Fabrication of Microfluidic Chips Based on an EHD-Assisted Direct Printing Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental System
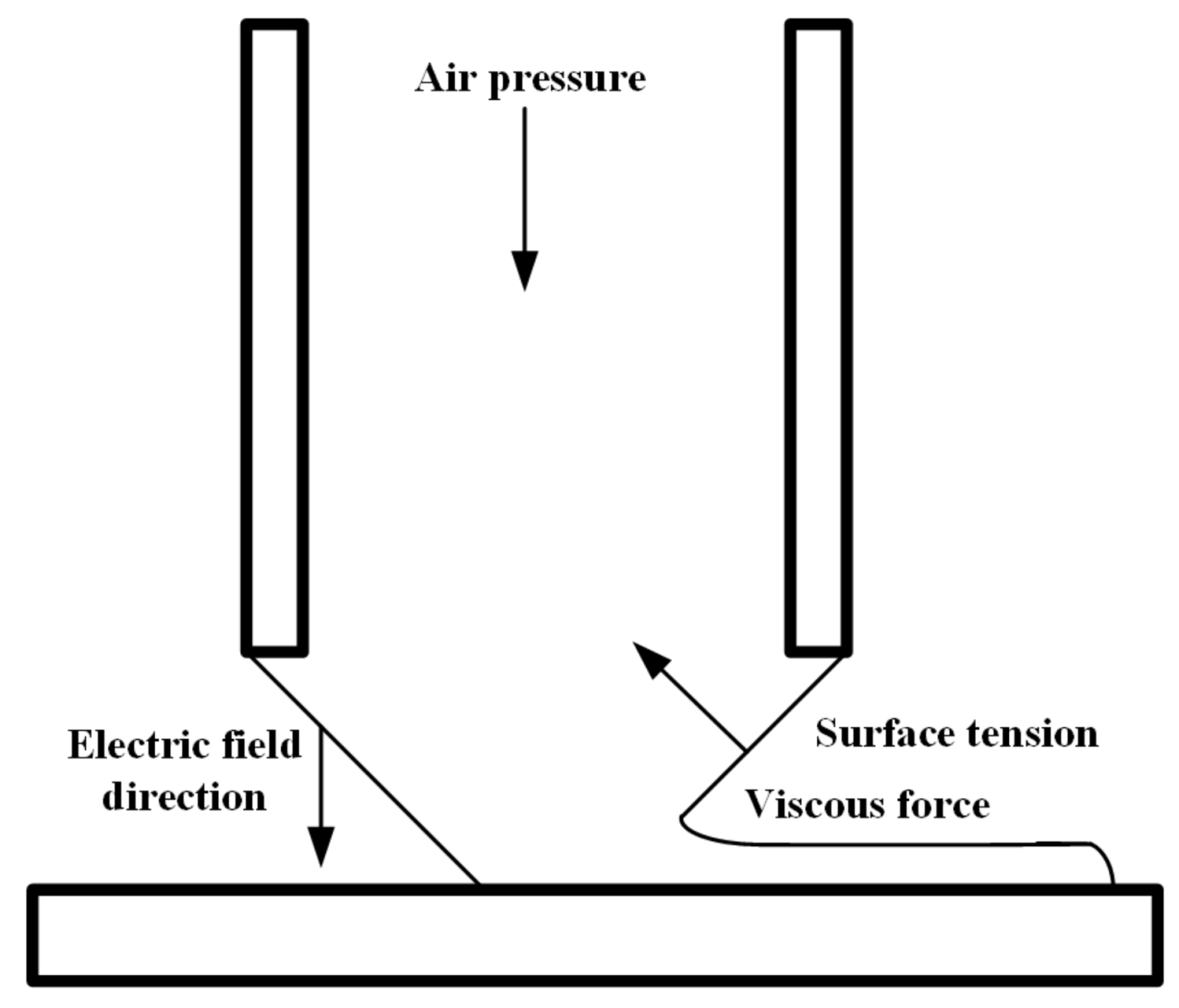
2.3. The EHD-Assisted Direct Printing Process
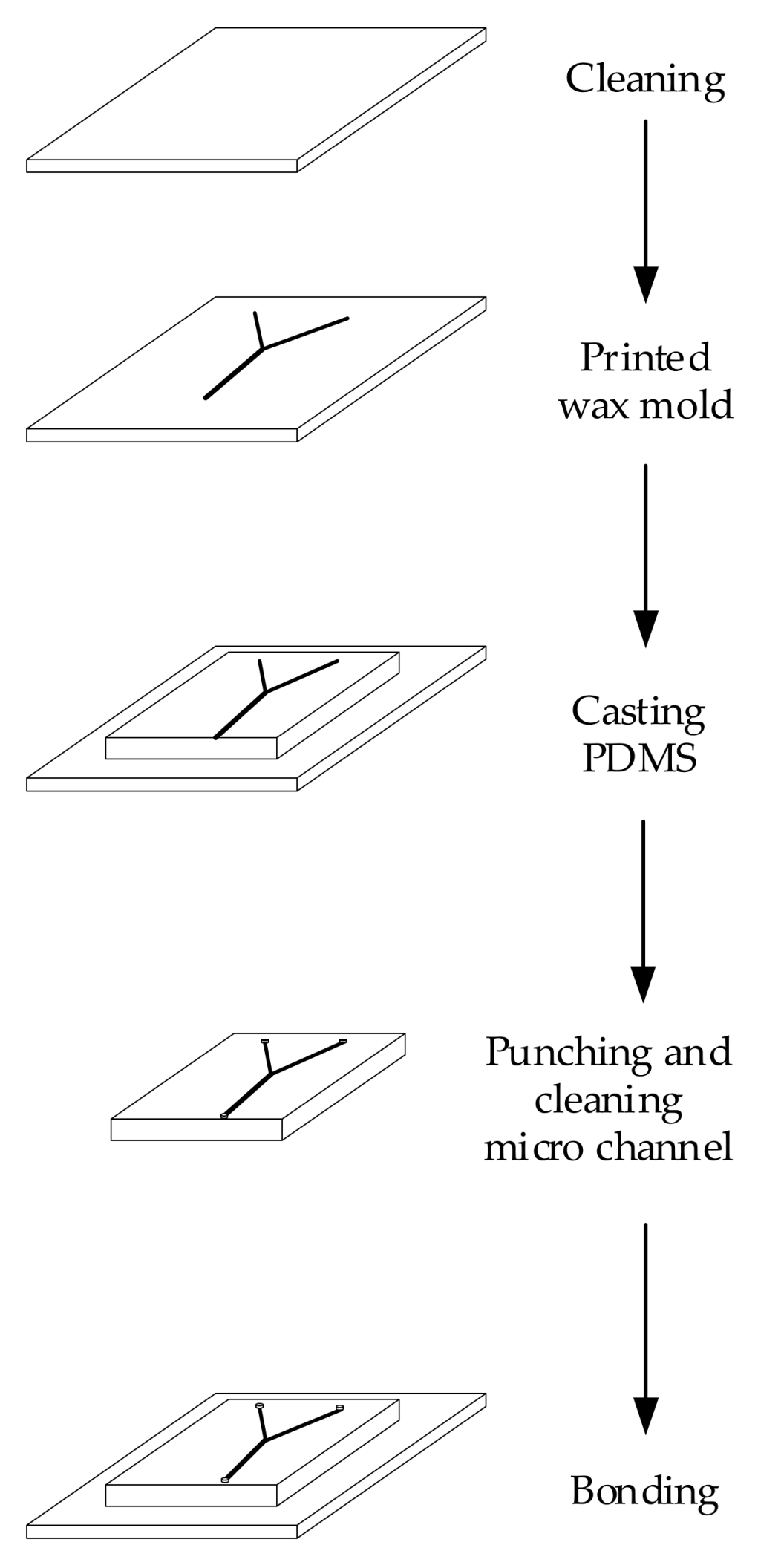
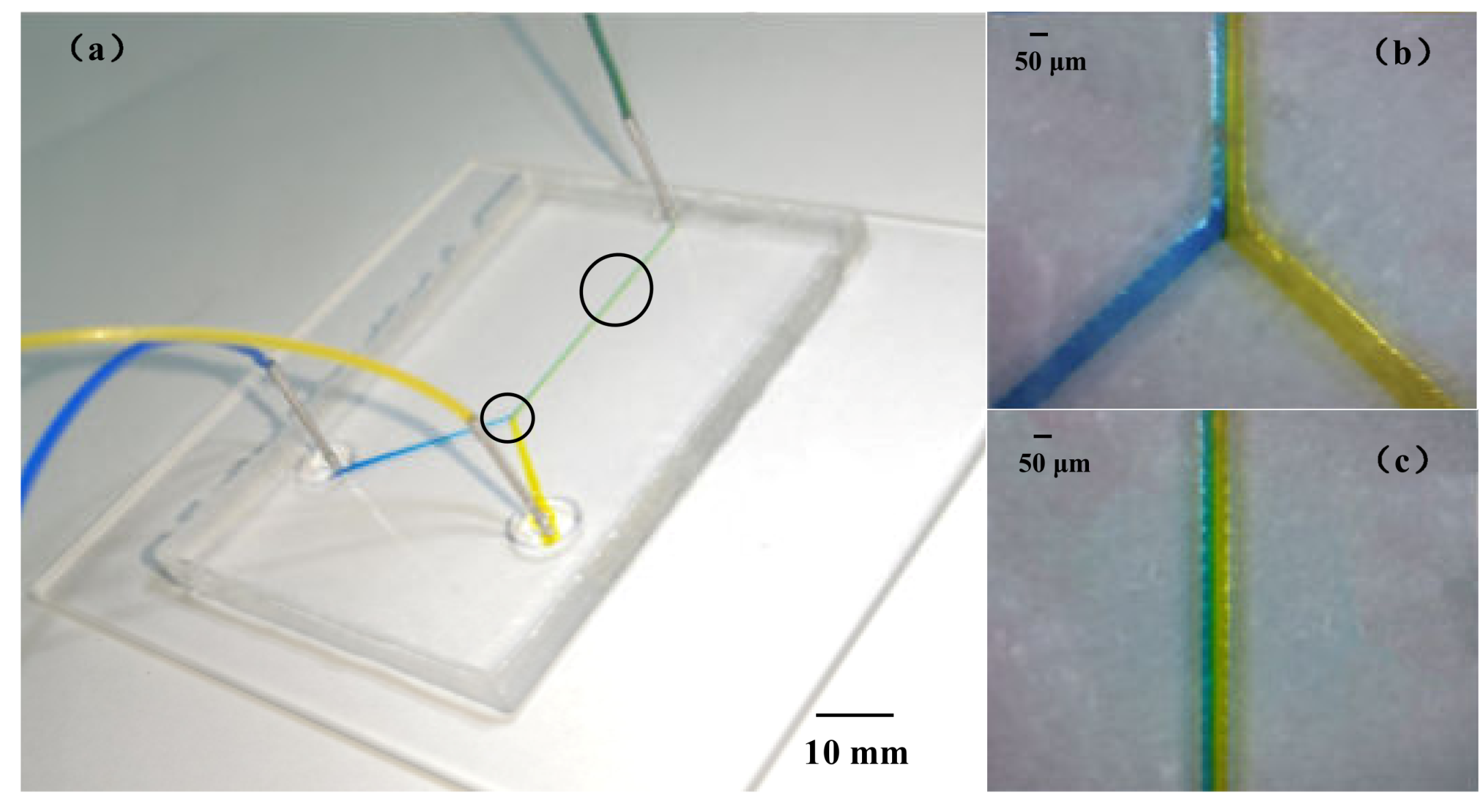
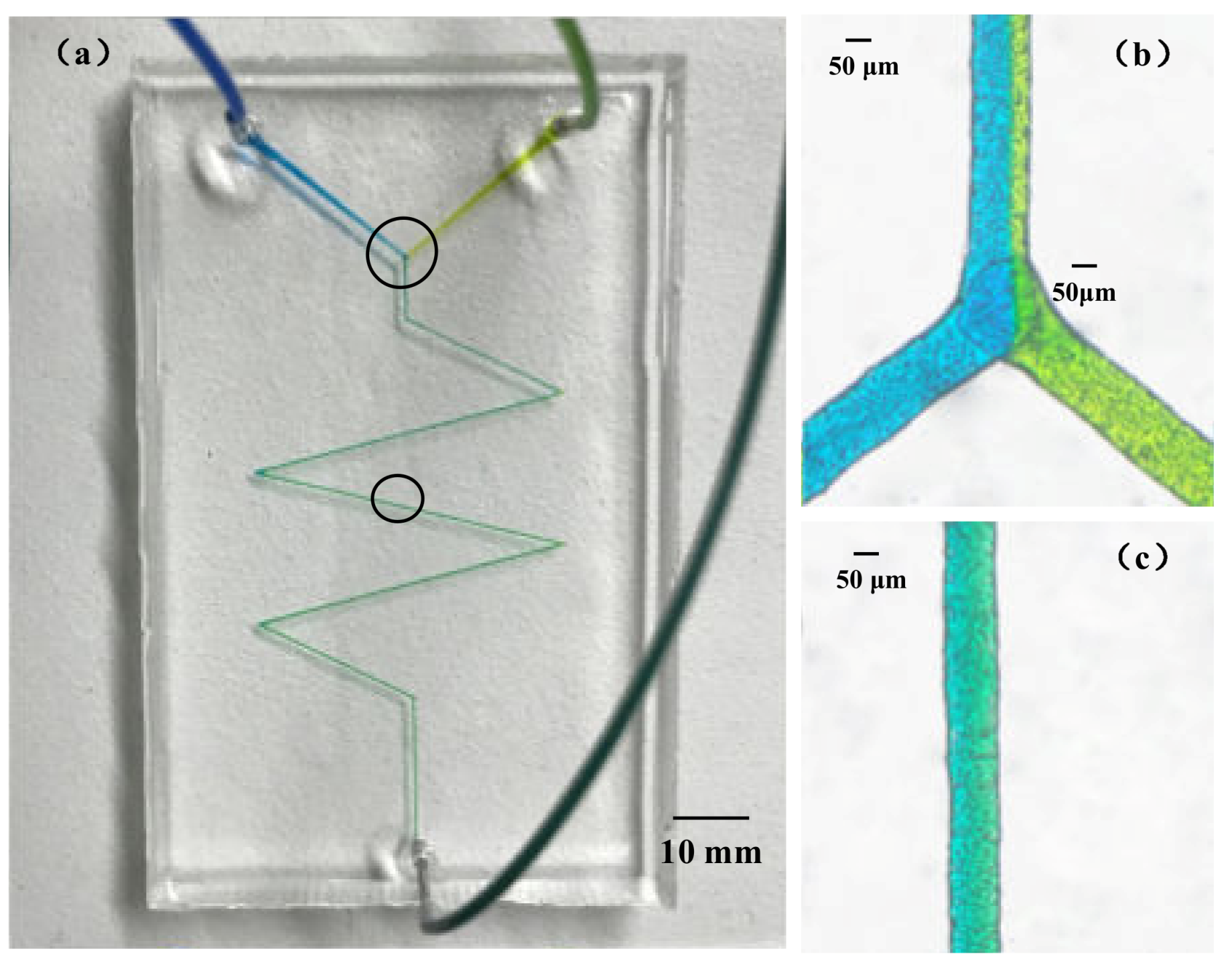
2.4. The Microfluidic Chip Fabricating Process
3. Results
3.1. Dynamic Changes of Droplets at the Nozzle
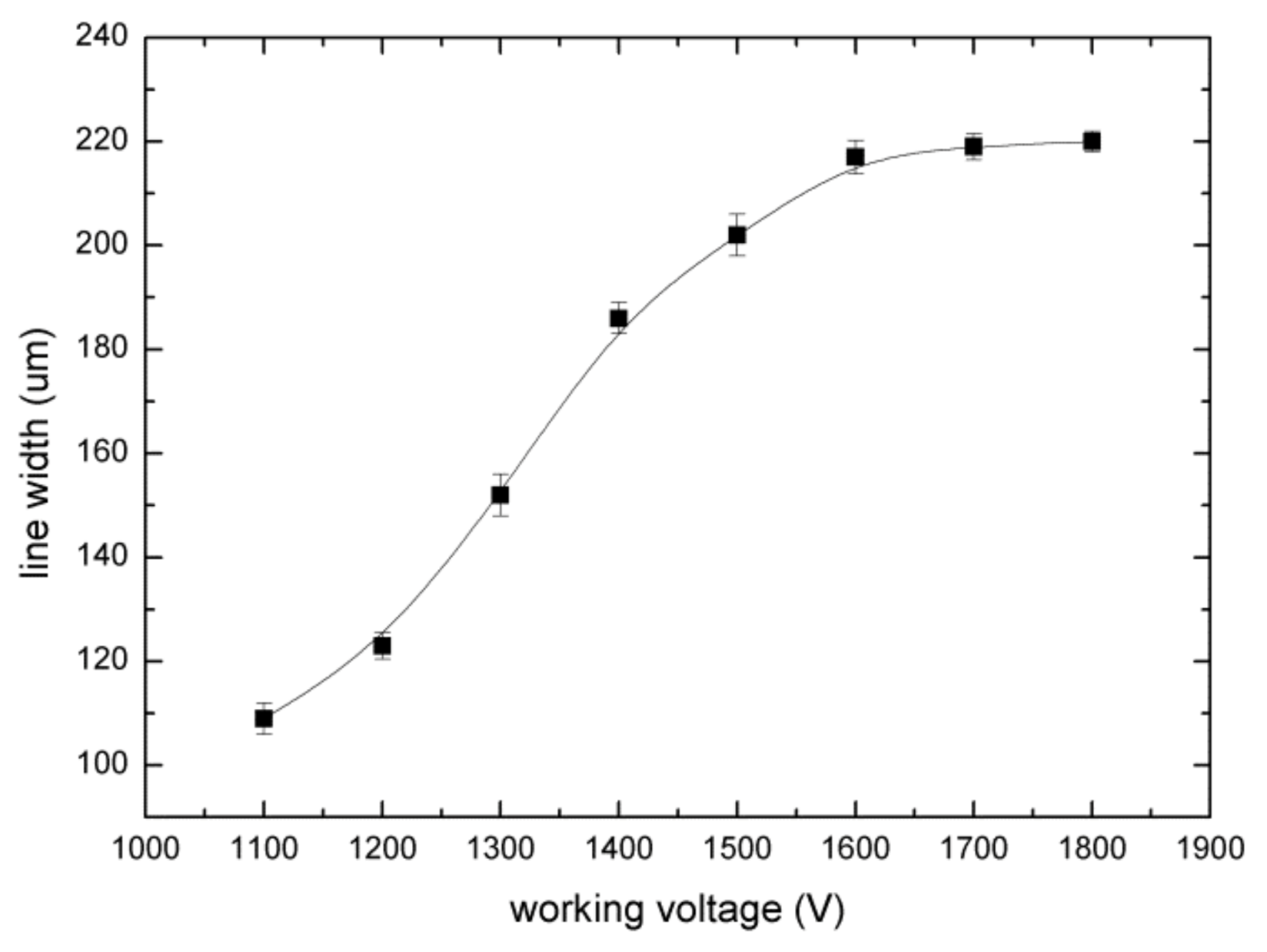
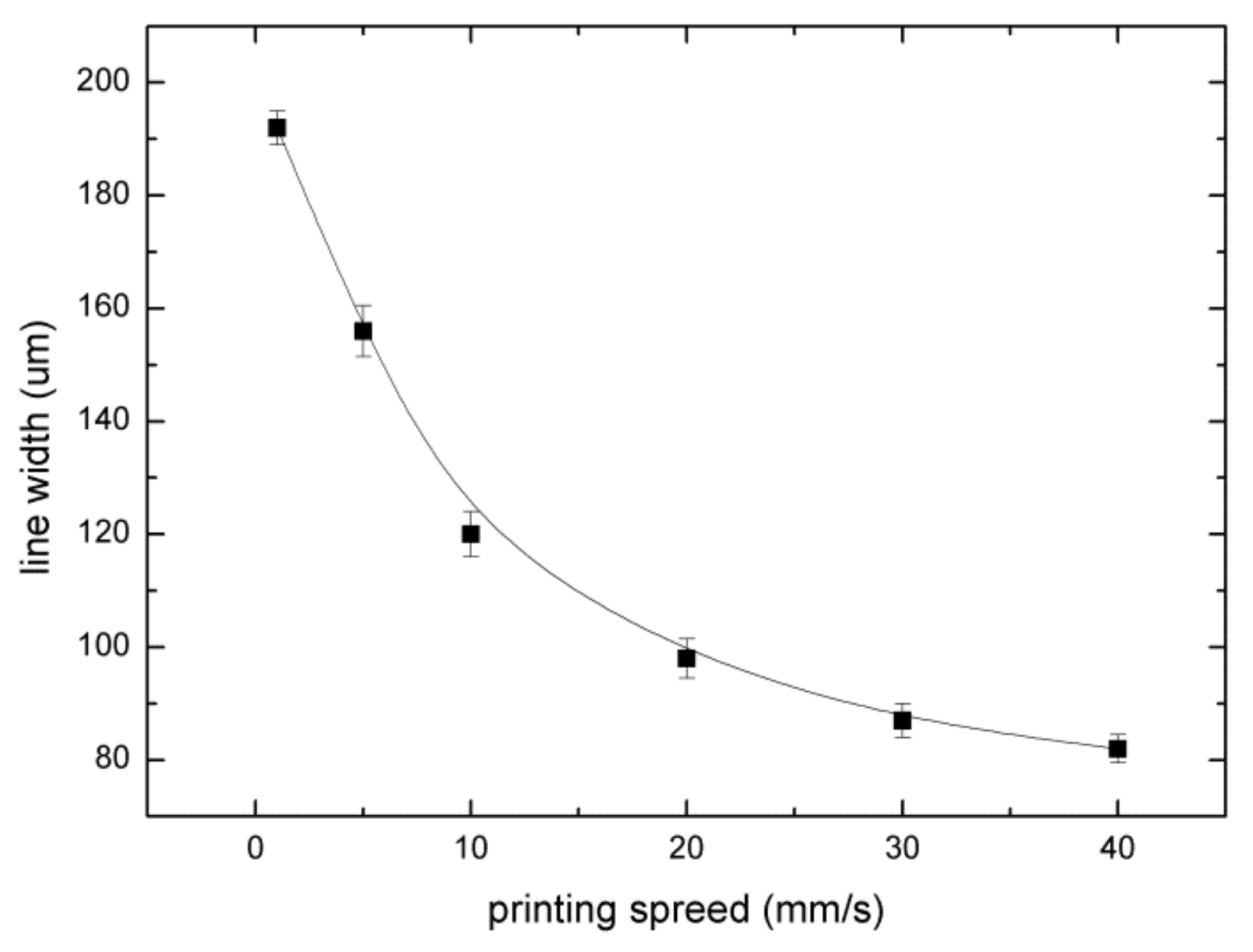
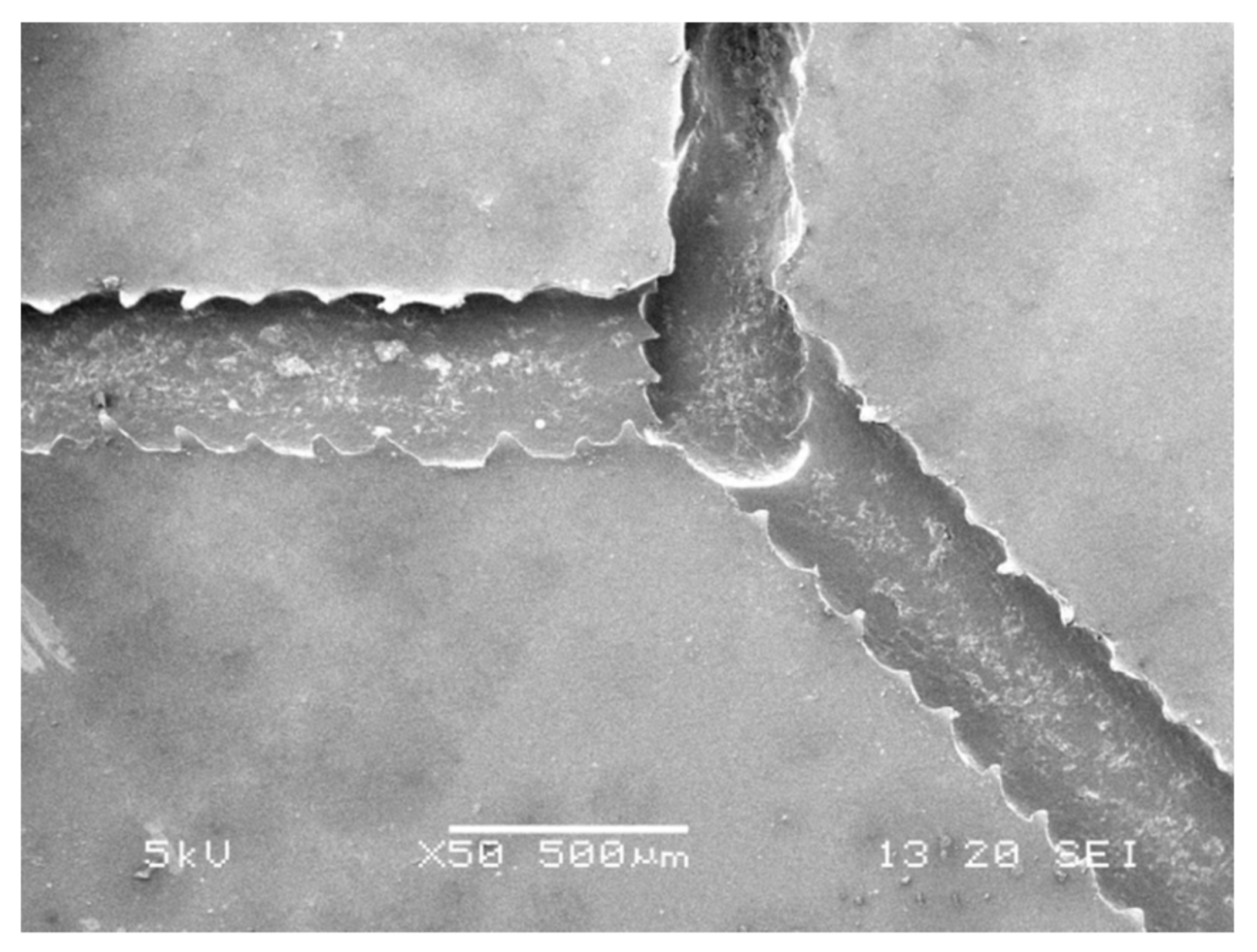
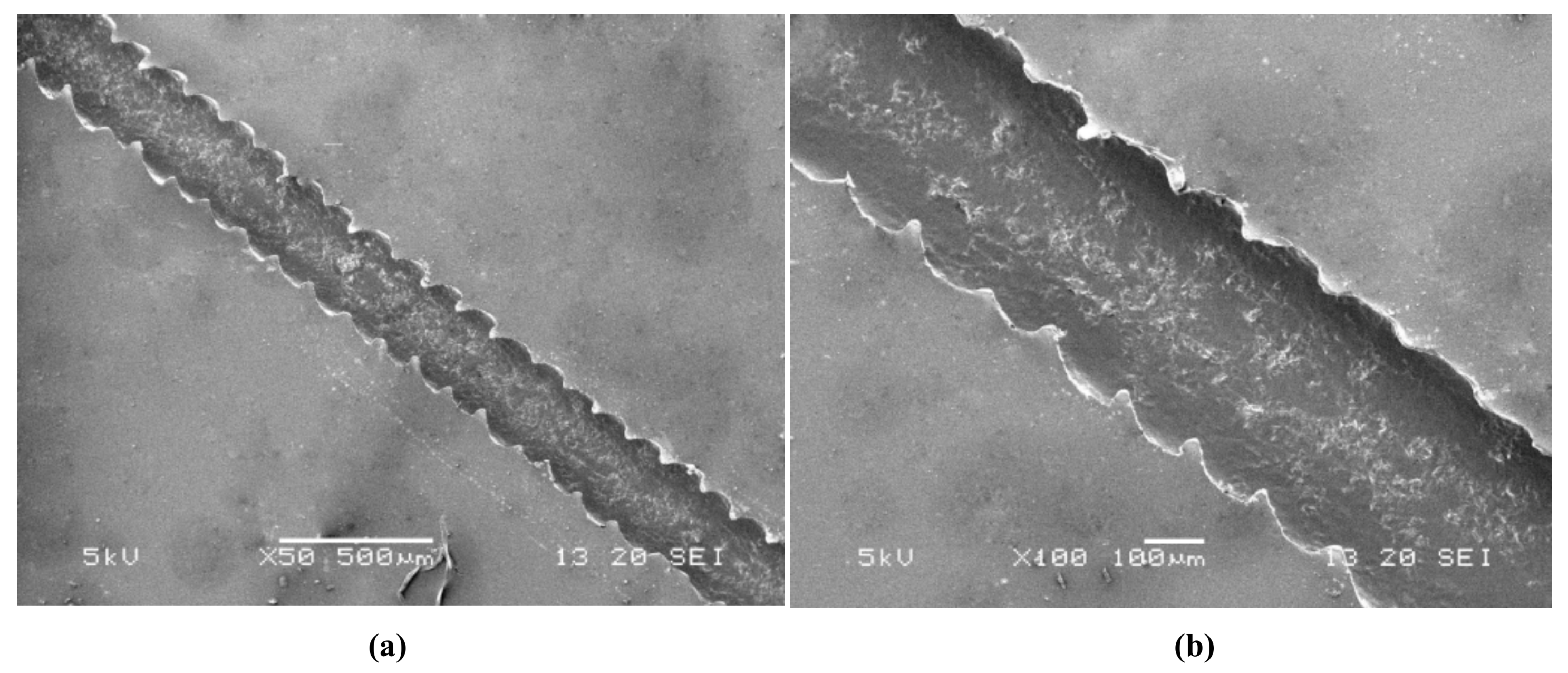
3.2. Analysis of Experimental Parameters
3.2.1. Working Voltage
3.2.2. Printing Speed
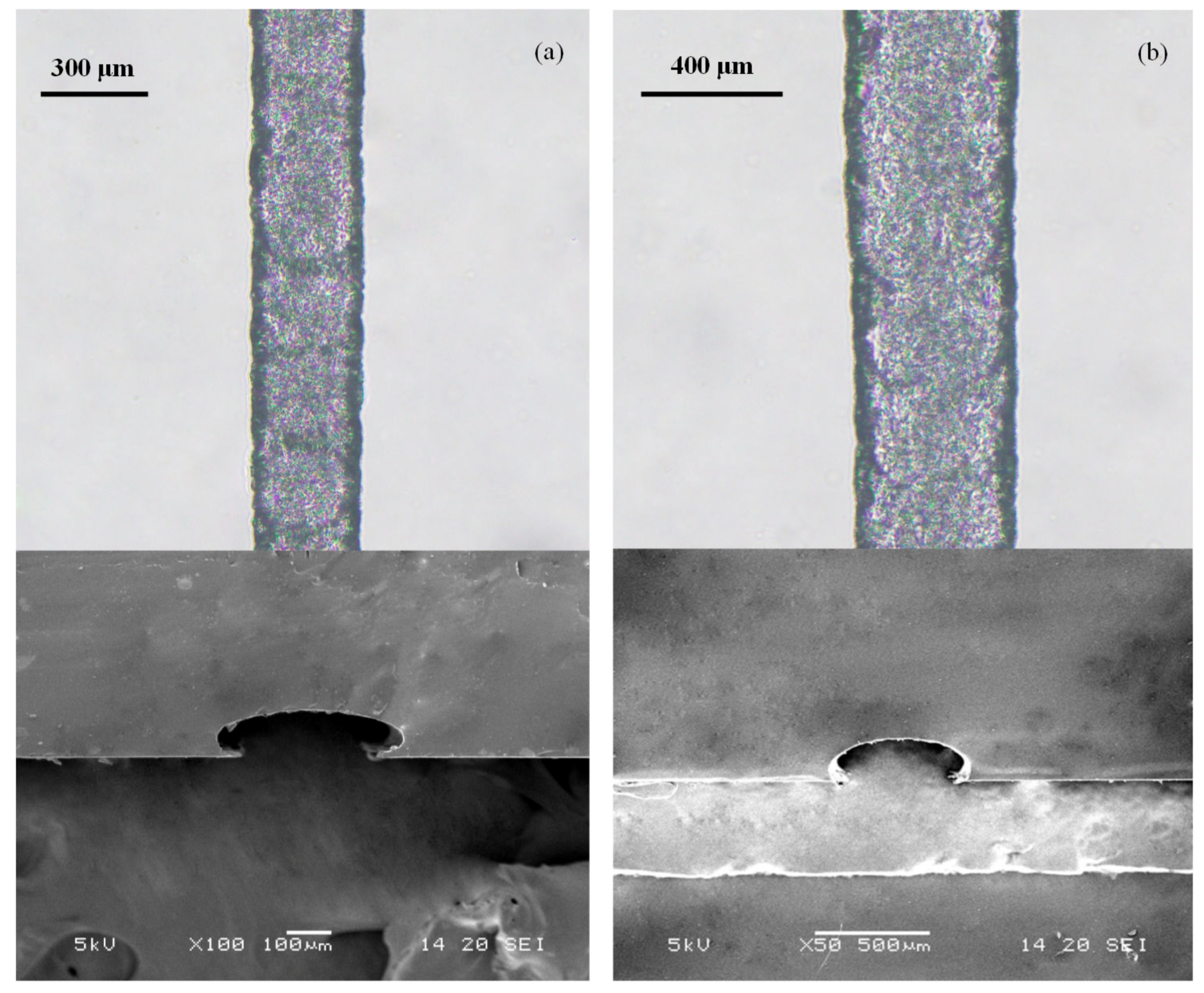
3.3. PDMS Microchannels Replicated Using the Paraffin Wax Mold
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hansen, F.A.; Sticker, D.; Kutter, J.P.; Petersen, N.J.; Pedersen-Bjergaard, S. Nanoliter-scale electromembrane extraction and enrichment in a microfluidic chip. Anal. Chem. 2018, 90, 9322–9329. [Google Scholar] [CrossRef] [PubMed]
- Elvira, K.S.; i Solvas, X.C.; Wootton, R.C.R.; Demello, A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat. Chem. 2013, 5, 905. [Google Scholar] [CrossRef] [PubMed]
- Alizadehgiashi, M.; Gevorkian, A.; Tebbe, M.; Seo, M.; Prince, E.; Kumacheva, E. 3D-Printed Microfluidic Devices for Materials Science. Adv. Mater. Technol. 2018, 3, 1800068. [Google Scholar] [CrossRef]
- Halldorsson, S.; Lucumi, E.; Gómez-Sjöberg, R.; Fleming, R.M. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens. Bioelectron. 2015, 63, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Becker, H.; Gärtner, C. Polymer microfabrication technologies for microfluidic systems. Anal. Bioanal. Chem. 2008, 390, 89–111. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998, 28, 153–184. [Google Scholar] [CrossRef]
- Waldbaur, A.; Rapp, H.; Länge, K.; Rapp, B.E. Let there be chip—Towards rapid prototyping of microfluidic devices: One-step manufacturing processes. Anal. Methods 2011, 3, 2681–2716. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.; Whitesides, G.M. Fabrication of microfluidic systems in poly (dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Faustino, V.; Catarino, S.O.; Lima, R.; Minas, G. Biomedical microfluidic devices by using low-cost fabrication techniques: A review. J. Biomech. 2016, 49, 2280–2292. [Google Scholar] [CrossRef]
- Abdelgawad, M.; Watson, M.W.L.; Young, E.W.K.; Mudrik, J.M.; Ungrin, M.D.; Wheeler, A.R. Soft lithography: Masters on demand. Lab Chip 2008, 8, 1379–1385. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, B.; Qin, J. Patterned paper as a low-cost, flexible substrate for rapid prototyping of PDMS microdevices via “liquid molding”. Anal. Chem. 2011, 83, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, Y.; Partlow, B.P.; Golding, A.S.; Tseng, P.; Coburn, J.; Applegate, M.B.; Moreau, J.E.; Omenetto, F.G.; Kaplan, D.L. Bio-functionalized silk hydrogel microfluidic systems. Biomaterials 2016, 93, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Koesdjojo, M.T.; Tennico, Y.H.; Rundel, J.T.; Remcho, V.T. Two-stage polymer embossing of co-planar microfluidic features for microfluidic devices. Sens. Actuators B Chem. 2008, 131, 692–697. [Google Scholar] [CrossRef]
- Grimes, A.; Breslauer, D.N.; Long, M.; Pegan, J.; Lee, L.P.; Khine, M. Shrinky-Dink microfluidics: Rapid generation of deep and rounded patterns. Lab Chip 2008, 8, 170–172. [Google Scholar] [CrossRef]
- Yue, W.; Li, C.W.; Xu, T.; Yang, M. Screen printing of solder resist as master substrates for fabrication of multi-level microfluidic channels and flask-shaped microstructures for cell-based applications. Biosens. Bioelectron. 2013, 41, 675–683. [Google Scholar] [CrossRef]
- Verma, M.K.S.; Majumder, A.; Ghatak, A. Embedded template-assisted fabrication of complex microchannels in PDMS and design of a microfluidic adhesive. Langmuir 2006, 22, 10291–10295. [Google Scholar] [CrossRef]
- Wu, J.; Wang, R.; Yu, H.; Li, G.; Xu, K.; Tien, N.C.; Roberts, R.C.; Li, D. Inkjet-printed microelectrodes on PDMS as biosensors for functionalized microfluidic systems. Lab Chip 2015, 15, 690–695. [Google Scholar] [CrossRef]
- Bonyár, A.; Sántha, H.; Varga, M.; Ring, B.; Vitéz, A.; Harsányi, G. Characterization of rapid PDMS casting technique utilizing molding forms fabricated by 3D rapid prototyping technology (RPT). Int. J. Mater. Form. 2014, 7, 189–196. [Google Scholar] [CrossRef]
- Tang, W.; Liu, H.; Zhu, L.; Shi, J.; Li, Z.; Xiang, N.; Yang, J. Fabrication of Different Microchannels by Adjusting the Extrusion Parameters for Sacrificial Molds. Micromachines 2019, 10, 544. [Google Scholar] [CrossRef]
- Parekh, D.P.; Ladd, C.; Panich, L.; Moussa, K.; Dickey, M.D. 3D printing of liquid metals as fugitive inks for fabrication of 3D microfluidic channels. Lab Chip 2016, 16, 1812–1820. [Google Scholar] [CrossRef]
- He, Y.; Qiu, J.; Fu, J.; Zhang, J.; Ren, Y.; Liu, A. Printing 3D microfluidic chips with a 3D sugar printer. Microfluid. Nanofluid. 2015, 19, 447–456. [Google Scholar] [CrossRef]
- Grimaldi, I.A.; Coppola, S.; Loffredo, F.; Villani, F.; Minarini, C.; Vespini, V.; Miccio, L.; Grilli, S.; Ferraro, P. Printing of polymer microlenses by a pyroelectrohydrodynamic dispensing approach. Opt. Lett. 2012, 37, 2460–2462. [Google Scholar] [CrossRef] [PubMed]
- Coppola, S.; Nasti, G.; Vespini, V.; Marchesano, V.; Ferraro, P. On the spraying modality of liquids by pyroelectrohydrodynamics. ACS Omega 2018, 3, 17707–17716. [Google Scholar] [CrossRef]
- Coppola, S.; Mecozzi, L.; Vespini, V.; Battista, L.; Grilli, S.; Nenna, G.; Loffredo, F.; Villani, F.; Minarini, C.; Ferraro, P. Nanocomposite polymer carbon-black coating for triggering pyro-electrohydrodynamic inkjet printing. Appl. Phys. Lett. 2015, 106, 261603. [Google Scholar] [CrossRef]
- Han, Y.; Wei, C.; Dong, J. Super-resolution electrohydrodynamic (EHD) 3D printing of micro-structures using phase-change inks. Manuf. Lett. 2014, 2, 96–99. [Google Scholar] [CrossRef]
- Lan, H. Active Mixing Nozzle for Multimaterial and Multiscale Three-Dimensional Printing. J. Micro Nano-Manuf. 2017, 5, 040904. [Google Scholar] [CrossRef]
- Coppola, S.; Nasti, G.; Todino, M.; Olivieri, F.; Vespini, V.; Ferraro, P. Direct writing of microfluidic footpaths by pyro-EHD printing. ACS Appl. Mater. Interfaces 2017, 9, 16488–16494. [Google Scholar] [CrossRef]
- Wong, S.H.; Bryant, P.; Ward, M.; Wharton, C. Investigation of mixing in a cross-shaped micromixer with static mixing elements for reaction kinetics studies. Sens. Actuators B Chem. 2003, 95, 414–424. [Google Scholar] [CrossRef]
- Lee, C.Y.; Wang, W.T.; Liu, C.C.; Fu, L.M. Passive mixers in microfluidic systems: A. review. Chem. Eng. J. 2016, 288, 146–160. [Google Scholar] [CrossRef]




| Molecular Weight | Density (g·mL−1) | Electrical Conductivity | Melting Point (°C) | Melt Viscosity (mm2·s−1) |
|---|---|---|---|---|
| 650 | 0.8–0.92 | insulation | 45–55 | 10–20 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, X.; Zhang, X.; Li, Z.; Yuan, Z.; Zhu, L.; Zhang, F.; Yang, J. Fabrication of Microfluidic Chips Based on an EHD-Assisted Direct Printing Method. Sensors 2020, 20, 1559. https://doi.org/10.3390/s20061559
Chi X, Zhang X, Li Z, Yuan Z, Zhu L, Zhang F, Yang J. Fabrication of Microfluidic Chips Based on an EHD-Assisted Direct Printing Method. Sensors. 2020; 20(6):1559. https://doi.org/10.3390/s20061559
Chicago/Turabian StyleChi, Xiang, Xinyu Zhang, Zongan Li, Zhe Yuan, Liya Zhu, Feng Zhang, and Jiquan Yang. 2020. "Fabrication of Microfluidic Chips Based on an EHD-Assisted Direct Printing Method" Sensors 20, no. 6: 1559. https://doi.org/10.3390/s20061559
APA StyleChi, X., Zhang, X., Li, Z., Yuan, Z., Zhu, L., Zhang, F., & Yang, J. (2020). Fabrication of Microfluidic Chips Based on an EHD-Assisted Direct Printing Method. Sensors, 20(6), 1559. https://doi.org/10.3390/s20061559







