Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instruments
2.3. Procedures
2.3.1. Synthesis and Characterization of Gold Nanoparticles
2.3.2. Manufacturing of the Sensor (Au-gr/CVE)
2.3.3. Electrochemical Measurements
2.4. Statistical Analysis and Data Treatment
3. Results
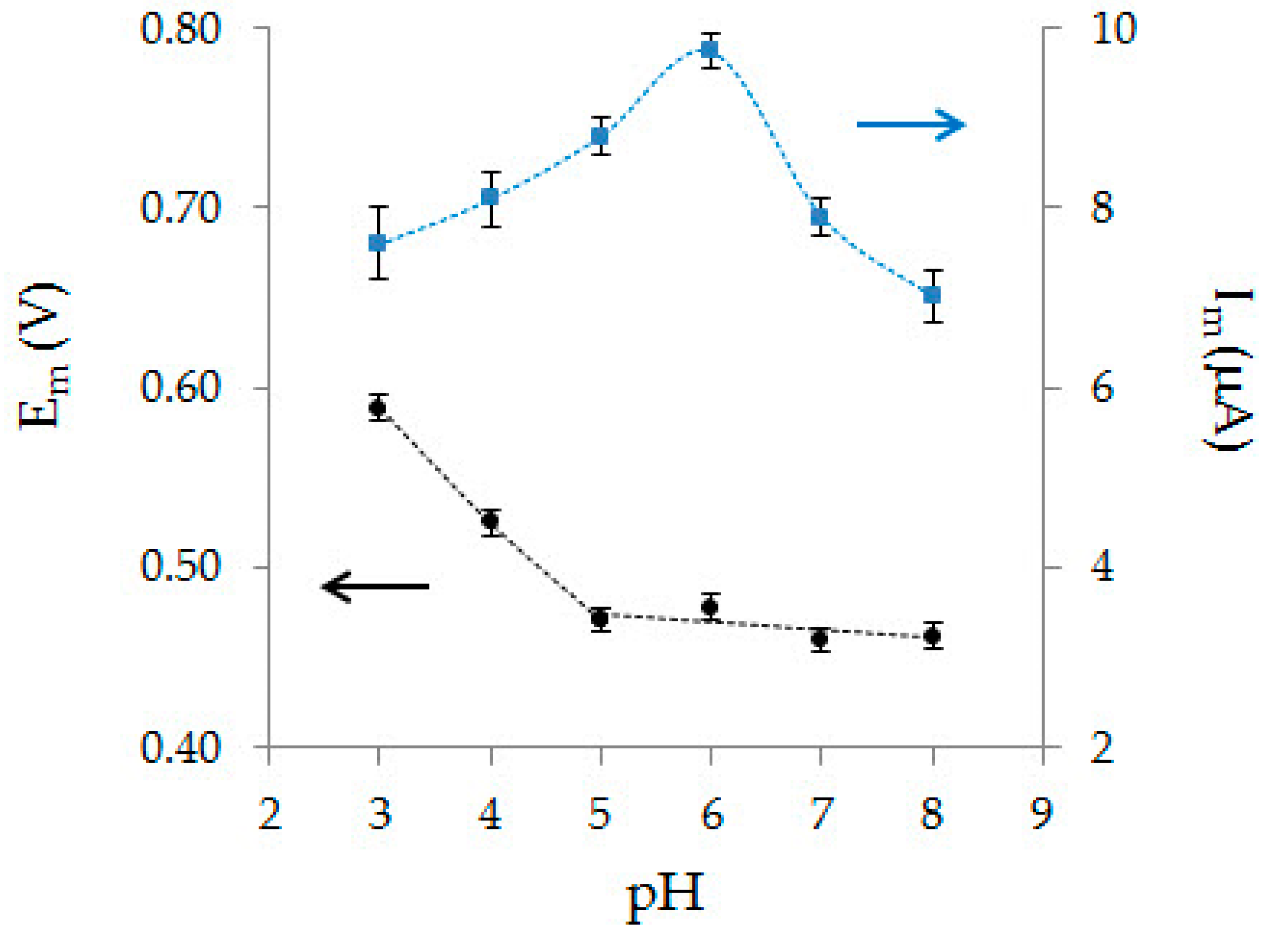
3.1. Electrochemical Behavior of Ascorbic Acid
3.2. Characterization of CVE and Au-gr/CVE
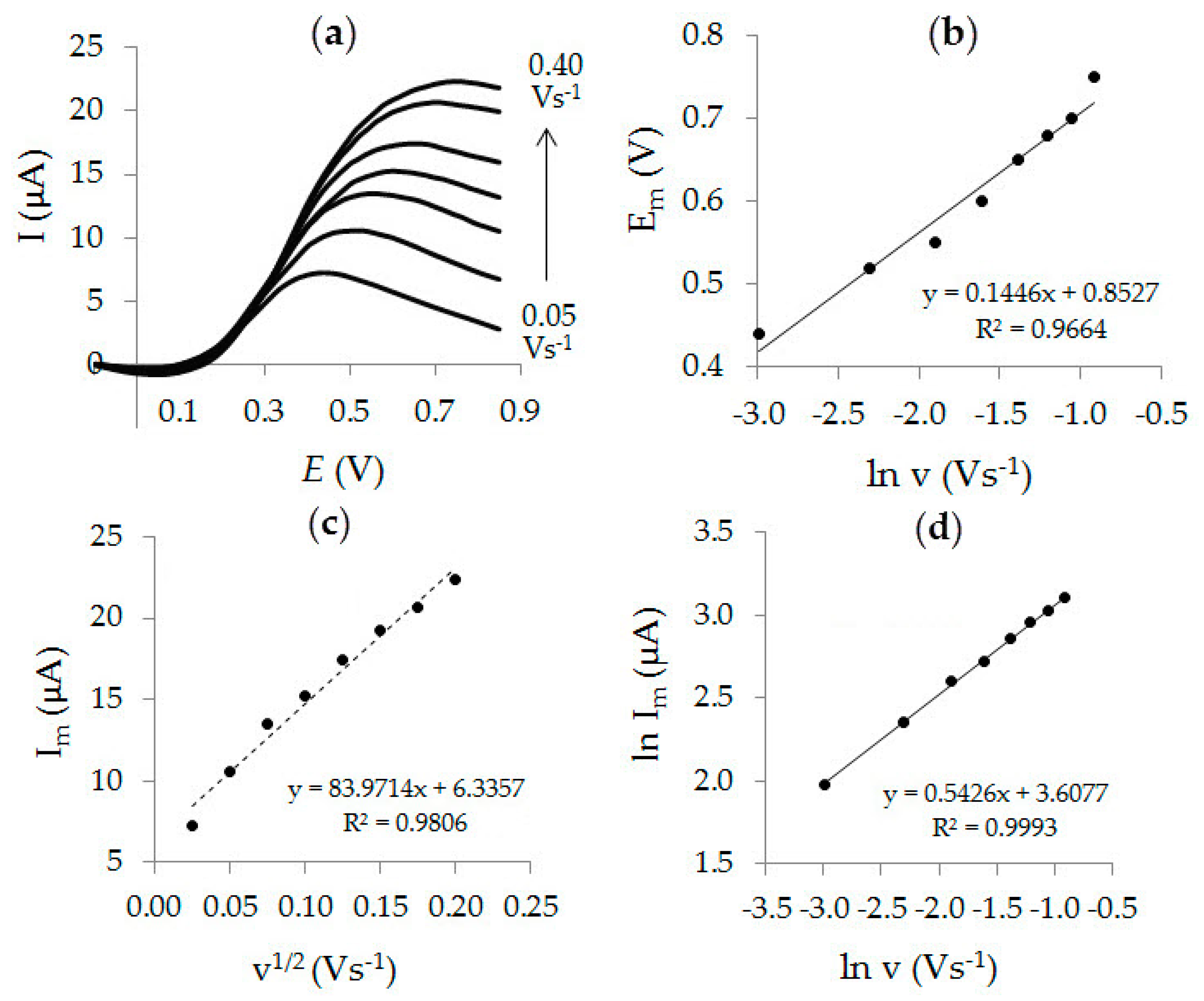
3.3. Electrooxidation of Ascorbic Acid on Au-gr/CVE
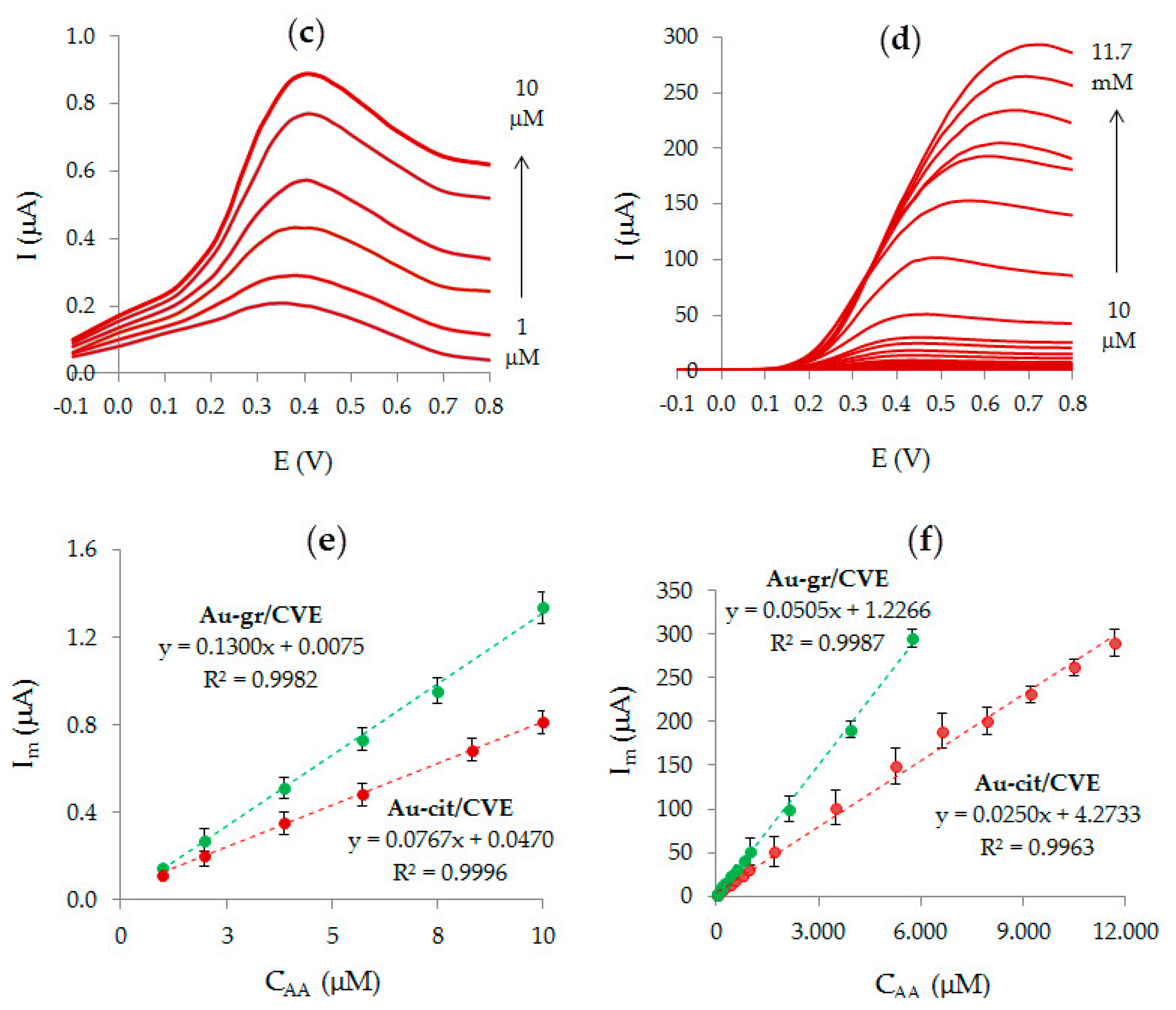
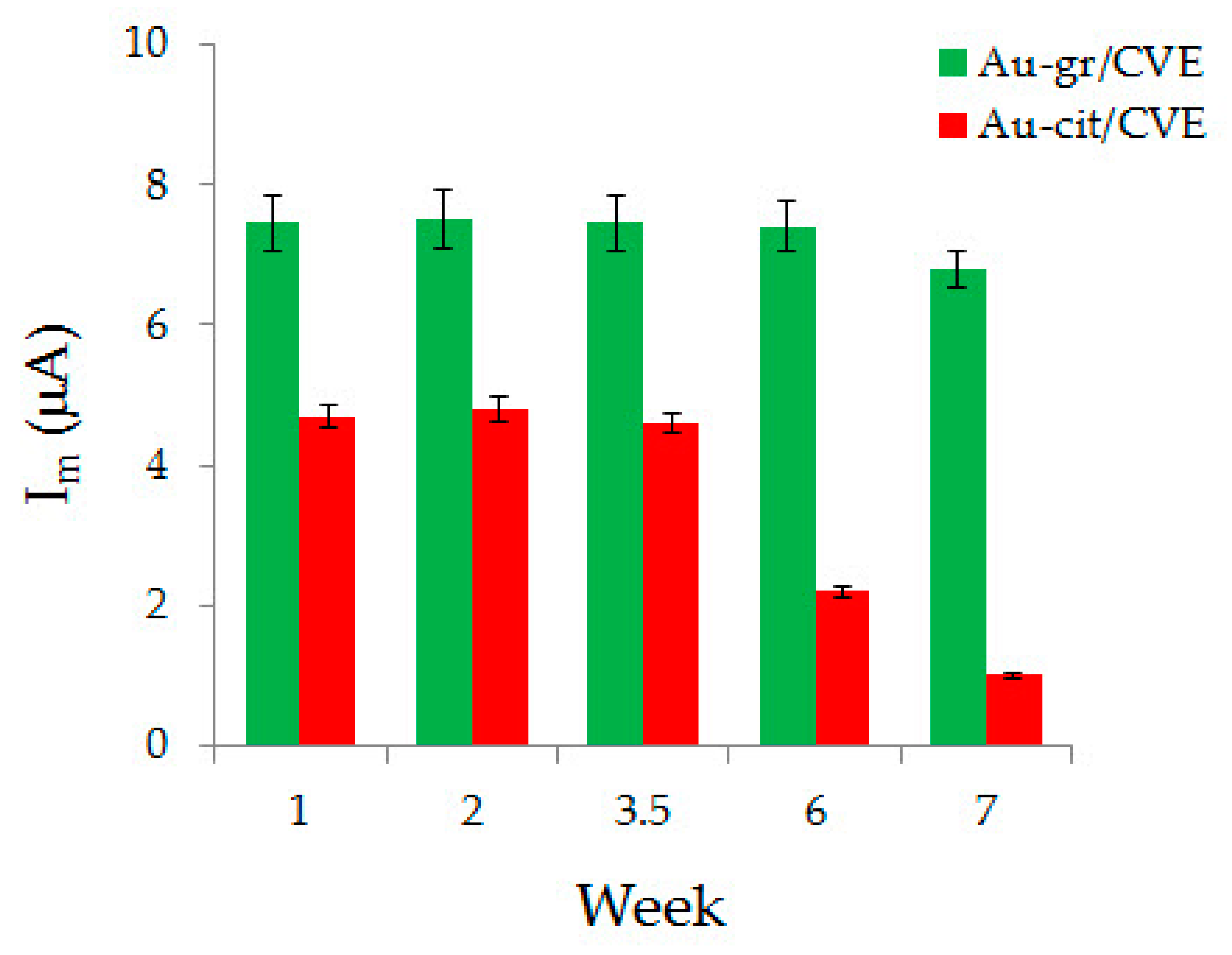
3.4. Analytic Characteristics of Au-gr/CVE
3.5. Determination of Ascorbic Acid in Fruit Juices
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brainina, K.; Tarasov, A.; Khamzina, E.; Stozhko, N.; Vidrevich, M. Contact hybrid potentiometric method for on-site and in situ estimation of the antioxidant activity of fruits and vegetables. Food Chem. 2020, 309, 125703. [Google Scholar] [CrossRef] [PubMed]
- Al Majidi, M.I.H.; Al-Qubury, H.Y. Determination of vitamin C (ascorbic acid) contents in various fruit and vegetable by UV-spectrophotometry and titration methods. J. Chem. Pharm. Sci. 2016, 9, 2972–2974. [Google Scholar]
- Da Silva, T.L.; Aguiar-Oliveira, E.; Mazalli, M.R.; Kamimura, E.S.; Maldonado, R.R. Comparison between titrimetric and spectrophotometric methods for quantification of vitamin C. Food Chem. 2017, 224, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Salkic, M.; Selimovic, A. Spectrophotometric determination of L-ascorbic acid in pharmaceuticals based on its oxidation by potassium peroxymonosulfate and hydrogen peroxide. Croat. Chem. Acta 2015, 88, 73–79. [Google Scholar] [CrossRef]
- Fatin Najwa, R.; Azrina, A. Comparison of vitamin C content in citrus fruits by titration and high performance liquid chromatography (HPLC) methods. Int. Food Res. J. 2017, 24, 726–733. [Google Scholar]
- Cunha-Santos, E.C.E.; Viganó, J.; Neves, D.A.; Martinez, J.; Godoy, H.T. Vitamin C in camu-camu: Evaluation of extraction and analytical methods. Food Res. Int. 2019, 115, 160–166. [Google Scholar] [CrossRef]
- Zuo, R.; Zhou, S.; Zuo, Y.; Deng, Y. Determination of creatinine, uric and ascorbic acid in bovine milk and orange juice by hydrophilic interaction HPLC. Food Chem. 2015, 182, 242–245. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, P.; Wang, X.; Chen, W.; Wu, L.; Wang, Q. Determination of vitamine C in fruits and vegetables by ultra performance liquid chromatography-quadrupole time-of-flight mass spectrometry. Chin. J. Chromatogr. 2016, 34, 1048–1054. [Google Scholar] [CrossRef]
- Kong, W.; Wu, D.; Li, G.; Chen, X.; Gong, P.; Sun, Z.; Chen, G.; Xia, L.; You, J.; Wu, Y. A facile carbon dots based fluorescent probe for ultrasensitive detection of ascorbic acid in biological fluids via non-oxidation reduction strategy. Talanta 2017, 165, 677–684. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Serban, A.I.; Fafaneata, C. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- Skrovankova, S.; Mlcek, J.; Sochor, J.; Baron, M.; Kynicky, J.; Jurikova, T. Determination of ascorbic acid by electrochemical techniques and other methods. Int. J. Electrochem. Sci. 2015, 10, 2421–2431. [Google Scholar]
- Chang, S.K.; Ismail, A.; Daud, Z.A.M. Ascorbic acid: Properties, determination and uses. In Encyclopedia of Food and Health, 1st ed.; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Wang, H.; Xiao, L.-G.; Chu, X.-F.; Chi, Y.-D.; Yang, X.-T. Rational design of gold nanoparticle/graphene hybrids for simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid. Chin. J. Anal. Chem. 2016, 44, e1617–e1625. [Google Scholar] [CrossRef]
- Ghanbari, K.; Hajian, A. Electrochemical characterization of Au/ZnO/PPy/RGO nanocomposite and its application for simultaneous determination of ascorbic acid, epinephrine, and uric acid. J. Electroanal. Chem. 2017, 801, 466–479. [Google Scholar] [CrossRef]
- Ji, D.Z.; Liu, Z.X.; Liu, L.; Low, S.S.; Lu, Y.L.; Yu, X.J.; Zhu, L.; Li, C.D.; Liu, Q.J. Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes. Biosens. Bioelectron. 2018, 119, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Scremin, J.; Barbosa, E.C.M.; Salamanca-Neto, C.A.R.; Camargo, P.H.C.; Sartori, E.R. Amperometric determination of ascorbic acid with a glassy carbon electrode modified with TiO2-gold nanoparticles integrated into carbon nanotubes. Microchim. Acta 2018, 185, 251. [Google Scholar] [CrossRef]
- Stozhko, N.Y.; Malakhova, N.A.; Byzov, I.V.; Brainina, K.Z. Electrodes in Stripping Voltammetry: From a Macro- to a Micro- and Nano-Structured Surface. J. Anal. Chem. 2009, 64, 1148–1157. [Google Scholar] [CrossRef]
- Kuang, H.Y.; He, J.H.; Xu, Q.; Song, Z.R. Determination of Ascorbic Acid Based on Gold Nanoparticles-L-Alanine/GCE. Adv. Mater. Res. 2011, 214, 498–502. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, F.; Xu, S.; Xia, Y.; Huang, W.; Li, Z. Fabrication of nanoflower-like dendritic Au and polyaniline composite nanosheets at gas/liquid interface for electrocatalytic oxidation and sensing of ascorbic acid. Electrochem. Commun. 2013, 30, 46–50. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Vikulova, E. Nanomaterials: Electrochemical properties and application in sensors. Phys. Sci. Rev. 2019, 3, 8050. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Galperin, L.G.; Bukharinova, M.A.; Stozhko, N.Y. Mathematical modeling and experimental study of electrode processes. J. Solid State Electrochem. 2015, 19, 599–606. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Stozhko, N.Y.; Bukharinova, M.A.; Galperin, L.G.; Vidrevich, M.B.; Murzakaev, A.M. Mathematical modeling and experimental data of the oxidation of ascorbic acid on electrodes modified by nanoparticles. J. Solid State Electrochem. 2016, 20, 2323–2330. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Stozhko, N.Y.; Shalygina, Z.V. Surface Microreliefs and Voltage–Current Characteristics of Gold Electrodes and Modified Thick-Film Graphite-Containing Electrodes. J. Anal. Chem. 2004, 59, 753–759. [Google Scholar] [CrossRef]
- Stozhko, N.; Bukharinova, M.; Galperin, L.; Brainina, K. Nanostructured Sensor Based on Gold Nanoparticles and Nafion for Determination of Uric Acid. Biosensors 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Stozhko, N.Y.; Bukharinova, M.A.; Khamzina, E.I.; Tarasov, A.V.; Vidrevich, M.B.; Brainina, K.Z. The Effect of the Antioxidant Activity of Plant Extracts on the Properties of Gold Nanoparticles. Nanomaterials 2019, 9, 1655. [Google Scholar] [CrossRef] [PubMed]
- Timoszyk, A. A review of the biological synthesis of gold nanoparticles using fruit extracts: Scientific potential and application. Bull. Mater. Sci. 2018, 41, 154. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Recent advances in plant-mediated engineered gold nanoparticles and their application in biological system. J. Trace Elem. Med. Biol. 2017, 40, 10–23. [Google Scholar] [CrossRef]
- Shin, D.; Shen, C.; Sanghadasa, M. Breathable 3D supercapacitors based on activated carbon fiber veil. Adv. Mater. Technol. 2018, 3, 1800209. [Google Scholar] [CrossRef]
- Ieropoulos, I.; Greenman, J.; Melhuish, C. Microbial fuel cells based on carbon veil electrodes: Stack configuration and scalability. Int. J. Energy Res. 2008, 32, 1228–1240. [Google Scholar] [CrossRef]
- Banerjee, R.; Bevilacqua, N.; Mohseninia, A.; Wiedemann, B.; Wilhelm, F.; Scholta, J.; Zeis, R. Carbon felt electrodes for redox flow battery: Impact of compression on transport on properties. J. Energy Storage 2019, 26, 100997. [Google Scholar] [CrossRef]
- Duarte, K.D.Z.; Frattini, D.; Kwon, Y. High performance yeast-based microbial fuels cells by surfacant-mediated gold nanoparticles grown atop a carbon felt anode. Appl. Energy 2019, 256, 113912. [Google Scholar] [CrossRef]
- Tools4FRP. Available online: https://www.tools4frp.com/carbon-fibre-veils/ (accessed on 3 December 2019).
- Zhang, X.F.; Li, D.; Liu, K.; Tong, J.; Yi, X.S. Flexible grapheme-coated carbon fober veil/polydimethylsiloxane mats as electrothermal materials with rapid responsiveness. Int. J. Lightweight Mater. Manuf. 2019, 2, 241–249. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Gu, Y.; Gong, Y.; Liang, J.; Wang, S.; Zhang, Z. Resistance heating forming process besed on carbon fiber veil for continuous glass fiber reinforced polypropylene. J. Reinf. Plast. Compos. 2018, 37, 366–380. [Google Scholar] [CrossRef]
- Wei, W.; Wu, S.-G. Study of electrooxidation behavior of nitrite on gold nanoparticles/graphitizing carbon felt electrode and its analytical application. Chin. J. Anal. Chem. 2019, 47, e19014–e19020. [Google Scholar] [CrossRef]
- Kuntolaksono, S.; Matsuura, H. Coulometric analysis of nitrite using electrochemically activated carbon felt electrode. Sens. Mater. 2019, 31, 1215–1224. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; Tricard, S.; Yu, L.; Fang, J. A sensitive and selective electrochemical sensor based on N, P-doped molybdenum carbide@carbon/Prussian blue/graphite felt composite electrode for the detection dopamine. Anal. Chim. Acta 2020, 1094, 80–89. [Google Scholar] [CrossRef]
- Manjubaashini, N.; Sephra, P.J.; Nehru, K.; Sivakumar, M.; Thangadurai, T.D. Electrochemical determination of ATP at rhodamine6G capped gold a nanoparticles modified carbon felt electrode at pH 7.2. Sens. Actuators B 2019, 281, 1054–1062. [Google Scholar] [CrossRef]
- Davies, T.J. Anodic stripping voltammetry with graphite felt electrodes for the trace analysis of silver. Analyst 2016, 141, 4742–4748. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Bukharinova, M.; Khamzina, E.; Vidrevich, M. Potentiometric method of plant microsuspensions antioxidant activity determination. Food Chem. 2019, 278, 653–658. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Galperin, L.G.; Vikulova, E.V.; Stozhko, N.Y.; Murzakaev, A.M.; Timoshenkova, O.R.; Kotov, Y.A. Gold nanoparticles electrooxidation: Comparison of theory and experiment. J. Solid State Electrochem. 2011, 15, 1049–1056. [Google Scholar] [CrossRef]
- Burns, D.T.; Danzer, K.; Townshend, A. Use of the terms “recovery” and “apparent recovery” in analytical procedures (IUPAC Recommendations 2002). Pure Appl. Chem. 2003, 74, 2201–2205. [Google Scholar] [CrossRef]
- Fotouhi, L.; Fatollahzadeh, M.; Heravi, M.M. Electrochemical Behavior and Voltammetric Determination of Sulfaguanidine at a Glassy Carbon Electrode Modified With a Multi-Walled Carbon Nanotube. Int. J. Electrochem. Sci. 2012, 7, 3919–3928. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 2001. [Google Scholar]
- Jayant, I. Gowda Sharanappa, T. Nandibewoor Electrochemical behavior of paclitaxel and its determination at glassy carbon electrode. Asian J. Pharm. Sci. 2014, 9, 42–49. [Google Scholar] [CrossRef]
- Eisele, T.A.; Drake, S.R. The partial compositional characteristics of apple juice from 175 apple varieties. J. Food Compos. Anal. 2005, 18, 213–221. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Negulescu, G.P.; Pisoschi, A. Determination of ascorbic acid content of some fruit juices and wine by voltammetry performed at Pt and carbon paste electrodes. Molecules 2011, 16, 1349. [Google Scholar] [CrossRef] [PubMed]
- Haile Reda, A.; Guesh, F. Determination of Ascorbic Acid in Citrus Sinensis and Ananas Comosus Using Poly(3, 4-Ethylenedioxythiophene) Modified Glassy Carbon Electrode. Am. J. Appl. Chem. 2016, 4, 1–7. [Google Scholar] [CrossRef]
- Wonsawat, W. Determination of Vitamin C (ascorbic acid) in orange juices product. Int. J. Mater. Metall. Eng. 2014, 8, 6. [Google Scholar]
- Bitew, Z.; Amare, M. Electrochemical determination of ascorbic acid in pharmaceutical tablets using carbon paste electrode. Org. Med. Chem. Int. J. 2019, 8, 555749. [Google Scholar] [CrossRef]
- Kong, Y.; Shan, X.; Ma, J.; Chen, M.; Chen, Z. A novel voltammetric sensor for ascorbic acid based on molecularly imprinted poly (o-phenylenediamine-co-o-aminophenol). Anal. Chim. Acta 2014, 809, 54–60. [Google Scholar] [CrossRef]
- Samseya, J.; Srinivasan, R.; Chang, Y.-T.; Tsao, C.-W.; Vasantha, V.S. Fabrication and characterization of high performance polypyrrole modified microarray sensor for ascorbic acid determination. Anal. Chim. Acta 2013, 793, 11–18. [Google Scholar] [CrossRef]
- Abraha, T.; Sergawie, A. Assessment of some selected beverages and fresh edible vegetables as nutritional source of vitamin C (ascorbic acid) by cyclic and square wave voltammetry. Int. J. Sci. Eng. Investig. 2014, 3, 39–49. [Google Scholar]
- Nezamzaden, A.; Amini, M.K.; Faghihian, H. Square-wave voltammetric determination of ascorbic acid based on its electrocatalytic oxidation at zeolite-modified carbon-paste electrode. Int. J. Electrochem. Sci. 2007, 2, 583–594. [Google Scholar]
- Tadese, A.; Subramanian, P.A.; Woldu, A. Electrochemical determination and comparison of ascorbic acid in freshly prepared and bottled fruit juices: A cyclic voltammetric study. J. Chem. Pharm. Res. 2014, 6, 880–888. [Google Scholar]
- State Standard 24556–89. Products of fruits and vegetables processing. In Methods for Determination of Vitamin C; Izdatelstvo Standartov: Moscow, Russian, 2003. [Google Scholar]
- Brainina, K.Z.; Ivanova, A.V.; Sharafutdinova, E.N.; Lozovskaya, E.L.; Shkarina, E.I. Potentiometry as a method of antioxidant activity investigation. Talanta 2007, 71, 13–18. [Google Scholar] [CrossRef]

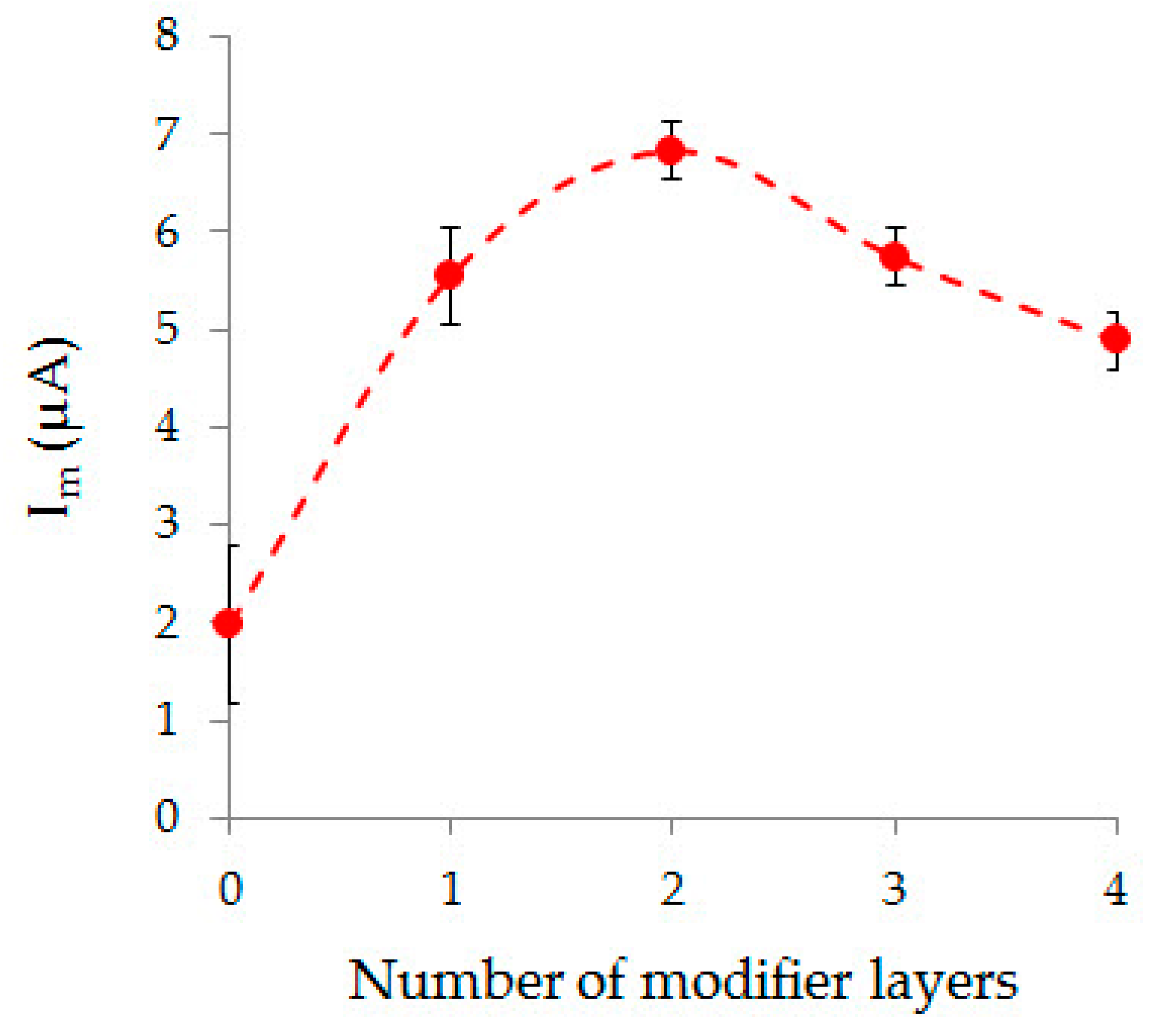

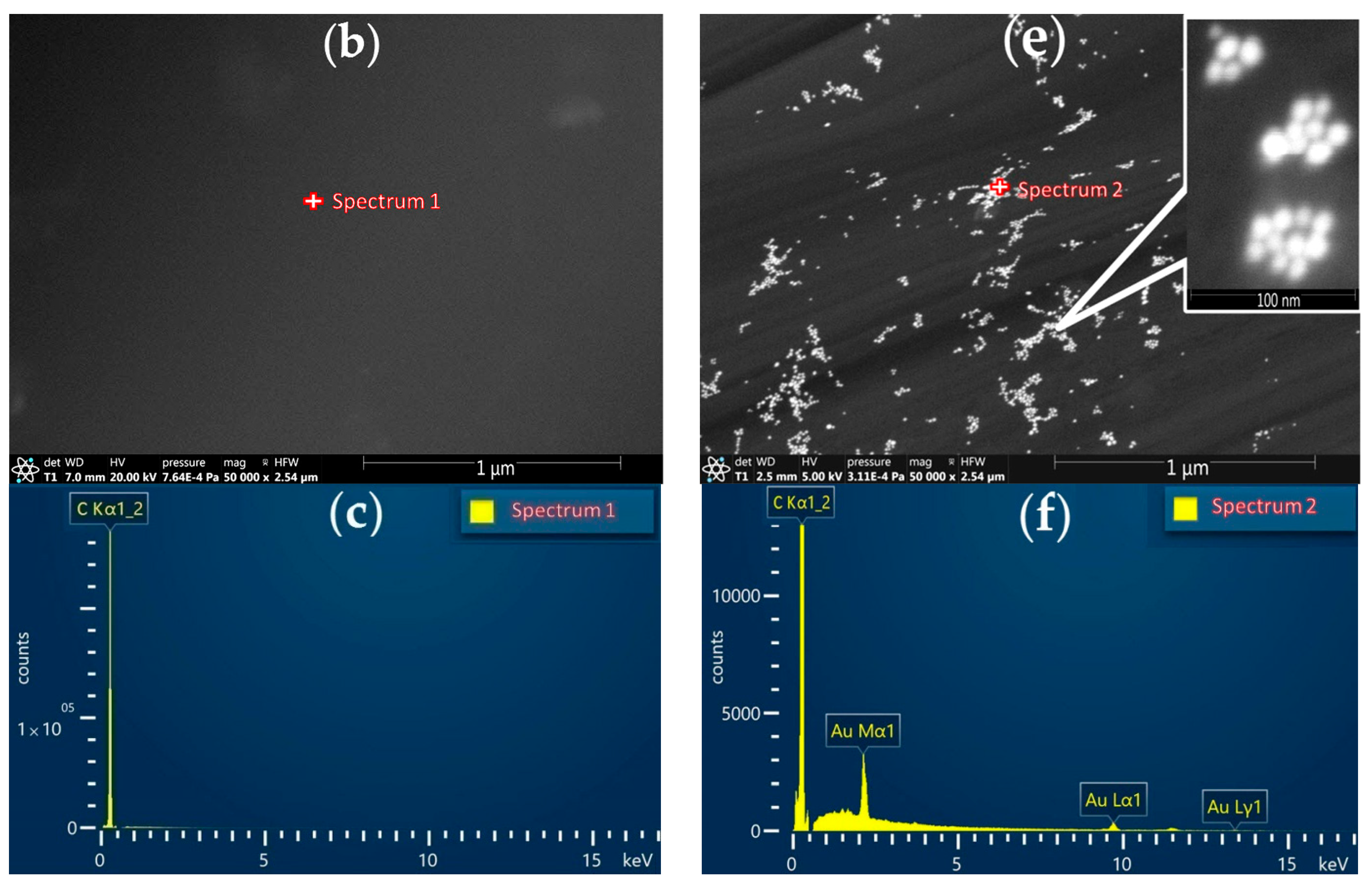
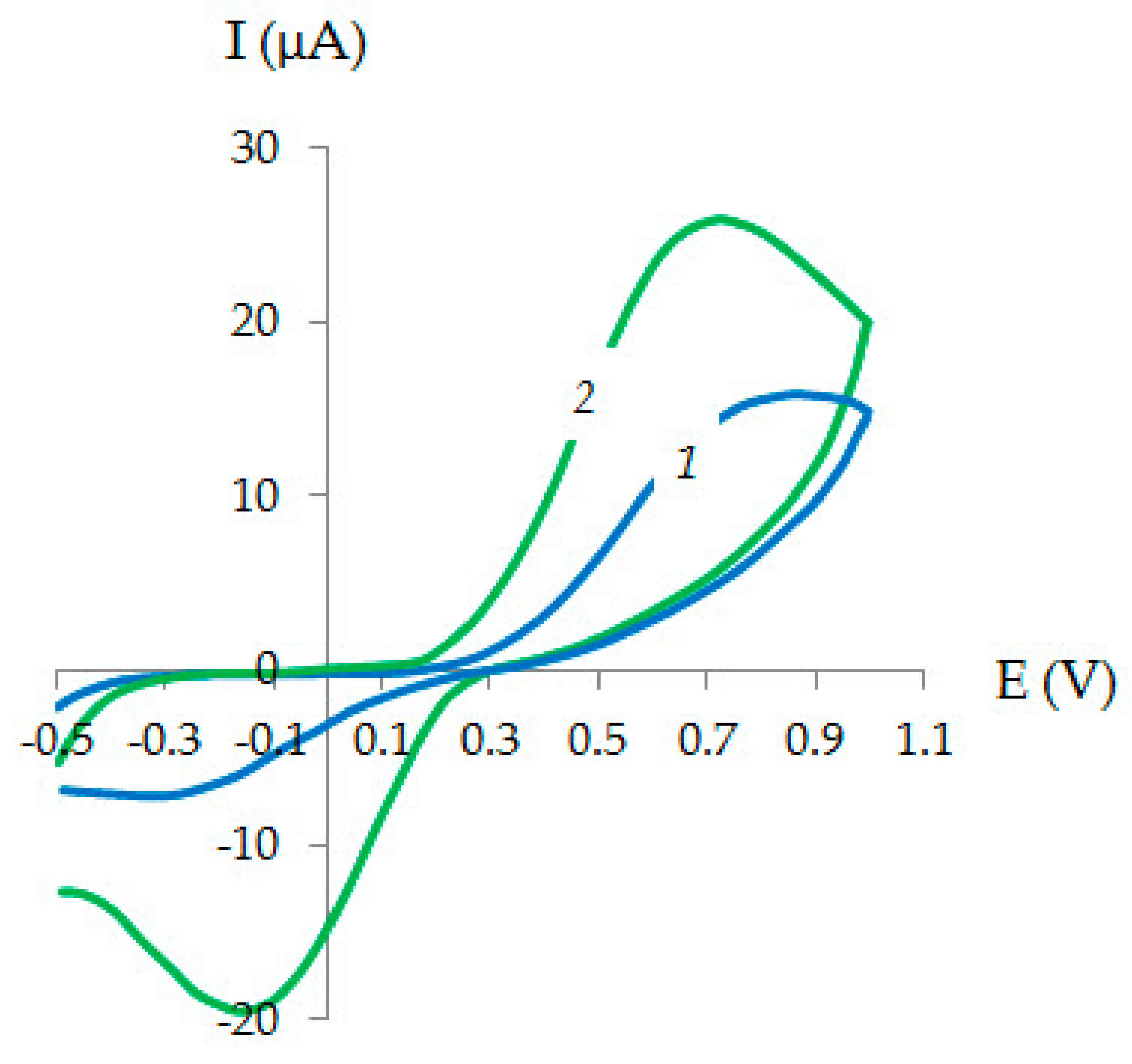
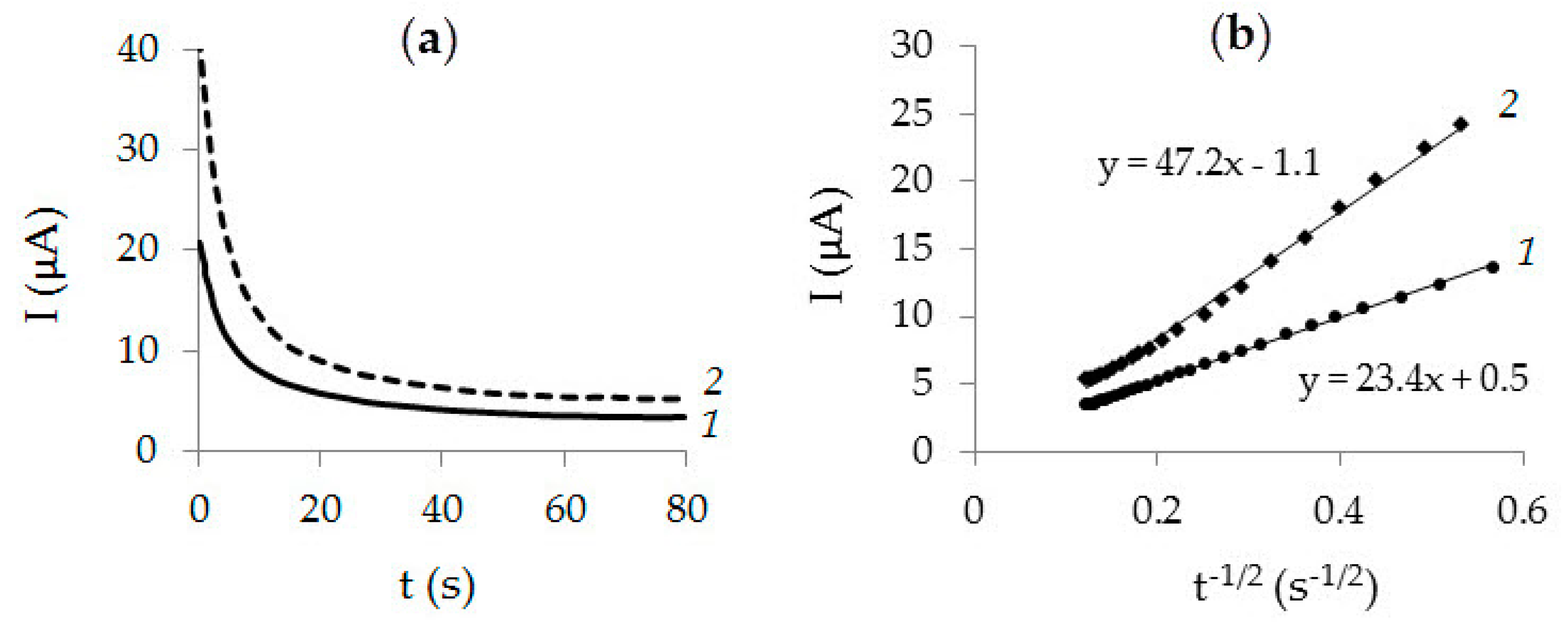


| Method | Parameter | Au-gr | Au-cit |
|---|---|---|---|
| TEM | Shape | Spherical | Spherical |
| d, nm | 14 ± 7 [25] | 13 and 38 (up to 10 %) [42] | |
| UV-Vis-spectrophotometry | d, nm | 10 ± 1 [25] | 20 [42] |
| LS Voltammetry | Im (Au), µA | 31 ± 5 | 27 ± 4 |
| Em (Au), V | 0.851 ± 0.002 | 0.877 ± 0.011 | |
| Em (AA), V | 0.420 ± 0.006 | 0.442 ± 0.007 |
| Interfering Substance | Concentration of Interfering Substance (CIS), mM | CIS: CAA | AA Response Change, % |
|---|---|---|---|
| Glucose | 1 | 100 | −9.5 |
| Sucrose | 5 | 500 | +4.2 |
| Fructose | 6 | 600 | +4.8 |
| Citric acid | 6 | 600 | −0.5 |
| Tartaric acid | 8 | 800 | −1.6 |
| Malic acid | 10 | 1000 | −0.5 |
| Sensor | LOD, μM | LR, μM | Technique | Object | Ref. |
|---|---|---|---|---|---|
| TiO2-Aunps-MWCNT-DHP/GCE | 1.20 | 5–51 | Am | pharmaceutical and orange juice samples | [16] |
| Aunps-PAN/GCE | 8.20 | 10–12000 | Am | medicine vitamin C tablets | [19] |
| Aunps-ZnO-PPy-RGO/GCE | 0.16 | 2–950 | DPV | vitamin C tablets | [14] |
| Aunps-L-Alanine/GCE | 10.00 | 12–160 | CV | – | [18] |
| Pt-electrode | 87.00 | 310–20000 | DPV | fruit juices, wine | [48] |
| CPE | 20.00 | 70–20000 | DPV | – | [48] |
| PEDOT/GCE | 23.30 | 50–90 | SWV | orange and pineapple juices | [49] |
| SPCE | 1360.00 | 0–10000 | CV | packed orange juice sample | [50] |
| CPE | 1.76 | 10–100 | SWV | pharmaceutical tablets | [51] |
| PoPDoAP/GCE | 36.40 | 100–1000 | DPV | vitamin C tablet and orange juices | [52] |
| Ppy/Au-MA | 5.00 | 10–2200 | SWV | lemon juice and celin tablet chewable | [53] |
| GCE | 11.50 | 8–80 | CV, SWV | beverages and fresh edible vegetables | [54] |
| Fe(III)-Y zeolite/CPE | 0.02 | 0.4–1200 | SWV | citrus juices | [55] |
| CPE | 22.10 | - | CV | fruit juices | [56] |
| Au-gr/CVE | 0.05 | 1–5750 | AV | fruit juice | [this work] |
| Sample | Found in the Sample, mM | Added, mM | Found in the Sample with Additive, mM | Found Additive, mM | R, % |
|---|---|---|---|---|---|
| Cherry-apple juice | 0.81 ± 0.06 | 1.94 | 2.96 ± 0.21 | 2.15 ± 0.23 | 111 |
| Apple juice for children | 0.47 ± 0.01 | 0.97 | 1.43 ± 0.03 | 0.96 ± 0.02 | 99 |
| Apple juice | 0.16 ± 0.01 | 0.25 | 0.418 ± 0.003 | 0.26 ± 0.01 | 104 |
| Apple nectar clarified. | 0.09 ± 0.01 | 0.12 | 0.21 ± 0.02 | 0.120 ± 0.003 | 100 |
| Samples | Found Using Au-gr/CVE | RSD, % | Found by Potentiometric Titration | RSD, % | F-Test | t-Test |
|---|---|---|---|---|---|---|
| Cherry-apple juice | 0.81 ± 0.06 | 6.8 | 0.76 ± 0.07 | 8.7 | 1.43 | 0.67 |
| Apple juice for children | 0.47 ± 0.01 | 2.2 | 0.42 ± 0.03 | 7.6 | 9.00 | 1.82 |
| Apple juice | 0.16 ± 0.01 | 5.0 | 0.16 ± 0.01 | 4.5 | 1.18 | 0.49 |
| Apple nectar clarified | 0.09 ± 0.01 | 6.5 | 0.09 ± 0.01 | 9.0 | 1.97 | 0.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brainina, K.Z.; Bukharinova, M.A.; Stozhko, N.Y.; Sokolkov, S.V.; Tarasov, A.V.; Vidrevich, M.B. Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid. Sensors 2020, 20, 1800. https://doi.org/10.3390/s20061800
Brainina KZ, Bukharinova MA, Stozhko NY, Sokolkov SV, Tarasov AV, Vidrevich MB. Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid. Sensors. 2020; 20(6):1800. https://doi.org/10.3390/s20061800
Chicago/Turabian StyleBrainina, Khiena Z., Maria A. Bukharinova, Natalia Yu. Stozhko, Sergey V. Sokolkov, Aleksey V. Tarasov, and Marina B. Vidrevich. 2020. "Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid" Sensors 20, no. 6: 1800. https://doi.org/10.3390/s20061800
APA StyleBrainina, K. Z., Bukharinova, M. A., Stozhko, N. Y., Sokolkov, S. V., Tarasov, A. V., & Vidrevich, M. B. (2020). Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid. Sensors, 20(6), 1800. https://doi.org/10.3390/s20061800







