An Au Nanofilm-Graphene/D-Type Fiber Surface Plasmon Resonance Sensor for Highly Sensitive Specificity Bioanalysis
Abstract
1. Introduction
2. Materials and Methods
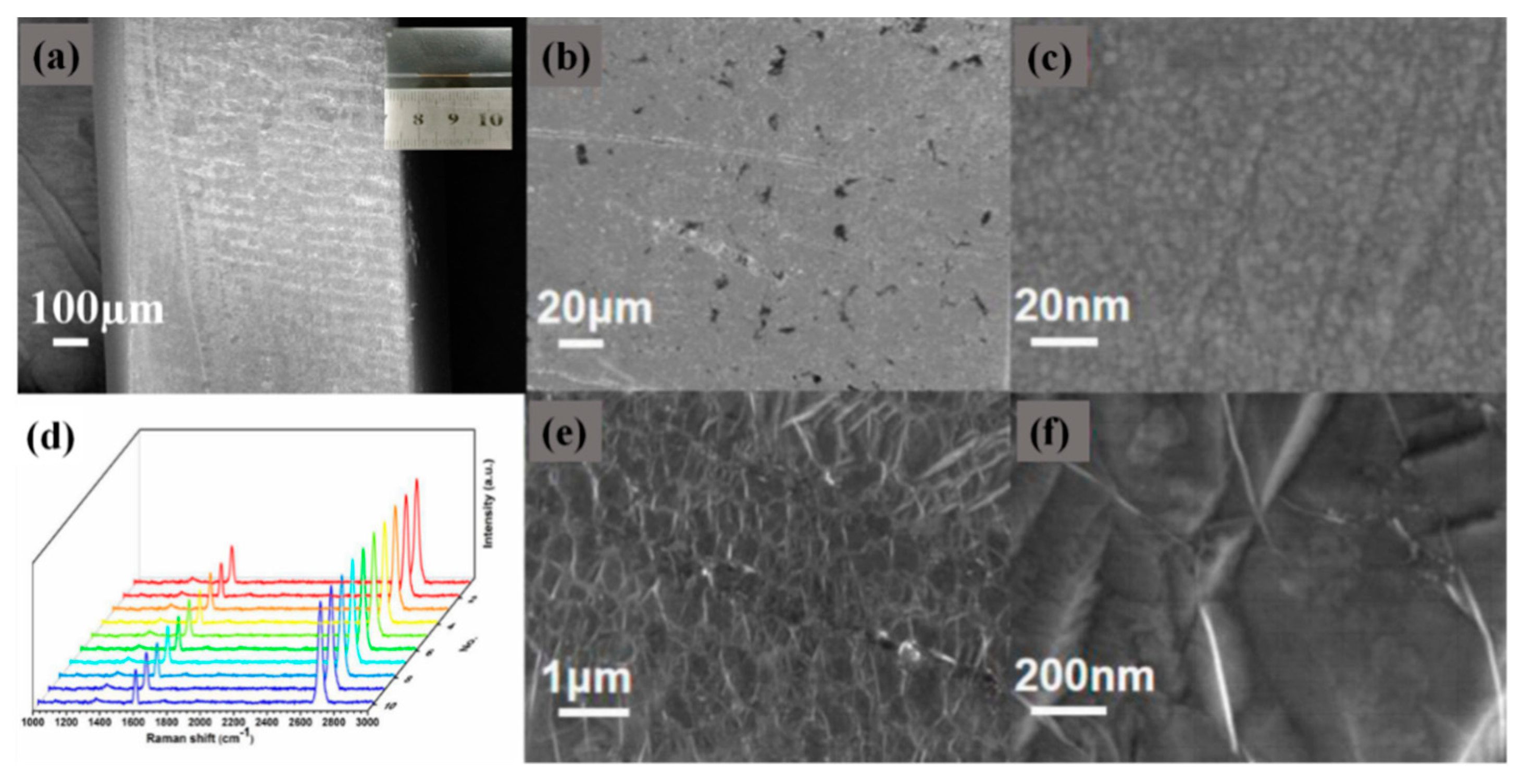
2.1. Production of D-Type Fiber
2.2. Preparation of Graphene-Gold Layer Structure and Synthesis of Novel D-Type Fiber Sensor
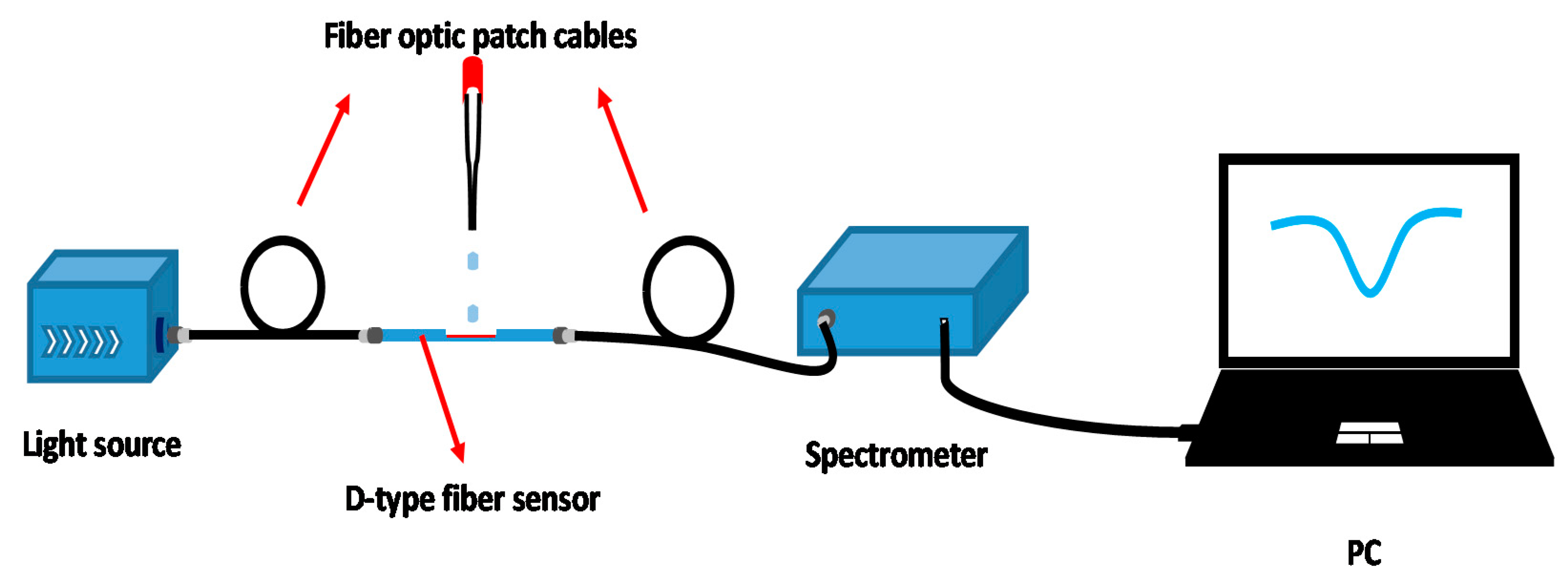
2.3. Optical Characterization and Measuring Instruments
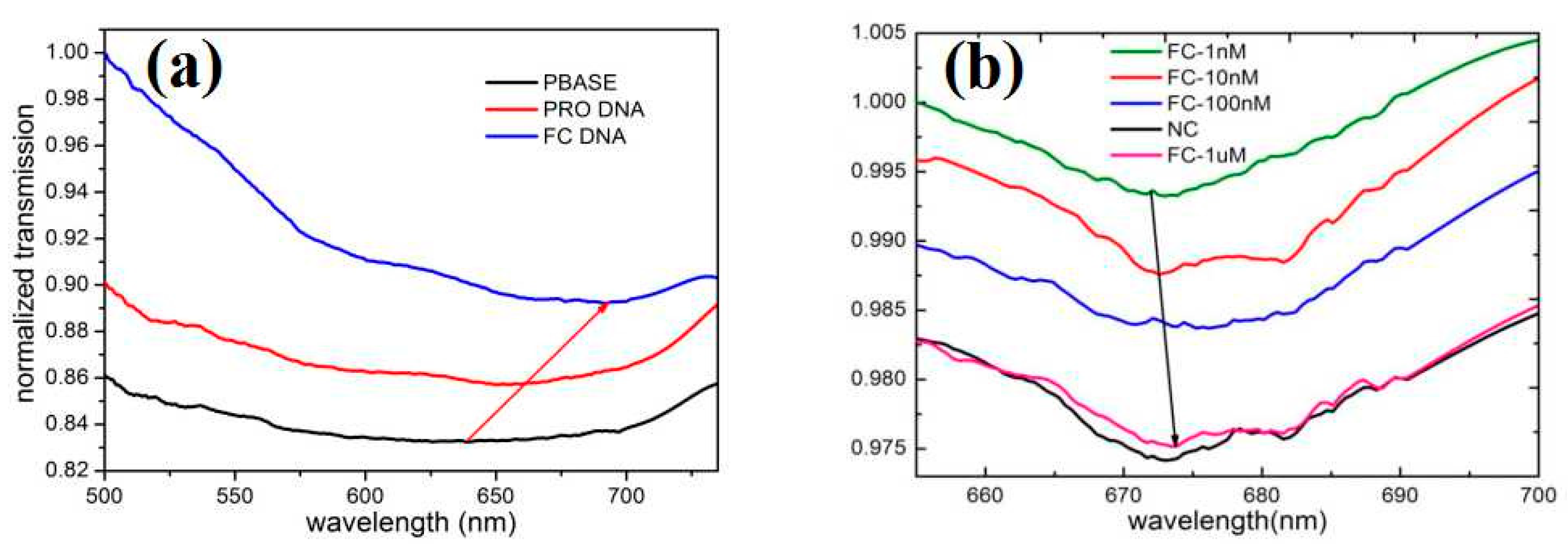
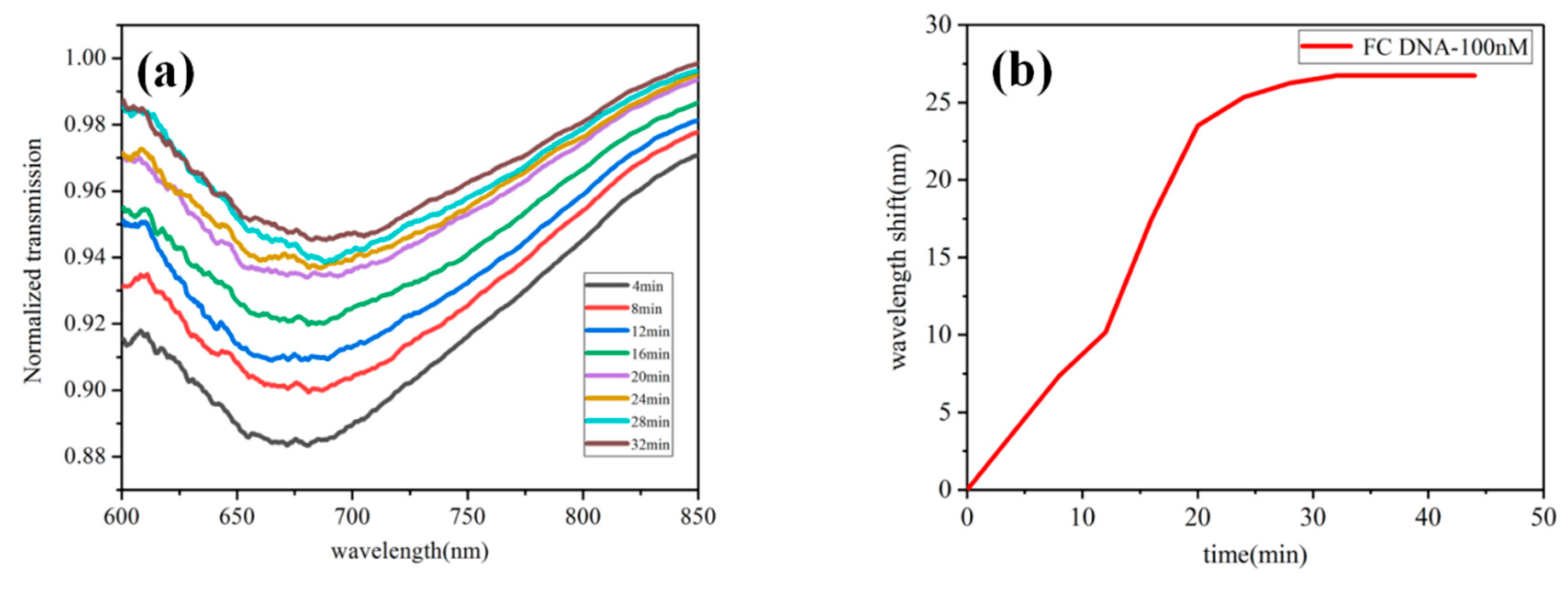
2.4. The Specific Detection of Biosensor
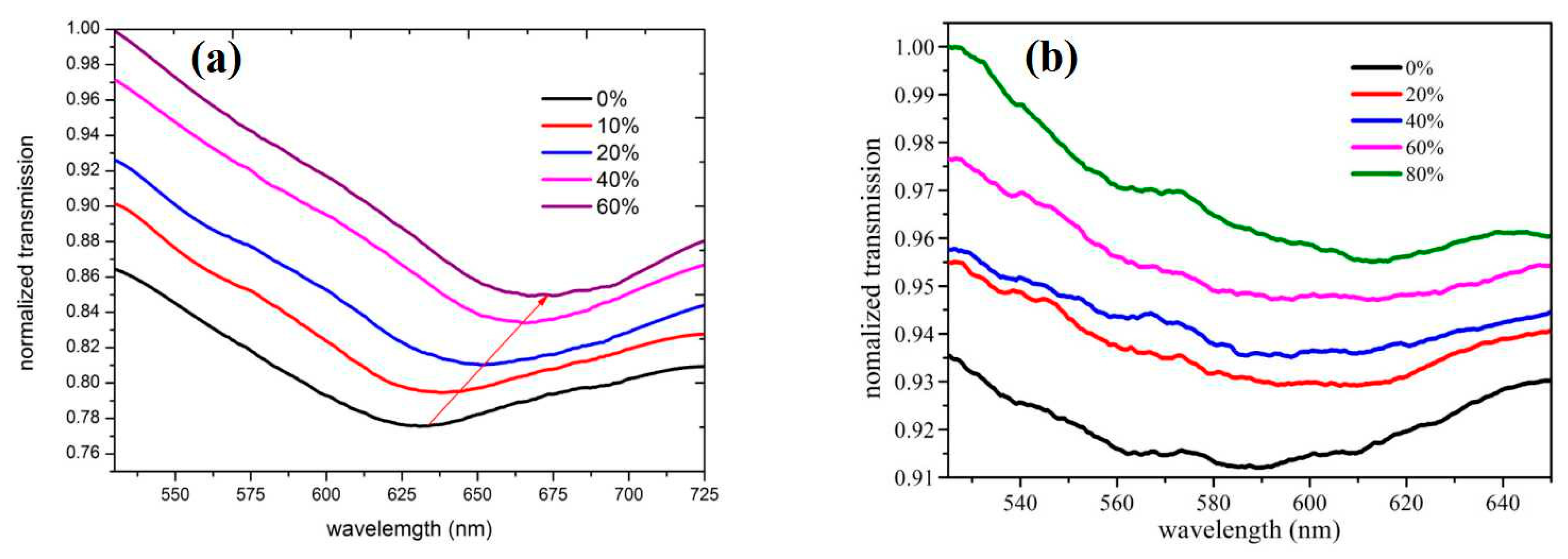
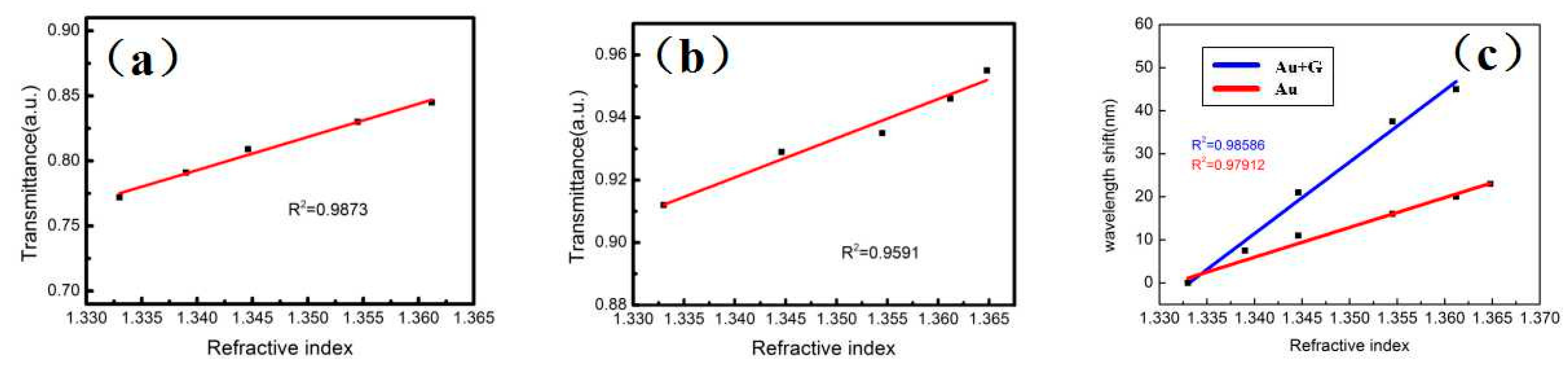
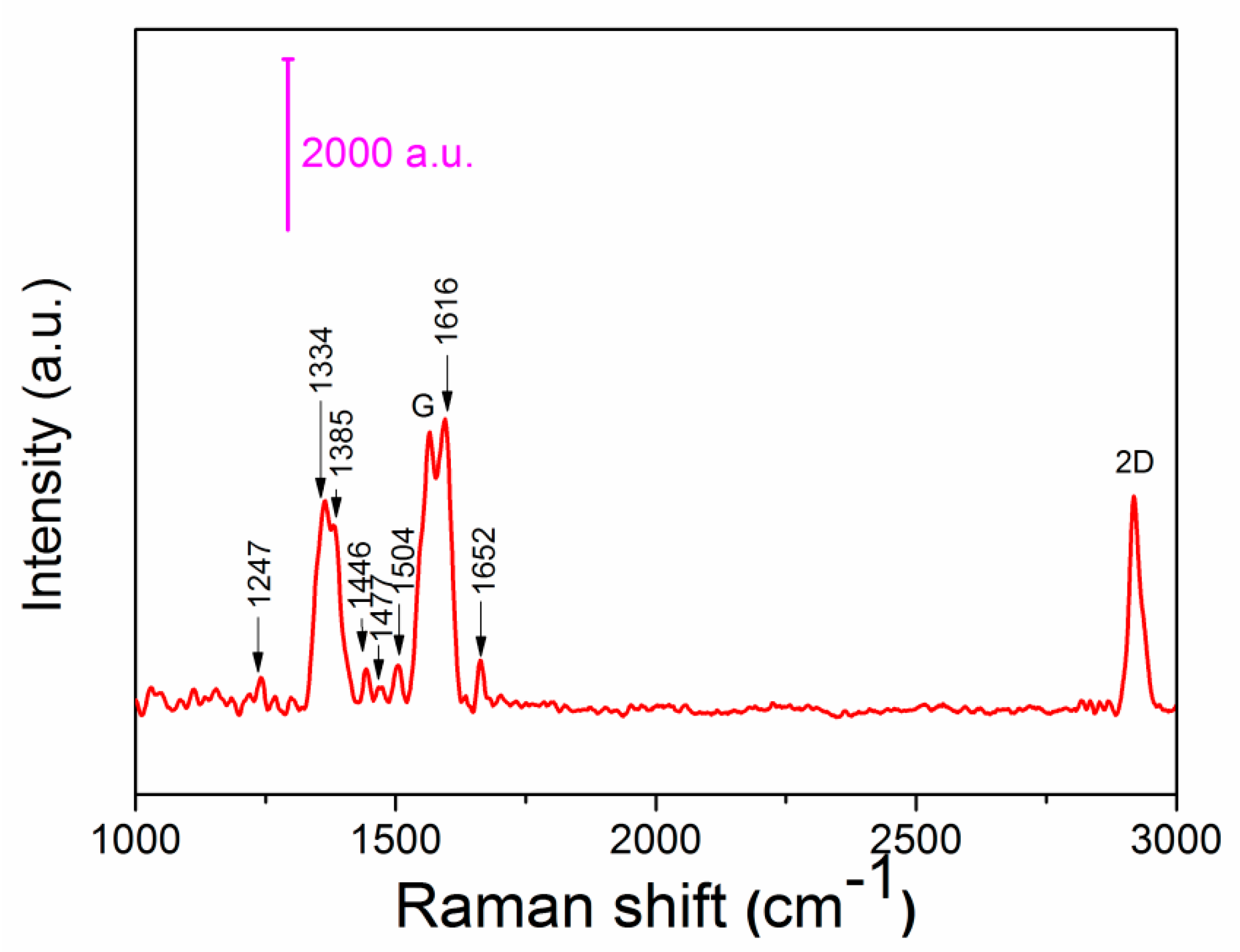
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ouyang, Q.L.; Zeng, S.W.; Jiang, L.; Hong, L.Y.; Xu, G.X.; Dinh, X.Q.Y.; Qian, J.; He, S.L.; Qu, J.L.; Coquet, P.; et al. Sensitivity enhancement of transition metal dichalcogenides/silicon nanostructure-based surface plasmon resonance biosensor. Sci. Rep. 2016, 6, 28190. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Kumari, D.; Gupta, B.D. Surface plasmon resonance based fiber optic ammonia gas sensor using ITO and polyaniline. Sens. Actuators B Chem. 2012, 171, 976–983. [Google Scholar] [CrossRef]
- Zeng, S.W.; Sreekanth, K.V.; Shang, J.Z.; Yu, T.; Chen, C.K.; Yin, F.; Baillargeat, D.; Coquet, P.; Ho, H.P.; Kabashin, A.V.; et al. Graphene–gold metasurface architectures for ultrasensitive plasmonic biosensing. Adv. Mater. 2015, 27, 6163–6169. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Sharma, N.K.; Sajal, V. SPR based fiber optic sensor with bi layers of indium tin oxide and platinum: A theoretical evaluation. Optik 2017, 135, 50–56. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Z.; Jiang, S.Z.; Li, C.H.; Xu, S.C.; Yu, J.; Li, Z.; Wang, M.H.; Liu, A.H.; Man, B.Y. U-bent fiber optic SPR sensor based on graphene/AgNPs. Sens. Actuators B Chem. 2017, 251, 127–133. [Google Scholar] [CrossRef]
- Nayak, J.K.; Jha, R. Numerical simulation on the performance analysis of a graphene-coated optical fiber plasmonic sensor at anti-crossing. Appl. Opt. 2017, 56, 3510–3517. [Google Scholar] [CrossRef]
- Diez, A.; Andres, M.V.; Cruz, J.L. In-line fiber-optic sensors based on the excitation of surface plasmon modes in metal-coated tapered fibers. Sens. Actuators B Chem. 2001, 73, 95–99. [Google Scholar] [CrossRef]
- Al-Qazwini, Y.; Noor, A.S.M.; Yaacob, M.H.; Harun, S.W.; Mahdi, M.A. Experimental realization and performance evaluation of refractive index SPR sensor based on unmasked short tapered multimode-fiber operating in aqueous environments. Sens. Actuators A Phys. 2015, 236, 38–43. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Pesavento, M.; Zeni, L. High selectivity and sensitivity sensor based on MIP and SPR in tapered plastic optical fibers for the detection of L-nicotine. Sens. Actuators B Chem. 2014, 191, 529–536. [Google Scholar] [CrossRef]
- Zhang, N.M.Y.; Li, K.W.; Shum, P.P.; Yu, X.C.; Zeng, S.W.; Wu, Z.F.; Wang, Q.J.; Yong, K.T.; Wei, L. Hybrid Graphene/Gold Plasmonic Fiber-Optic Biosensor. Adv. Mater. Technol. 2012, 2, 1600185. [Google Scholar] [CrossRef]
- Wu, L.M.; Guo, J.; Wang, Q.K.; Lu, S.B.; Dai, X.Y.; Xiang, Y.J.; Fan, D.Y. Sensitivity enhancement by using few-layer black phosphorus-graphene/TMDCs heterostructure in surface plasmon resonance biochemical sensor. Sens. Actuators B Chem. 2017, 249, 542–548. [Google Scholar] [CrossRef]
- Rezaei, N.; Yahaghi, A. A high sensitivity surface plasmon resonance D-shaped fiber sensor based on a waveguide-coupled bimetallic structure: Modeling and optimization. IEEE Sens. J. 2014, 14, 3611–3615. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Li, Z.; Zhang, C.; Gao, S.S.; Li, Z.; Qiu, H.W.; Li, C.H.; Yang, C.; Liu, M.; Liu, Y.J. A novel U-bent plastic optical fibre local surface plasmon resonance sensor based on a graphene and silver nanoparticle hybrid structure. J. Phys. D Appl. Phys. 2017, 50, 165105. [Google Scholar] [CrossRef]
- Paul, D.; Dutta, S.; Biswas, R. LSPR enhanced gasoline sensing with a U-bent optical fiber. J. Phys. D Appl. Phys. 2016, 49, 305104. [Google Scholar] [CrossRef]
- Xu, S.C.; Jiang, S.Z.; Zhang, C.; Yue, W.W.; Zou, Y.; Wang, G.Y.; Liu, H.L.; Zhang, X.M.; Li, M.Z.; Zhu, Z.S.; et al. Ultrasensitive label-free detection of DNA hybridization by sapphire-based graphene field-effect transistor biosensor. Appl. Surf. Sci. 2018, 427, 1114–1119. [Google Scholar] [CrossRef]
- Zeng, S.W.; Hu, S.Y.; Xia, J.; Anderson, T.; Dinh, X.Q.; Meng, X.M.; Coquet, P.; Yong, K.T. Graphene–MoS2 hybrid nanostructures enhanced surface plasmon resonance biosensors. Sens. Actuators B Chem. 2015, 207, 801–810. [Google Scholar] [CrossRef]
- Yu, L.L.; Lee, Y.H.; Ling, X.; Santos, E.J.G.; Shin, Y.C.; Lin, Y.X.; Dubey, M.; Kaxiras, E.; Kong, J.; Wang, H.; et al. Graphene/MoS2 hybrid technology for large scale two-dimensional electronics. Nano Lett. 2014, 14, 3055–3063. [Google Scholar] [CrossRef]
- Rahman, M.S.; Anower, M.S.; Hasan, M.R.; Hossain, M.B.; Haque, M.I. Design and numerical analysis of highly sensitive Au-MoS2-graphene based hybrid surface plasmon resonance biosensor. Opt. Commun. 2017, 396, 36–43. [Google Scholar] [CrossRef]
- Yue, W.W.; Tang, C.Y.; Wang, C.X.; Bai, C.J.; Liu, S.Y.; Xie, X.H.; Hua, H.L.; Zhang, Z.; Li, D.W. An electricity-fluorescence double-checking biosensor based on graphene for detection of binding kinetics of DNA hybridization. RSC Adv. 2017, 7, 44559–44567. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, S.Z.; Huo, Y.Y.; Liu, A.H.; Xu, S.C.; Liu, X.Y.; Sun, Z.C.; Xu, Y.Y.; Li, Z.; Man, B.Y. SERS detection of R6G based on a novel graphene oxide/silver nanoparticles/silicon pyramid arrays structure. Opt. Express 2015, 23, 24811–24821. [Google Scholar] [CrossRef]
- Lee, S.; Lee, M.H.; Shin, H.J.; Choi, D. Control of density and LSPR of Au nanopar-ticles on graphene. Nanotechnology. 2013, 24, 275702. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, C.; Han, Y.S.; Gao, S.S.; Sheng, Y.Q.; Zhang, S.Z.; Lu, Z.Y.; Man, B.Y.; Jiao, Y.; Jiang, S.Z. Evanescent wave absorption sensor with direct-growth MoS2 film based on U-bent tapered multimode fiber. J. Phys. D Appl. Phys. 2017, 50, 315302. [Google Scholar] [CrossRef]
- Qiu, H.W.; Gao, S.S.; Chen, P.X.; Li, Z.; Liu, X.Y.; Zhang, C.; Xu, Y.Y.; Jiang, S.Z.; Yang, C.; Huo, Y.Y.; et al. Evanescent wave absorption sensor based on tapered multimode fiber coated with monolayer graphene film. Opt. Commun. 2016, 366, 275–281. [Google Scholar] [CrossRef]
- Xu, H.L.; Wu, L.M.; Dai, X.Y.; Gao, Y.X.; Xiang, Y.J. An ultra-high sensitivity surface plasmon resonance sensor based on graphene-aluminum-graphene sandwich-like structure. J. Appl. Phys. 2016, 120, 053101. [Google Scholar] [CrossRef]
- Kim, J.A.; Hwang, T.; Dugasani, S.R.; Amin, R.; Kulkarni, A.; Park, S.H.; Kim, T. Graphene based fiber optic surface plasmon resonance for bio-chemical sensor applications. Sens. Actuators B Chem. 2013, 187, 426–433. [Google Scholar] [CrossRef]
- Galatus, R.; Szolga, L.; Voiculescu, E. Sensitivity enhancement of D-shape SPR-POF low coast sensor using graphene. Int. J. Adv. Res. Eng. 2013, 1, 1–6. [Google Scholar]
- Hossain, M.B.; Rana, M.M. DNA hybridization detection based on resonance frequency readout in graphene on Au SPR biosensor. J. Sens. 2016, 2016, 6070742. [Google Scholar] [CrossRef]
- Johansen, K.; Arwin, H.; Lundstrm, I.; Liedberg, B. Imaging surface plasmon resonance sensor based on multiple wavelengths: Sensitivity considerations. Rev. Sci. Instrum. 2000, 71, 3530–3538. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Hao, Y.F.; Adogla, E.A.; Yan, J.; Li, D.C.; Xu, K.X.; Wang, Q.; Hone, J.; Lin, Q. A graphene-based affinity nanosensor for detection of low-charge and low-molecular-weight molecules. Nanoscale 2016, 8, 5815–5819. [Google Scholar] [CrossRef]
- Guo, S.R.; Lin, J.; Penchev, M.; Yengel, E.; Ghazinejad, M.; Ozkan, C.S.; Ozkan, M. Label free DNA detection using large area graphene based field effect transistor biosensors. J. Nanosci. Nanotechnol. 2011, 11, 5258–5263. [Google Scholar] [CrossRef]
- Xu, S.; Zhan, J.; Man, B.; Jiang, S.; Yue, W.; Gao, S.; Guo, C.; Liu, H.; Li, Z.; Wang, J.; et al. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor. Nat. Commun. 2017, 8, 14902. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Peng, Z.S.; Li, H.M.; Wang, Z.Q.; Mu, Y.Q.; Zhang, G.P.; Chen, S.; Liu, S.Y.; Wang, G.T.; Liu, C.D.; et al. Suspended CNT-Based FET sensor for ultrasensitive and label-free detection of DNA hybridization. Biosens. Bioelectron. 2019, 137, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.T.; Wang, Z.J.; Ping, J.L.; Ban, D.K.; Shiah, Z.C.; Antonschmidt, L.; Lee, J.; Liu, Y.S.; Karkisaval, A.G.; Johnson, A.T.C.; et al. DNA Nanotweezers and Graphene Transistor Enable Label-Free Genotyping. Adv. Mater. 2018, 30, 1802440. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Agostino, G.D.; Donà, A.; Dacarro, G.; Pallavicini, P.; Pesavento, M.; Zeni, L.G. Localized Surface Plasmon Resonance with Five-Branched Gold Nanostars in a Plastic Optical Fiber for Bio-Chemical Sensor Implementation. Sensors 2013, 13, 14676–14686. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, Z.Y.; Owens, A.C.E.; Kulaots, I.; Chen, Y.T.; Kane, A.B.; Hurt, R.H. Antioxidant chemistry of graphene-based materials and its role in oxidation protection technology. Nanoscale 2014, 6, 11744–11755. [Google Scholar]
- Ruiz, V.; Yate, L.; Garcia, I.; Cabanero, G.; Grande, H.J. Tuning the antioxidant activity of graphene quantum dots: Protective nanomaterials against dye decoloration. Carbon 2017, 116, 366–374. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, X.; Xu, J.; Li, S.; Song, J.; Yang, W.; Sun, Y.; Jiang, S.; Han, Y.; Fan, X. An Au Nanofilm-Graphene/D-Type Fiber Surface Plasmon Resonance Sensor for Highly Sensitive Specificity Bioanalysis. Sensors 2020, 20, 991. https://doi.org/10.3390/s20040991
Xi X, Xu J, Li S, Song J, Yang W, Sun Y, Jiang S, Han Y, Fan X. An Au Nanofilm-Graphene/D-Type Fiber Surface Plasmon Resonance Sensor for Highly Sensitive Specificity Bioanalysis. Sensors. 2020; 20(4):991. https://doi.org/10.3390/s20040991
Chicago/Turabian StyleXi, Xiangtai, Jihua Xu, Shuanglu Li, Jingyi Song, Wen Yang, Yang Sun, Shouzhen Jiang, Yanshun Han, and Xiuwei Fan. 2020. "An Au Nanofilm-Graphene/D-Type Fiber Surface Plasmon Resonance Sensor for Highly Sensitive Specificity Bioanalysis" Sensors 20, no. 4: 991. https://doi.org/10.3390/s20040991
APA StyleXi, X., Xu, J., Li, S., Song, J., Yang, W., Sun, Y., Jiang, S., Han, Y., & Fan, X. (2020). An Au Nanofilm-Graphene/D-Type Fiber Surface Plasmon Resonance Sensor for Highly Sensitive Specificity Bioanalysis. Sensors, 20(4), 991. https://doi.org/10.3390/s20040991




