Plutonium (IV) Quantification in Technologically Relevant Media Using Potentiometric Sensor Array
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Sensor Preparation and Potentiometric Measurements
2.3. UV-Vis-NIR Spectroscopic Measurements
2.4. Multivariate Data Processing
3. Results
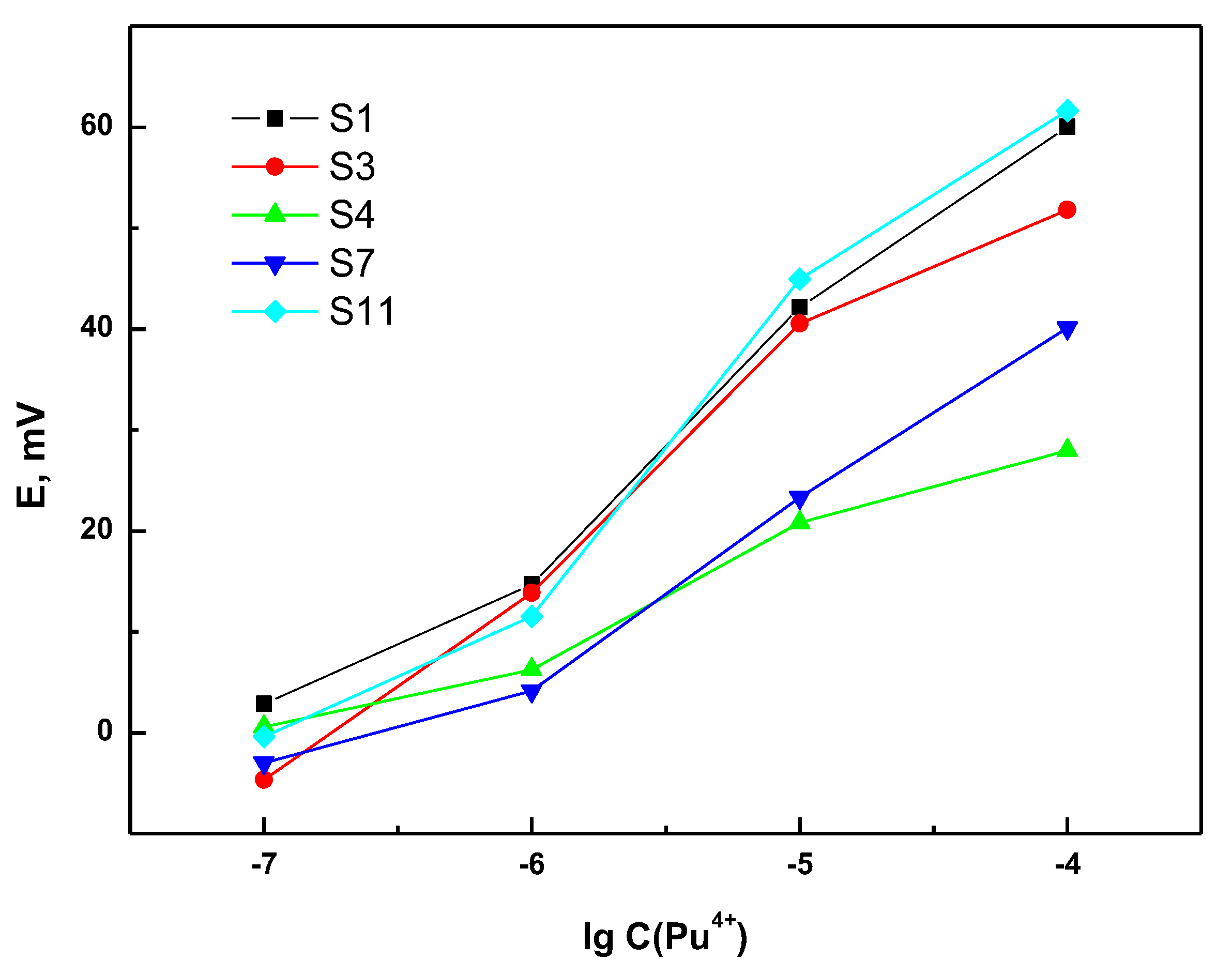
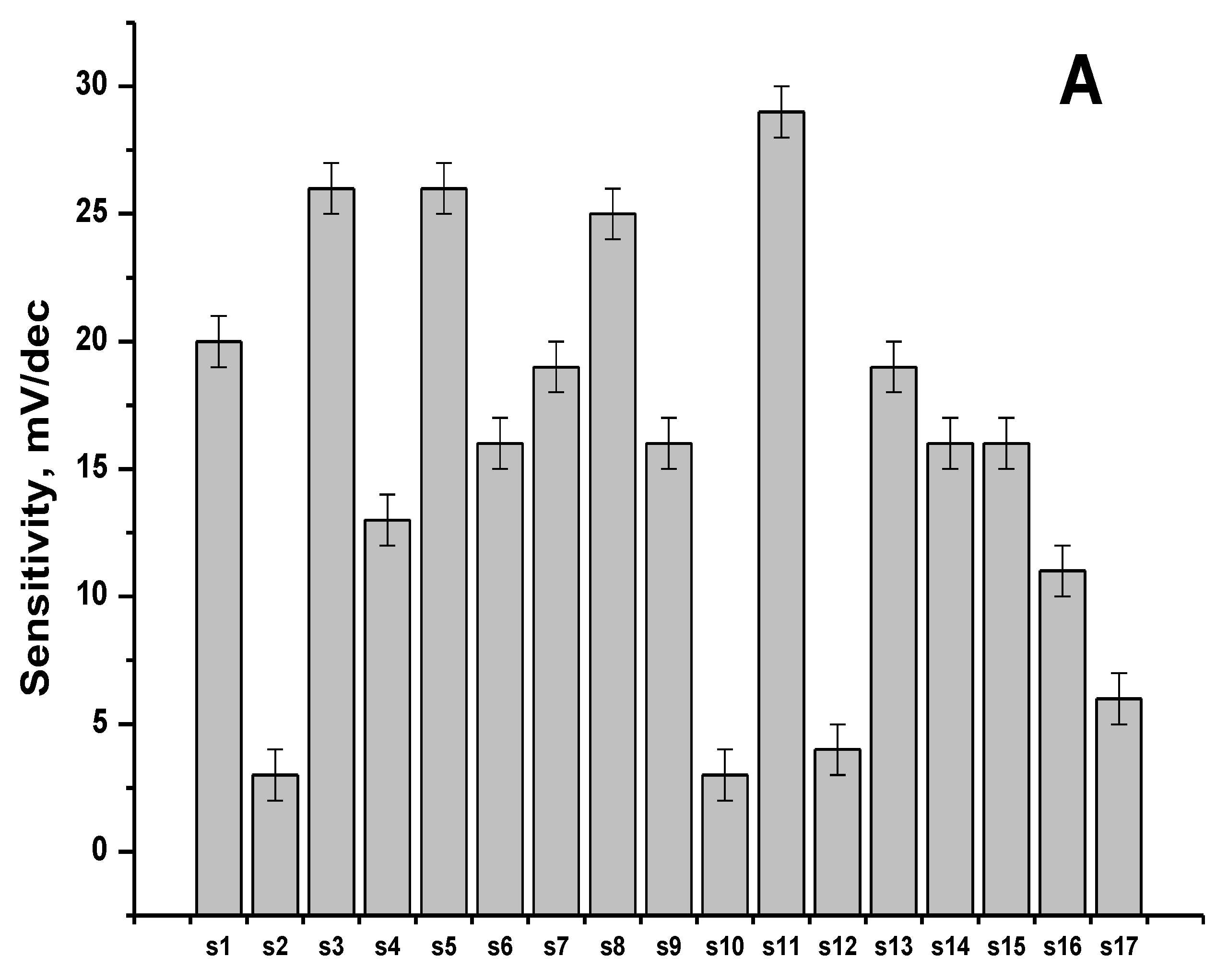
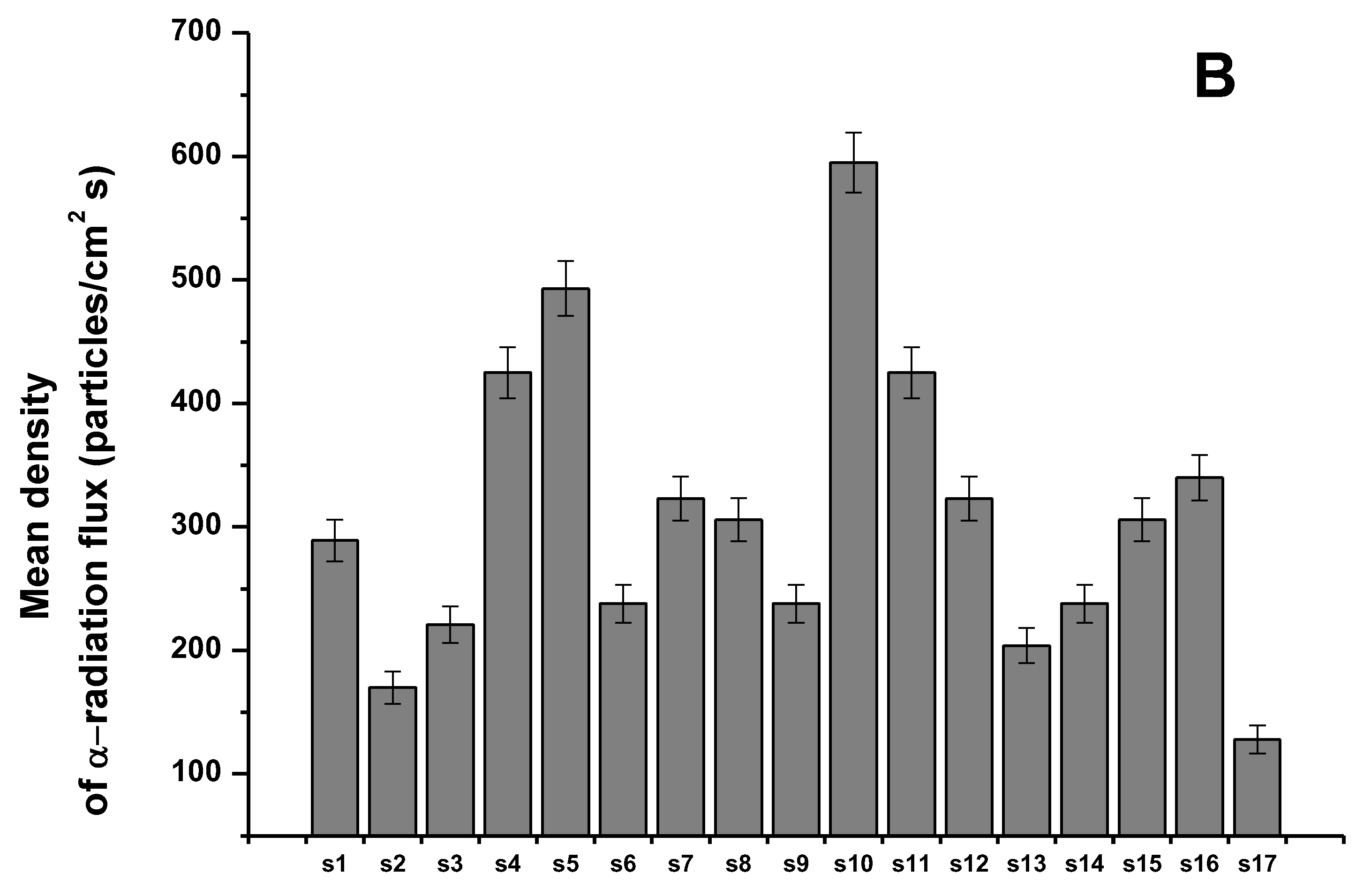
3.1. Sensitivity and Selectivity of the Individual Sensors
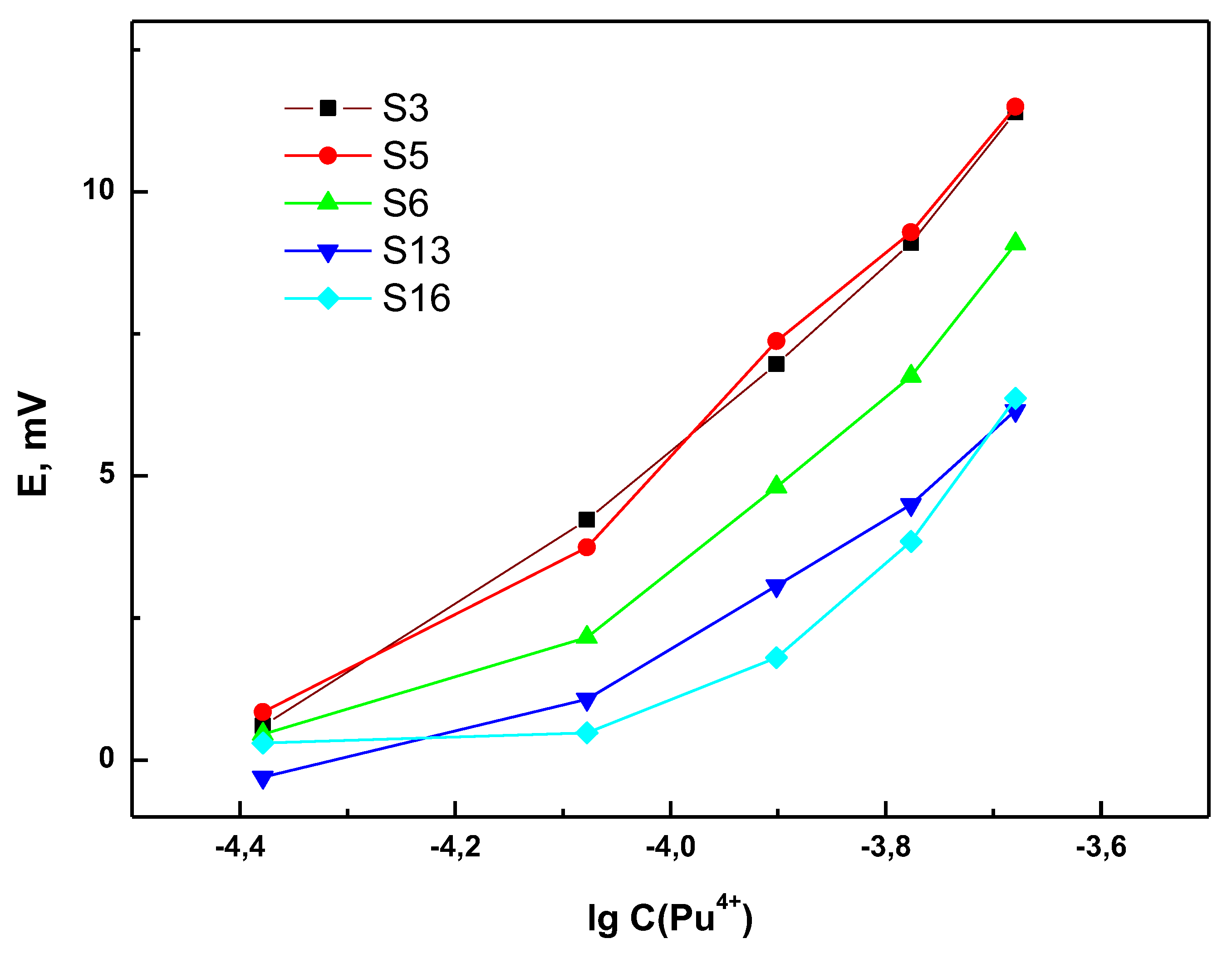
3.2. Sensor Response towards Pu4+ in Presence of UO22+
3.3. Multisensor Measurements in Simulated PUREX Solutions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paiva, A.P.; Malik, P. Recent advances on the chemistry of solvent extraction applied to the reprocessing of spent nuclear fuels and radioactive wastes. J. Radioanal. Nucl. Chem. 2004, 261, 485–496. [Google Scholar] [CrossRef]
- Kulkarni, M.J.; Thulasidas, S.K.; Goyal, N.; Page, A.G.; Sastry, M.D. Inter-element interference studies in the determination of trace amounts of plutonium by inductively coupled plasma-atomic emission spectrometry. Anal. Lett. 1990, 23, 2095–2106. [Google Scholar] [CrossRef]
- Men, W.; Zheng, J.; Wang, H.; Ni, Y.; Aono, T.; Maxwell, S.L.; Tagami, K.; Uchida, S.; Yamada, M. Establishing rapid analysis of Pu isotopes in seawater to study the impact of Fukushima nuclear accident in the Northwest Pacific. Sci. Rep. 2018, 8, 1892. [Google Scholar] [CrossRef]
- Bürck, J. Spectrophotometric determination of uranium and nitric acid by applying partial least-squares regression to uranium(VI) absorption spectra. Anal. Chim. Acta 1991, 254, 159–165. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Ye, G.-A. Simultaneous spectrophotometric determination of uranium, nitric acid and nitrous acid by least-squares method in PUREX process. J. Radioanal. Nucl. Chem. 2013, 295, 325–330. [Google Scholar] [CrossRef]
- Suresh Kumar, K.; Magesvaran, P.; Sreejeya, D.; Kumar, T.; Shreekumar, B.; Dey, P.K. Simultaneous determination of U and Pu in Pu-stream of PUREX process by visible spectrophotometry. J. Radioanal. Nucl. Chem. 2010, 284, 457–460. [Google Scholar] [CrossRef]
- Casella, A.; Lines, A.; Nelson, G.; Bello, J.; Bryan, S. MicroRaman Measurements for Nuclear Fuel Reprocessing Applications. Procedia Chem. 2016, 21, 466–472. [Google Scholar] [CrossRef]
- Bryan, S.A.; Levitskaia, T.G.; Johnsen, A.M.; Orton, C.R.; Peterson, J.M. Spectroscopic monitoring of spent nuclear fuel reprocessing streams: An evaluation of spent fuel solutions via Raman, visible, and near-infrared spectroscopy. Radiochim. Acta 2011, 99, 563–571. [Google Scholar] [CrossRef]
- Bai, X.; Li, D.-M.; Chang, Z.-Y.; Fan, D.-J.; Liu, J.-P.; Wang, H. On-line monitoring of the U(VI) concentration in 30 vol.% TBP/kerosene: An evaluation of real-time analysis in polyetheretherketone (PEEK) containers via Raman spectroscopy. J. Radioanal. Nucl. Chem. 2015, 305, 643–652. [Google Scholar] [CrossRef]
- Kirsanov, D.; Babain, V.; Agafonova-Moroz, M.; Lumpov, A.; Legin, A. Approach to on-line monitoring of PUREX process using chemometric processing of the optical spectral data. Radiochim. Acta 2013, 101, 149–154. [Google Scholar] [CrossRef]
- Casella, A.J.; Levitskaia, T.G.; Peterson, J.M.; Bryan, S.A. Water O–H Stretching Raman Signature for Strong Acid Monitoring via Multivariate Analysis. Anal. Chem. 2013, 85, 4120–4128. [Google Scholar] [CrossRef]
- Nee, K.; Bryan, S.A.; Levitskaia, T.G.; Kuo, J.W.J.; Nilsson, M. Combinations of NIR, Raman spectroscopy and physicochemical measurements for improved monitoring of solvent extraction processes using hierarchical multivariate analysis models. Anal. Chim. Acta 2018, 1006, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Nash, K.L.; Braley, J.C. Advanced Separation Techniques for Nuclear Fuel Reprocessing and Radioactive Waste Treatment; Woodhead Publishing: Sawston, UK, 2011. [Google Scholar]
- Harvey, B.G.; Heal, H.G.; Maddock, A.G.; Rowley, E.L. The chemistry of plutonium. J. Chem. Soc. (Resumed) 1947, 188, 1010–1021. [Google Scholar] [CrossRef] [PubMed]
- Plock, C.E. Anodic voltammetric determination of plutonium: Diffusion coefficients of Putonium(III) in mineral acids. Anal. Chim. Acta 1970, 491, 83–87. [Google Scholar] [CrossRef]
- Murray, R.W.; Ewing, A.G.; Durst, R.A. Chemically modified electrodes. Molecular design for electroanalysis. Anal. Chem. 1987, 59, 379A–390A. [Google Scholar]
- Gupta, R.; Jayachandran, K.; Aggarwal, S.K. Single-walled carbon nanotube (SWCNT) modified gold (Au) electrode for simultaneous determination of plutonium and uranium. RSC Adv. 2013, 3, 13491–13496. [Google Scholar] [CrossRef]
- Gupta, R.; Gamare, J.S.; Pandey, A.K.; Tyagi, D.; Kamat, J.V. Highly Sensitive Detection of Arsenite Based on Its Affinity toward Ruthenium Nanoparticles Decorated on Glassy Carbon Electrode. Anal. Chem. 2016, 88, 2459–2465. [Google Scholar] [CrossRef]
- Kitatsuji, Y.; Aoyagi, H.; Yoshida, Z.; Kihara, S. Plutonium(III)-ion selective electrode of liquid membrane type using multidentate phosphine oxide ionophore. Anal. Chim. Acta 1999, 387, 181–187. [Google Scholar] [CrossRef]
- Kirsanov, D.; Khaydukova, M.; Tkachenko, L.; Legin, A.; Babain, V. Potentiometric Sensor Array for Analysis of Complex Rare Earth Mixtures. Electroanal 2012, 24, 121–130. [Google Scholar] [CrossRef]
- Kirsanov, D.; Cetó, X.; Khaydukova, M.; Blinova, Y.; del Valle, M.; Babain, V.; Legin, A. A combination of dynamic measurement protocol and advanced data treatment to resolve the mixtures of chemically similar analytes with potentiometric multisensor system. Talanta 2014, 119, 226–231. [Google Scholar] [CrossRef]
- Vinogradov, A.P. Analytical Chemistry of Uranium; The Russian Academy of Sciences: Moscow, Russia, 1962. [Google Scholar]
- Kirsanov, D.; Panchuk, V.; Agafonova-Moroz, M.; Khaydukova, M.; Lumpov, A.; Semenov, V.; Legin, A. A sample-effective calibration design for multiple components. Analyst 2014, 139, 4303–4309. [Google Scholar] [CrossRef] [PubMed]
- Bogomolov, A. Diagonal designs for a multi-component calibration experiment. Anal. Chim. Acta 2017, 951, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Oleneva, E.; Savosina, J.; Agafonova-Moroz, M.; Lumpov, A.; Babain, V.; Jahatspanian, I.; Legin, A.; Kirsanov, D. Potentiometric multisensor system for tetra- and hexavalent actinide quantification in complex rare earth metal mixtures related to spent nuclear fuel reprocessing. Sens. Actuators B Chem. 2019, 288, 155–162. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Turanov, A.N.; Karandashev, V.K.; Fedoseev, A.M.; Rodygina, N.I.; Baulin, V.E. Effect of the Structure of o-Methyleneoxyphenyldiphosphine Dioxides on Their Extractive Power and Selectivity in Nitric Acid Solutions. Radiochemistry 2005, 47, 177–180. [Google Scholar] [CrossRef]
- Rozen, A.M.; Nikolotova, Z.I.; Kartasheva, N.A. Complexation of plutonium with alkylene diphosphine dioxides. Radiokhimiya 1975, 17, 772–777. [Google Scholar]
- Turanov, A.N.; Karandashev, V.K.; Yarkevich, A.N.; Safronova, Z.V.; Kharitonov, A.V.; Radygina, N.I.; Fedoseev, A.M. Extraction of U(VI), Th(IV), Pu(IV), Am(III), and rare-earth elements from nitric acid solutions with diphenyl(dialkylcarbamoylmethyl)phosphine oxides substituted in the methylene bridge. Radiochemistry 2004, 46, 461–467. [Google Scholar] [CrossRef]
- Alyapyshev, M.; Babain, V.; Tkachenko, L.; Kenf, E.; Voronaev, I.; Dar’in, D.; Matveev, P.; Petrov, V.; Kalmykov, S.; Ustynyuk, Y. Extraction of actinides with heterocyclic dicarboxamides. J. Radioanal. Nucl. Chem. 2018, 316, 419–428. [Google Scholar] [CrossRef]
- Manchanda, V.K.; Pathak, P.N. Amides and diamides as promising extractants in the back end of the nuclear fuel cycle: An overview. Sep. Purif. Technol. 2004, 35, 85–103. [Google Scholar] [CrossRef]
- Mittal, S.K.; Kumar, S.K.A.; Sharma, H.K. PVC-based dicyclohexano-18-crown-6 sensor for La(III) ions. Talanta 2004, 62, 801–805. [Google Scholar] [CrossRef]
- Shamsipur, M.; Yousefi, M.; Ganjali, M.R. PVC-Based 1,3,5-Trithiane Sensor for Cerium(III) Ions. Anal. Chem. 2000, 72, 2391–2394. [Google Scholar] [CrossRef] [PubMed]
- Zamani, H.A.; Arvinfar, A.; Rahimi, F.; Imani, A.; Ganjali, M.R.; Meghdadi, S. Praseodymium analysis in aqueous solution by Pr3+–PVC membrane sensor based on N,N′-bis(4-hydroxysalicylidene)-1-3-phenylenediamine. Mater. Sci. Eng. C 2011, 31, 307–312. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Memari, Z.; Faridbod, F.; Dinarvand, R.; Norouzi, P. Sm3+ Potentiometric Membrane Sensor as a Probe for Determination of Some Pharmaceutics. Electroanalysis 2008, 20, 2663–2670. [Google Scholar] [CrossRef]
- Gupta, V.K.; Singh, A.K.; Kumawat, L.K. A novel gadolinium ion-selective membrane electrode based on 2-(4-phenyl-1, 3-thiazol-2-yliminomethyl) phenol. Electrochim. Acta 2013, 95, 132–138. [Google Scholar] [CrossRef]

| # Mixture | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Pu | 0 | 0 | 0 | 10 | 10 | 10 | 20 | 20 | 20 | 30 |
| U | 10 | 400 | 1000 | 200 | 600 | 1000 | 10 | 400 | 800 | 200 |
| # Mixture | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| Pu | 30 | 30 | 40 | 40 | 40 | 50 | 50 | 50 | 50 | 50 |
| U | 600 | 1000 | 10 | 400 | 800 | 10 | 200 | 400 | 600 | 1000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savosina, J.; Agafonova-Moroz, M.; Yaroshenko, I.; Ashina, J.; Babain, V.; Lumpov, A.; Legin, A.; Kirsanov, D. Plutonium (IV) Quantification in Technologically Relevant Media Using Potentiometric Sensor Array. Sensors 2020, 20, 1604. https://doi.org/10.3390/s20061604
Savosina J, Agafonova-Moroz M, Yaroshenko I, Ashina J, Babain V, Lumpov A, Legin A, Kirsanov D. Plutonium (IV) Quantification in Technologically Relevant Media Using Potentiometric Sensor Array. Sensors. 2020; 20(6):1604. https://doi.org/10.3390/s20061604
Chicago/Turabian StyleSavosina, Julia, Marina Agafonova-Moroz, Irina Yaroshenko, Julia Ashina, Vasily Babain, Alexander Lumpov, Andrey Legin, and Dmitry Kirsanov. 2020. "Plutonium (IV) Quantification in Technologically Relevant Media Using Potentiometric Sensor Array" Sensors 20, no. 6: 1604. https://doi.org/10.3390/s20061604
APA StyleSavosina, J., Agafonova-Moroz, M., Yaroshenko, I., Ashina, J., Babain, V., Lumpov, A., Legin, A., & Kirsanov, D. (2020). Plutonium (IV) Quantification in Technologically Relevant Media Using Potentiometric Sensor Array. Sensors, 20(6), 1604. https://doi.org/10.3390/s20061604





