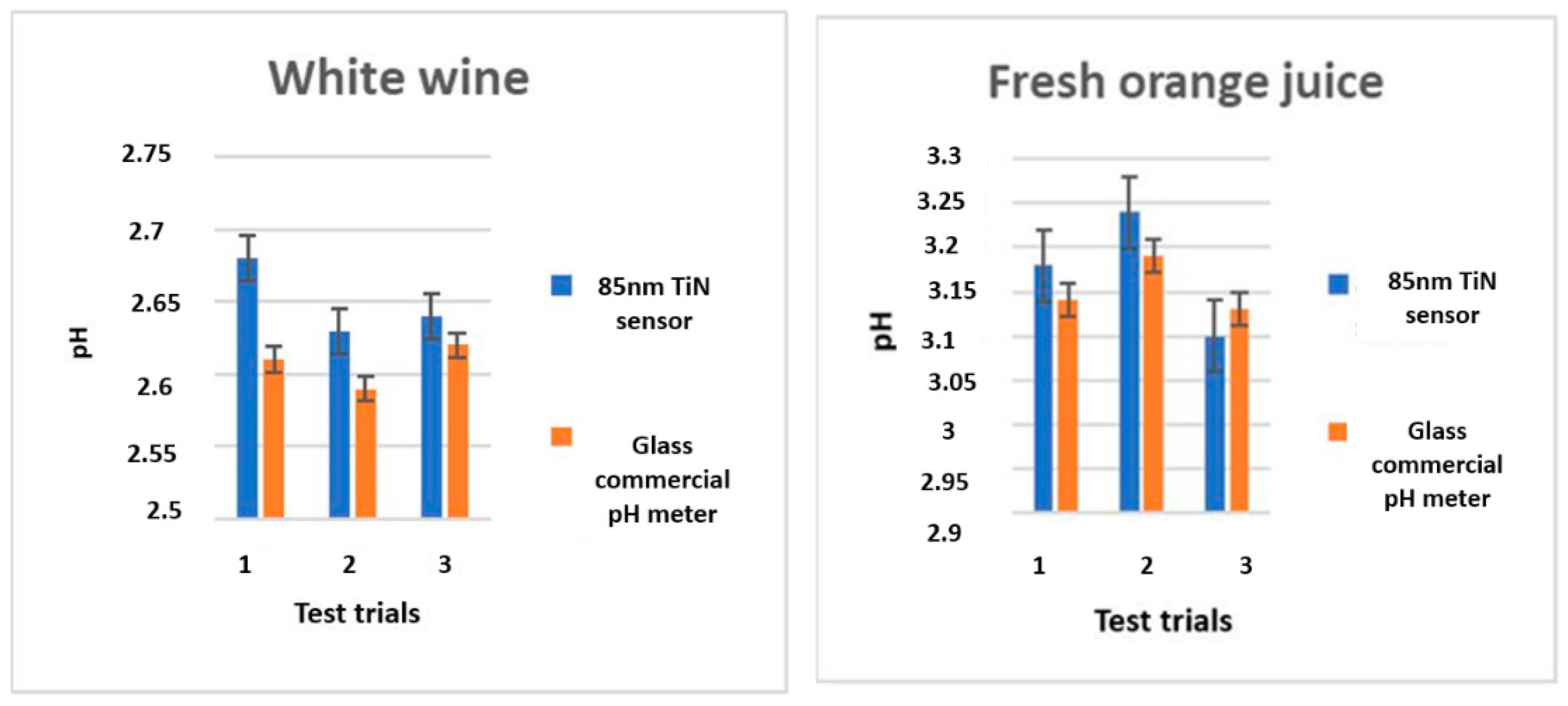
3.7. Sample Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yao, S.; Wang, M.; Madou, M. A pH Electrode Based on Melt-Oxidized Iridium Oxide. J. Electrochem. Soc. 2001, 148, H29. [Google Scholar] [CrossRef]
- Han, S.-T.; Peng, H.; Sun, Q.; Venkatesh, S.; Chung, K.-S.; Lau, S.C.; Zhou, Y.; Roy, V.A.L. An Overview of the Development of Flexible Sensors. Adv. Mater. 2017, 29, 1700375. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yuan, H.; Lu, X.; Zhou, Z.; Xiao, D. All Solid-State pH Electrode Based on Titanium Nitride Sensitive Film. Electroanalysis 2006, 18, 1493–1498. [Google Scholar] [CrossRef]
- Fog, A.; Buck, R.P. Electronic semiconducting oxides as pH sensors. Sens. Actuators 1984, 5, 137–146. [Google Scholar] [CrossRef]
- Schirrmann, M.; Gebbers, R.; Kramer, E.; Seidel, J. Soil pH Mapping with an On-The-Go Sensor. Sensors 2011, 11, 573–598. [Google Scholar] [CrossRef]
- Raven, J.; Caldeira, K.; Elderfield, H.; Hoegh-Guldberg, O.; Liss, P.; Riebesell, U.; Shepherd, J.; Turley, C.; Watson, A. Ocean Acidification due to Increasing Atmospheric Carbon Dioxide; Policy document 12/05; The Royal Society: London, UK, 2005. [Google Scholar]
- Briggs, D.E.; Boulton, C.A.; Brookes, P.A.; Stevens, R. Brewing Science and Practice eBook-EEn; Elsevier: Cambridge, UK, 2004. [Google Scholar]
- Hamm, L.L.; Nakhoul, N.; Hering-Smith, K.S. Acid-Base Homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2232–2242. [Google Scholar] [CrossRef]
- Tian, Y.; Su, F.; Weber, W.; Nandakumar, V.; Shumway, B.R.; Jin, Y.; Zhou, X.; Holl, M.R.; Johnson, R.H.; Meldrum, D.R. A series of naphthalimide derivatives as intra and extracellular pH sensors. Biomaterials 2010, 31, 7411–7422. [Google Scholar] [CrossRef]
- Salvo, P.; Dini, V.; Kirchhain, A.; Janowska, A.; Oranges, T.; Chiricozzi, A.; Lomonaco, T.; Di Francesco, F.; Romanelli, M. Sensors and Biosensors for C-Reactive Protein, Temperature and pH, and Their Applications for Monitoring Wound Healing: A Review. Sensors 2017, 17, 2952. [Google Scholar] [CrossRef]
- Morris, D.; Coyle, S.; Wu, Y.; Lau, K.T.; Wallace, G.; Diamond, D. Bio-sensing textile based patch with integrated optical detection system for sweat monitoring. Sens. Actuators B Chem. 2009, 139, 231–236. [Google Scholar] [CrossRef]
- Benčina, M. Illumination of the Spatial Order of Intracellular pH by Genetically Encoded pH-Sensitive Sensors. Sensors 2013, 13, 16736–16758. [Google Scholar] [CrossRef]
- Bousse, L.; Bergveld, P. The role of buried OH sites in the response mechanism of inorganic-gate pH-sensitive ISFETs. Sens. Actuators 1984, 6, 65–78. [Google Scholar] [CrossRef]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J. Review on Hydrogel-based pH Sensors and Microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Xia, H.; He, Z.; Wang, Z. Design of a Water Environment Monitoring System Based on Wireless Sensor Networks. Sensors 2009, 9, 6411–6434. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Mukherjee, K.; Shoukat, R.; Dong, H. A review on pH sensitive materials for sensors and detection methods. Microsyst. Technol. 2017, 23, 4391–4404. [Google Scholar] [CrossRef]
- Lawand, N.S.; French, P.J.; Briaire, J.J.; Frijns, J.H.M. Thin Titanium Nitride Films Deposited using DC Magnetron Sputtering used for Neural Stimulation and Sensing Purposes. Procedia Eng. 2012, 47, 726–729. [Google Scholar] [CrossRef]
- Lonsdale, W.; Wajrak, M.; Alameh, K. Manufacture and application of RuO2 solid-state metal-oxide pH sensor to common beverages. Talanta 2018, 180, 277–281. [Google Scholar] [CrossRef]
- Uria, N.; Abramova, N.; Bratov, A.; Muñoz-Pascual, F.-X.; Baldrich, E. Miniaturized metal oxide pH sensors for bacteria detection. Talanta 2016, 147, 364–369. [Google Scholar] [CrossRef]
- Liu, M.; Ma, Y.; Su, L.; Chou, K.-C.; Hou, X. A titanium nitride nanotube array for potentiometric sensing of pH. Analyst 2016, 141, 1693–1699. [Google Scholar] [CrossRef]
- Meng, L.-J.; Santos, M.P. dos Characterization of titanium nitride films prepared by d.c. reactive magnetron sputtering at different nitrogen pressures. Surf. Coat. Technol. 1997, 90, 64–70. [Google Scholar] [CrossRef]
- Maurya, D.; Sardarinejad, A.; Alameh, K. Recent Developments in R.F. Magnetron Sputtered Thin Films for pH Sensing Applications—An Overview. Coatings 2014, 4, 756–771. [Google Scholar] [CrossRef]
- Chang, Y.-H.; Lu, Y.-S.; Hong, Y.-L.; Gwo, S.; Yeh, J.A. Highly Sensitive pH Sensing Using an Indium Nitride Ion-Sensitive Field-Effect Transistor. IEEE Sens. J. 2011, 11, 1157–1161. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Chou, J.-C. Fabrication and Characterization of a Ruthenium Nitride Membrane for Electrochemical pH Sensors. Sensors 2009, 9, 2478–2490. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; Huang, B.R.; Chattopadhyay, S.; Chen, K.H.; Chen, L.C. Amorphous boron carbon nitride as a pH sensor. Appl. Phys. Lett. 2004, 84, 2676–2678. [Google Scholar] [CrossRef]
- Yusof, K.A.; Noh, N.I.; Herman, S.H.; Abdullah, A.Z.; Zolkapli, M.; Abdullah, W.F. pH sensing characteristics of silicon nitride thin film and silicon nitride-based ISFET sensor. In Proceedings of the 2013 IEEE 4th Control and System Graduate Research Colloquium, IEEE, Shah Alam, Malaysia, 19–20 August 2013; pp. 132–135. [Google Scholar]
- Chin, Y.-L.; Chou, J.-C.; Lei, Z.-C.; Sun, T.-P.; Chung, W.-Y.; Hsiung, S.-K. Titanium Nitride Membrane Application to Extended Gate Field Effect Transistor pH Sensor Using VLSI Technology. Jpn. J. Appl. Phys. 2001, 40, 6311–6315. [Google Scholar] [CrossRef]
- Bazhanov, D.I.; Knizhnik, A.A.; Safonov, A.A.; Bagatur’yants, A.A.; Stoker, M.W.; Korkin, A.A. Structure and electronic properties of zirconium and hafnium nitrides and oxynitrides. J. Appl. Phys. 2005, 97, 044108. [Google Scholar] [CrossRef]
- Babinova, R.V.; Smirnov, V.V.; Useenov, A.S.; Kravchuk, K.S.; Gladkikh, E.V.; Shapovalov, V.I.; Mylnikov, I.L. Mechanical properties of titanium nitride films obtained by reactively sputtering with hot target. J. Phys. Conf. Ser. 2017, 872, 012035. [Google Scholar] [CrossRef]
- Huang, J.-H.; Lau, K.-W.; Yu, G.-P. Effect of nitrogen flow rate on structure and properties of nanocrystalline TiN thin films produced by unbalanced magnetron sputtering. Surf. Coat. Technol. 2005, 191, 17–24. [Google Scholar] [CrossRef]
- Nur-E-Alam, M.; Rahman, M.M.; Basher, M.K.; Vasiliev, M.; Alameh, K. Optical and Chromaticity Properties of Metal-Dielectric Composite-Based Multilayer Thin-Film Structures Prepared by RF Magnetron Sputtering. Coatings 2020, 10, 251. [Google Scholar] [CrossRef]
- Lovetskiy, K.P.; Sevastianov, L.A.; Nikolaev, N.E. Numerical modeling of color perception of optical radiation. Math. Model. Geom. 2018, 6. [Google Scholar] [CrossRef]
- Study Effects of Thin Film Thickness on the Behavior of cus EGFET Implemented as pH Sensor|Request PDF. Available online: https://www.researchgate.net/publication/307588929_Study_effects_of_thin_film_thickness_on_the_behavior_of_cus_EGFET_implemented_as_pH_sensor (accessed on 11 December 2020).
- Jeyachandran, Y.L.; Narayandass, S.K.; Mangalaraj, D.; Areva, S.; Mielczarski, J.A. Properties of titanium nitride films prepared by direct current magnetron sputtering. Mater. Sci. Eng. A 2007, 445–446, 223–236. [Google Scholar] [CrossRef]
- Lengauer, W. The Reactivity of Some Transition Metal Nitrides and Carbides; Wiley: Hoboken, NJ, USA, 2015; pp. 1–24. [Google Scholar] [CrossRef]
- Gray, B.M.; Hector, A.L.; Jura, M.; Owen, J.R.; Whittam, J. Effect of oxidative surface treatments on charge storage at titanium nitride surfaces for supercapacitor applications. J. Mater. Chem. A 2017, 5, 4550–4559. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- (PDF) XPS Structure Analysis of TiN/TiC Bilayers Produced by Pulsed Vacuum Arc Discharge. Available online: https://www.researchgate.net/publication/49598678_XPS_structure_analysis_of_TiNTiC_bilayers_produced_by_pulsed_vacuum_arc_discharge (accessed on 11 December 2020).
- Zgrabik, C.M.; Hu, E.L. Optimization of sputtered titanium nitride as a tunable metal for plasmonic applications. Opt. Mater. Express 2015, 5, 2786. [Google Scholar] [CrossRef]
- Mohammed, W.M.; Gumarov, A.I.; Vakhitov, I.R.; Yanilkin, I.V.; Kiiamov, A.G.; Kharintsev, S.S.; Nikitin, S.I.; Tagirov, L.R.; Yusupov, R. V Electrical properties of titanium nitride films synthesized by reactive magnetron sputtering. J. Phys. Conf. Ser. 2017, 927, 012036. [Google Scholar] [CrossRef]
- Lonsdale, W.; Wajrak, M.; Alameh, K. Effect of conditioning protocol, redox species and material thickness on the pH sensitivity and hysteresis of sputtered RuO2 electrodes. Sens. Actuators B Chem. 2017, 252, 251–256. [Google Scholar] [CrossRef]
- Lonsdale, W. Development, Manufacture and Application of a Solid-State pH Sensor Using Ruthenium Oxide. Ph.D. Thesis, Edith Cowan University, Joondalup, Australia, 2018. Available online: https://ro.ecu.edu.au/theses/2095 (accessed on 10 October 2020).
- Lonsdale, W.; Shylendra, S.P.; Brouwer, S.; Wajrak, M.; Alameh, K. Application of ruthenium oxide pH sensitive electrode to samples with high redox interference. Sens. Actuators B Chem. 2018, 273, 1222–1225. [Google Scholar] [CrossRef]
- Dura, J.A.; Murthi, V.S.; Hartman, M.; Satija, S.K.; Majkrzak, C.F. Multilamellar Interface Structures in Nafion. Macromolecules 2009, 42, 4769–4774. [Google Scholar] [CrossRef]
- Zhong, L.; Song, Y.; Zhou, S. The Effectiveness of Nafion-Coated Stainless Steel Surfaces for Inhibiting Bacillus Subtilis Biofilm Formation. Appl. Sci. 2020, 10, 5001. [Google Scholar] [CrossRef]


| Process Parameters | Values and Comments |
|---|---|
| Sputtering target stoichiometry | Titanium Nitride (TiN, 99.95%) |
| Base pressure (Torr) | 4–5 × 10−6 |
| Argon (Ar) and Nitrogen (Ni) pressure (during deposition) | ≈2 mTorr |
| Argon (Ar) and Nitrogen (Ni) gas flow ration | Ar:10 sccm and N2; 2 sccm |
| RF power densities | 300 W |
| Substrate stage temperature (°C) | Room Temperature (21–23 °C) |
| Substrate stage rotation rate (rpm) | 10–11 |
| Substrates to target distance | 15.5–16 cm |
| Film Color | Sensitivity (mV/pH) | Hysteresis (mV) | Drift (mv/h) | E0 (Potential) (mV) |
|---|---|---|---|---|
| Green | −59 | 2.8 | 8 | 476 |
| Gold | −59.1 | 1.2 | 3.9 | 483 |
| Grey | −55.2 | 9.1 | 3.4 | 423 |
| Film Colors | Gas Ratio (Ar: N2) (Sccm) | Sputter Pressure (Milli Torr) | Sputter Power (Watts) |
|---|---|---|---|
| Green | 09:01 | 10 | 200 |
| Gold | 10:02 | 2 | 350 |
| Grey | 10:10 | 10 | 350 |
| TiN Films | Simulated Hunter Values | Measured Hunter Values | ||||
|---|---|---|---|---|---|---|
| L | a | b | L | a | b | |
| Gold film 1 | 43.85 | 5.1 | 12.43 | 43.75 | 7.23 | 8.69 |
| Gold film 2 | 40.67 | 7.8 | 6.07 | 40.60 | 6.68 | 6.96 |
| Thickness (nm) | Sensitivity (mV/pH) | E0 (mV) | R2 | Hysteresis (mV) | Drift (mV/h) | Precision (pH) |
|---|---|---|---|---|---|---|
| 20 nm | −49.2 | 498 | 0.9972 | 5.8 | 18 | 0.1 |
| 50 nm | −57.5 | 335.1 | 0.9991 | 5.5 | 9 | 0.1 |
| 85 nm | −59.1 | 483 | 0.9997 | 1.2 | 3.9 | 0.06 |
| 100 nm | −58.3 | 450 | 0.9996 | 1.8 | 4.8 | 0.03 |
| 200 nm | −57.5 | 411.3 | 0.9999 | 0.8 | 5.4 | 0.01 |
| 300 nm | −58.2 | 399.4 | 0.9998 | 1.3 | 11.8 | 0.2 |
| 500 nm | −58.5 | 442.8 | 0.9995 | 2.4 | 15.5 | 0.2 |
| Slope (mV/pH) | E0 (mV) | R2 | Hysteresis (mV) | Drift (mV/h) | Resolution (pH) |
|---|---|---|---|---|---|
| −59.1 ± 0.1 | 483 ± 2 | 0.9997 | 1.2 ± 0 | 3.9 | 0.06 |
| Matrix | Titanium Nitride | Iridium Oxide | ||||
|---|---|---|---|---|---|---|
| (mV/pH) | E0 (mV) | R2 | (mV/pH) | E0 (mV) | R2 | |
| pH 7 | −59.1 | 483 | 0.9997 | −57.9 | 590 | 0.9997 |
| Reduced (ascorbic acid) | −56.9 | 451 | 0.9992 | −55.2 | 444 | 0.9965 |
| Oxidized (KMnO4) | −54 | 902 | 0.9974 | −55 | 889 | 0.9683 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul Shylendra, S.; Lonsdale, W.; Wajrak, M.; Nur-E-Alam, M.; Alameh, K. Titanium Nitride Thin Film Based Low-Redox-Interference Potentiometric pH Sensing Electrodes. Sensors 2021, 21, 42. https://doi.org/10.3390/s21010042
Paul Shylendra S, Lonsdale W, Wajrak M, Nur-E-Alam M, Alameh K. Titanium Nitride Thin Film Based Low-Redox-Interference Potentiometric pH Sensing Electrodes. Sensors. 2021; 21(1):42. https://doi.org/10.3390/s21010042
Chicago/Turabian StylePaul Shylendra, Shimrith, Wade Lonsdale, Magdalena Wajrak, Mohammad Nur-E-Alam, and Kamal Alameh. 2021. "Titanium Nitride Thin Film Based Low-Redox-Interference Potentiometric pH Sensing Electrodes" Sensors 21, no. 1: 42. https://doi.org/10.3390/s21010042
APA StylePaul Shylendra, S., Lonsdale, W., Wajrak, M., Nur-E-Alam, M., & Alameh, K. (2021). Titanium Nitride Thin Film Based Low-Redox-Interference Potentiometric pH Sensing Electrodes. Sensors, 21(1), 42. https://doi.org/10.3390/s21010042