Highly Selective Adsorption on SiSe Monolayer and Effect of Strain Engineering: A DFT Study
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Adsorption on SiSe Monolayer
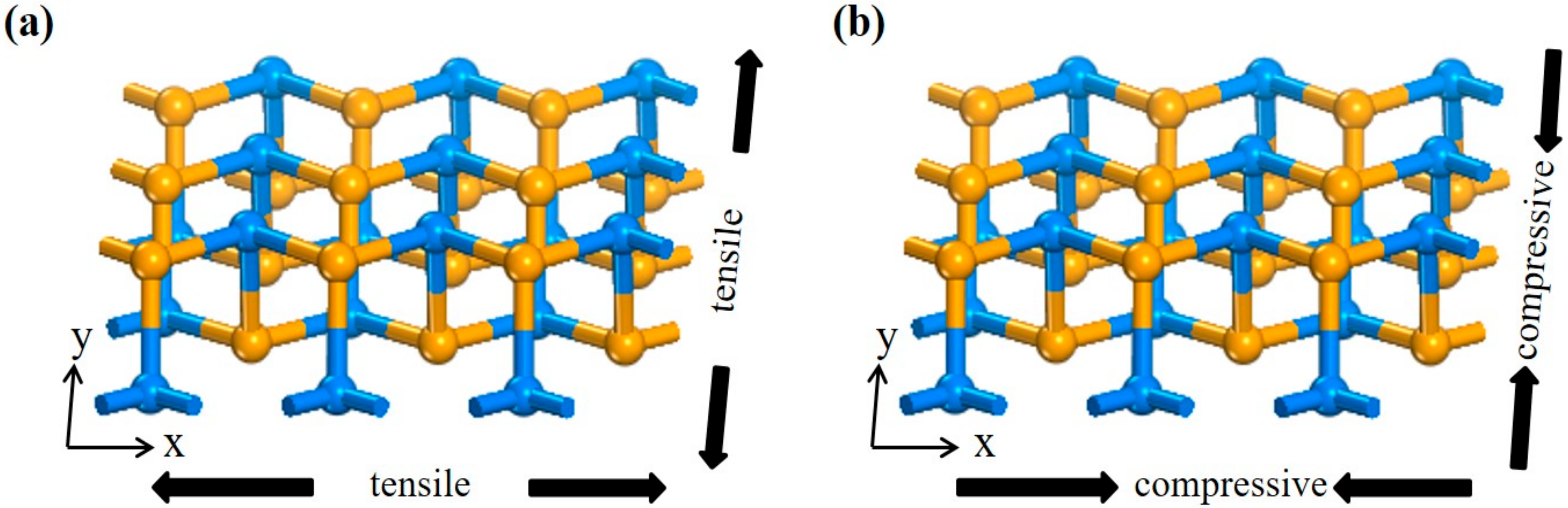
3.2. Adsorption Mechanism of NH3 Under Stress
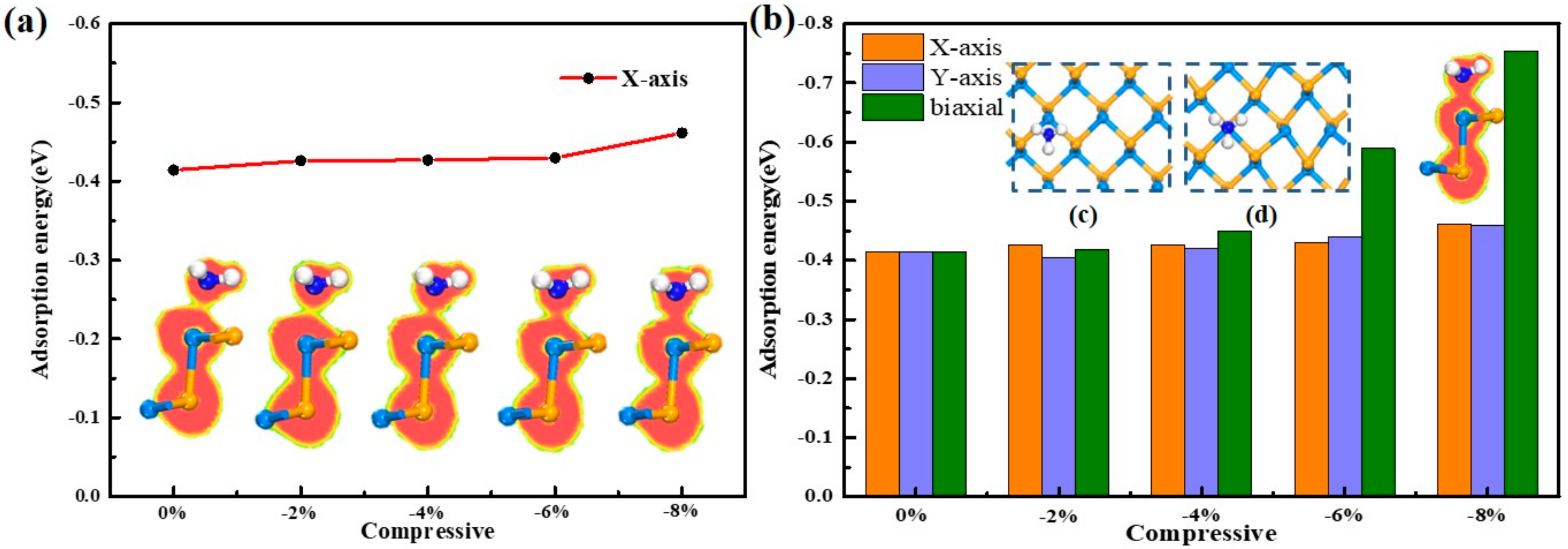
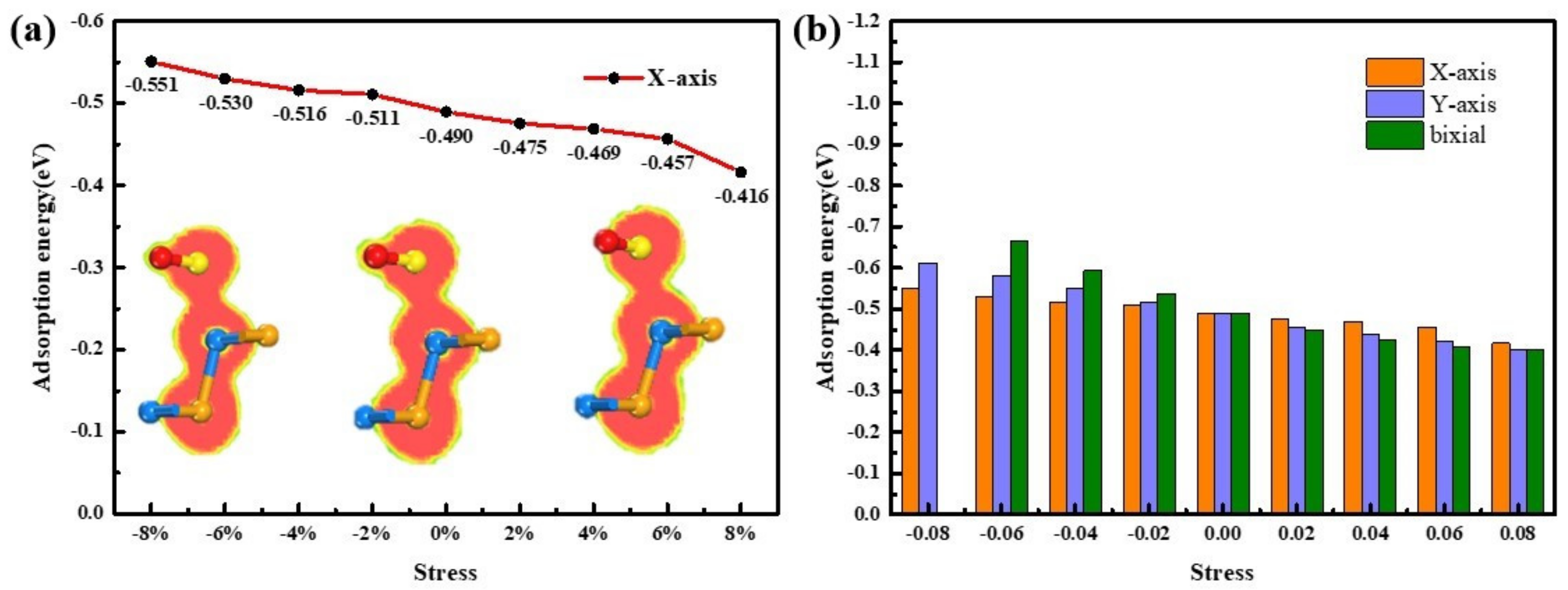
3.2.1. Compressive Direction
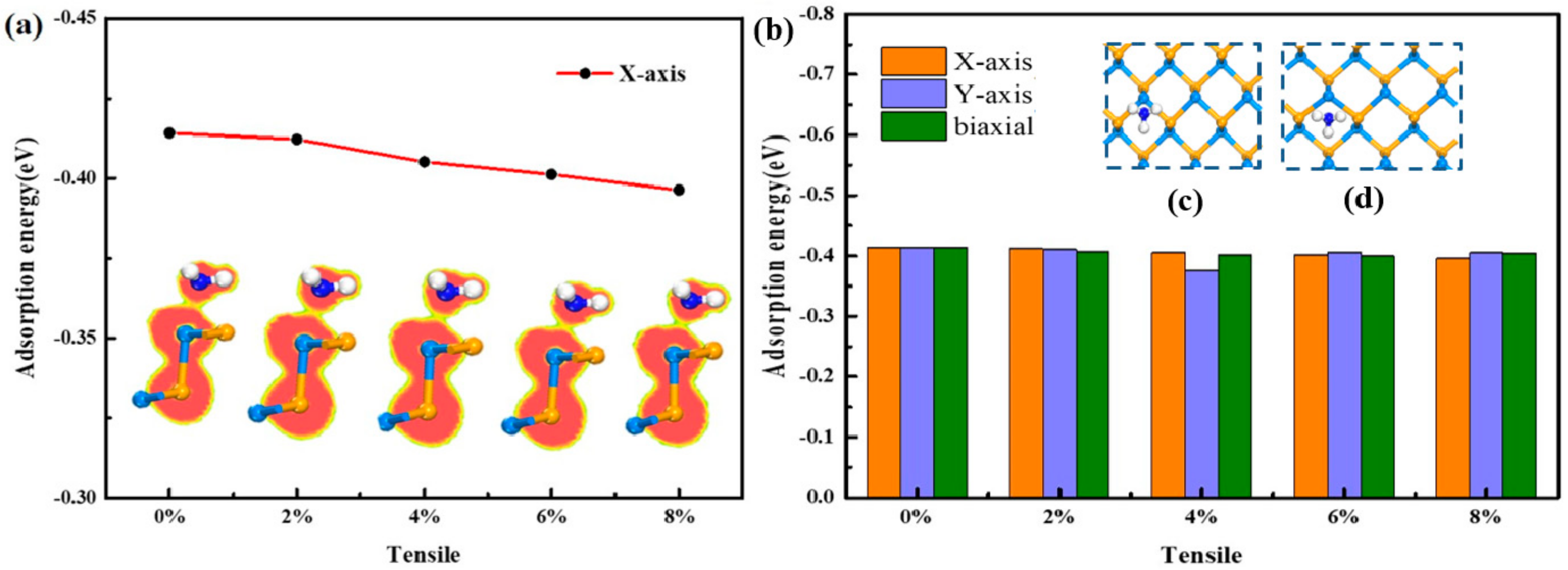
3.2.2. Tensile Direction
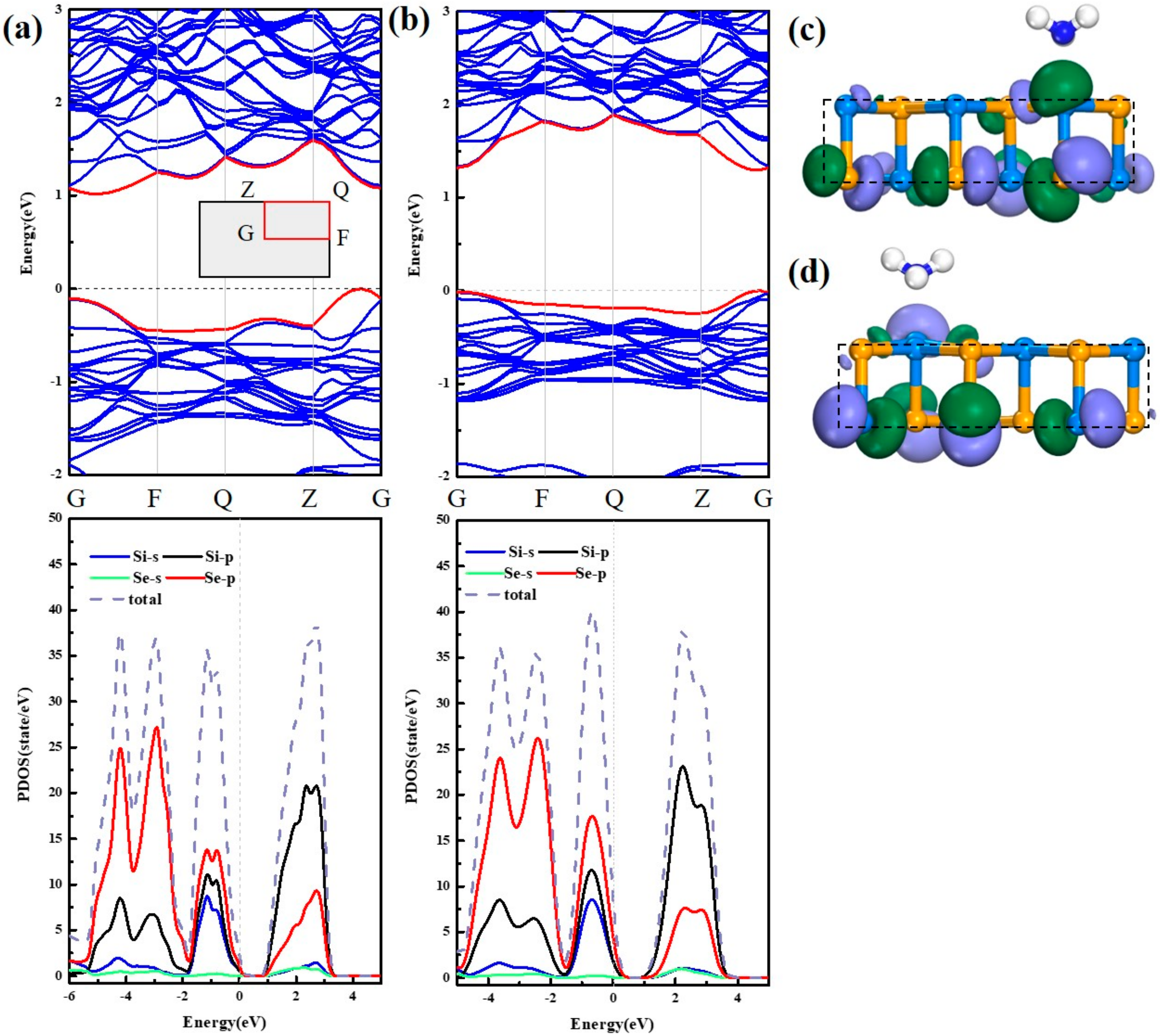
3.3. Adsorption Mechanism of SO2 Under Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Qin, Z.; Zeng, D.; Xie, C. Metal-oxide-semiconductor based gas sensors: Screening, preparation, and integration. Phys. Chem. Chem. Phys. 2017, 19, 6313–6329. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A review on chemiresistive room temperature gas sensors based on metal oxide nanostructures, graphene and 2D transition metal dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef] [PubMed]
- Wella, S.A.; Aditya, I.D.; Wungu, T.D.K. Density Functional Theory (DFT) Study: Electronic Properties of Silicene under Uniaxial Strain as H2S Gas Sensor. Key Eng. Mater. 2016, 675–676, 15–18. [Google Scholar]
- Ma, S.; Yuan, D.; Wang, Y.; Jiao, Z. Monolayer GeS as a potential candidate for NO2 gas sensor and capturer. J. Mater. Chem. C 2018, 6, 8082–8091. [Google Scholar] [CrossRef]
- Ahn, M.W.; Park, K.S.; Heo, J.H.; Park, J.G.; Kim, D.W.; Choi, K.J.; Lee, J.H.; Hong, S.H. Gas Sensing Properties of Defect-Controlled ZnO-Nanowire Gas Sensor. Appl. Phys. Lett. 2008, 93, 1502. [Google Scholar] [CrossRef]
- Ren, J.; Kong, W.; Ni, J. The Potential Application of BAs for a Gas Sensor for Detecting SO2 Gas Molecule: A DFT Study. Nanoscale Res. Lett. 2019, 14, 133. [Google Scholar] [CrossRef]
- Guo, S.; Lu, Y.; Liu, X.; Zhou, W.; Song, X.; Zhang, S. First-principles study of SO2 sensors based on phosphorene and its isoelectronic counterparts: GeS, GeSe, SnS, SnSe. Chem. Phys. Lett. 2017, 686, 83–87. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Int. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Huiru, Y.; Zeping, W.; Huaiyu, Y.; Kai, Z.; Xianping, C.; Guoqi, Z. Promoting sensitivity and selectivity of HCHO sensor based on strained InP3 monolayer: A DFT study. Appl. Surf. Sci. 2018, 459, 554–561. [Google Scholar]
- Bo, X.; Li, Y.C.; Yu, X.F.; Cheng, J.B. MXenes: Reusable materials for NH3 sensor or capturer by controlling the charge injection. Sens. Actuators B Chem. 2016, 235, 103–109. [Google Scholar]
- Yamazoe, N. Toward innovations of gas sensor technology. Sens. Actuators B Chem. 2005, 108, 2–14. [Google Scholar] [CrossRef]
- Choi, J.K.; Hwang, I.S.; Kim, S.J.; Park, J.S.; Park, S.S.; Jeong, U.; Yun, C.K.; Lee, J.H. Design of selective gas sensors using electrospun Pd-doped SnO2 hollow nanofibers. Sens. Actuators B Chem. 2010, 150, 191–199. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, C.; Wei, S. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Kannan, P.K.; Late, D.J.; Morgan, H.; Rout, C.S. Recent developments in 2D layered inorganic nanomaterials for sensing. Nanoscale 2015, 7, 13293–13312. [Google Scholar] [CrossRef] [PubMed]
- Peyghan, A.A.; Rastegar, S.F.; Hadipour, N.L. DFT study of NH3 adsorption on pristine, Ni- and Si-doped graphynes. Phys. Lett. A 2014, 378, 2184–2190. [Google Scholar] [CrossRef]
- Liang, X.Y.; Ding, N.; Ng, S.P.; Wu, C.M.L. Adsorption of gas molecules on Ga-doped graphene and effect of applied electric field: A DFT study. Appl. Surf. Sci. 2017, 411, 11–17. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, W.; Ning, L.; Zhuo, Z.; Xiao, C.Z.; Wu, X.; Yang, J. CO2 Capture on h-BN Sheet with High Selectivity Controlled by External Electric Field. J. Phys. Chem. C 2015, 119, 6912–6917. [Google Scholar] [CrossRef]
- Zhu, J.; Ha, E.; Zhao, G.; Zhou, Y. Recent advance in MXenes: A promising 2D material for catalysis, sensor and chemical adsorption. Coord. Chem. Rev. 2017, 352, 306–327. [Google Scholar] [CrossRef]
- Yu, X.F.; Li, Y.C.; Cheng, J.B.; Liu, Z.B.; Li, Q.Z.; Li, W.Z.; Yang, X.; Xiao, B. Monolayer Ti2CO2: A Promising Candidate for NH3 Sensor or Capturer with High Sensitivity and Selectivity. ACS Appl. Mater. Interfaces 2015, 7, 13707–13713. [Google Scholar] [CrossRef]
- Ma, S.H.; Yuan, D.; Jiao, Z.Y.; Wang, T.X.; Dai, X. Monolayer Sc2CO2: A Promising Candidate as SO2 Gas Sensor or Capturer. J. Phys. Chem. C 2017, 121, 24077–24084. [Google Scholar] [CrossRef]
- Park, M.; Park, Y.J.; Chen, X.; Park, Y.K.; Kim, M.S.; Ahn, J.H. MoS2-Based Tactile Sensor for Electronic Skin Applications. Adv. Mater. 2016, 28, 2556–2562. [Google Scholar] [CrossRef] [PubMed]
- Wasey, A.H.M.A.; Chakrabarty, S.; Das, G.P. Quantum size effects in layered VX2 (X=S, Se, Te) materials: Manifestation of metal to semimetal or semiconductor transition. J. Appl. Phys. 2015, 117, 064313. [Google Scholar] [CrossRef]
- Docherty, C.J.; Lin, C.T.; Joyce, H.J.; Nicholas, R.J.; Herz, L.M.; Li, L.J.; Johnston, M.B. Extreme sensitivity of graphene photoconductivity to environmental gases. Nat. Commun. 2012, 3, 1228. [Google Scholar] [CrossRef] [PubMed]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Cui, S.; Pu, H.; Wells, S.A.; Wen, Z.; Mao, S.; Chang, J.; Hersam, M.C.; Chen, J. Ultrahigh sensitivity and layer-dependent sensing performance of phosphorene-based gas sensors. Nat. Commun. 2015, 6, 8632. [Google Scholar] [CrossRef]
- Kou, L.; Frauenheim, T.; Chen, C. Phosphorene as a Superior Gas Sensor: Selective Adsorption and Distinct I-V Response. J. Phys. Chem. Lett. 2014, 5, 2675–2681. [Google Scholar] [CrossRef]
- Chen, P.F.; Li, N.; Chen, X.Z.; Ong, W.J.; Zhao, X.J. The rising star of 2D black phosphorus beyond graphene: Synthesis, properties and electronic applications. 2D Mater. 2018, 5, 21. [Google Scholar] [CrossRef]
- Abbas, A.N.; Liu, B.; Chen, L.; Ma, Y.; Cong, S.; Aroonyadet, N.; KoPf, M.; Nilges, T.; Zhou, C. Black phosphorus gas sensors. Acs Nano 2015, 9, 5618–5624. [Google Scholar] [CrossRef]
- Wei, Q.; Peng, X. Superior mechanical flexibility of phosphorene and few-layer black phosphorus. Appl. Phys. Lett. 2014, 104, 372–398. [Google Scholar] [CrossRef]
- Yang, G.; Ma, T.; Peng, X. Superior mechanical flexibility and strained-engineered direct-indirect band gap transition of green phosphorene. Appl. Phys. Lett. 2018, 112, 241904. [Google Scholar] [CrossRef]
- Gomes, L.C.; Carvalho, A. Phosphorene analogues: Isoelectronic two-dimensional group-IV monochalcogenides with orthorhombic structure. Phys. Rev. B 2015, 92, 085406. [Google Scholar] [CrossRef]
- Li, Z.Y.; Liu, M.Y.; Huang, Y.; Chen, Q.Y.; Cao, C.; He, Y. Tuning the electronic properties of bilayer group-IV monochalcogenides by stacking order, strain and an electric field: A computational study. Phys. Chem. Chem. Phys. 2017, 20, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Sa, B.; Sun, Z.; Wu, B. The development of two dimensional group IV chalcogenides, blocks for van der Waals heterostructures. Nanoscale 2016, 8, 1169–1178. [Google Scholar] [CrossRef]
- Yang, J.H.; Yuan, Q.; Deng, H.; Wei, S.H.; Yakobson, B.I. Earth-Abundant and Non-Toxic SiX (X = S, Se) Monolayers as Highly Efficient Thermoelectric Materials. J. Phys. Chem. C 2017, 121, 123–128. [Google Scholar] [CrossRef]
- Jiang, H.R.; Zhao, T.S.; Ren, Y.X.; Zhang, R.H.; Wu, M.C. Ab initio prediction and characterization of phosphorene-like SiS and SiSe as anode materials for sodium-ion batteries. Sci. Bull. 2017, 62, 572–578. [Google Scholar] [CrossRef]
- Karmakar, S.; Chowdhury, C.; Datta, A. Two-Dimensional Group IV Monochalcogenides: Anode Materials for Li-Ion Batteries. J. Phys. Chem. C 2016, 120, 14522–14530. [Google Scholar] [CrossRef]
- Mao, Y.L.; Ben, J.; Yuan, J.M.; Zhong, J.X. Tuning the electronic property of two dimensional SiSe monolayer by in-plane strain. Chem. Phys. Lett. 2018, 705, 12–18. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Appalakondaiah, S.; Vaitheeswaran, G.; Lebègue, S.; Christensen, N.E.; Svane, A. Effect of van der Waals interactions on the structural and elastic properties of black phosphorus. Phys. Rev. B 2012, 86, 035105. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Q.; Ye, H.Y.; Chen, X.P.; Zhang, G.Q. Adsorption of gases on monolayer GeSe: A first principle study. In Proceedings of the 18th International Conference on Thermal, Mechanical and Multi-Physics Simulation and Experiments in Microelectronics and Microsystems (EuroSimE), Dresden, Germany, 3–5 April 2017. [Google Scholar]
- Ye, H.; Liu, L.; Xu, Y.; Wang, L.; Chen, X.; Zhang, K.; Liu, Y.F.; Koh, S.W.; Zhang, G. SnSe monolayer: A promising candidate of SO2 sensor with high adsorption quantity. Appl. Surf. Sci. 2019, 484, 33–38. [Google Scholar] [CrossRef]
- Rodin, A.S.; Carvalho, A.; Castro Neto, A.H. Strain-Induced Gap Modification in Black Phosphorus. Phys. Rev. Lett. 2014, 112, 176801. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.V.; Hieu, N.N. Effect of biaxial strain and external electric field on electronic properties of MoS 2 monolayer: A first-principle study. Chem. Phys. 2016, 468, 9–14. [Google Scholar] [CrossRef]
- Naumis, G.G.; Barraza-Lopez, S.; Oliva-Leyva, M.; Terrones, H. A review of the electronic and optical properties of strained graphene and other similar 2D materials. Rep. Prog. Phys. 2017, 80, 096501. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Z.K.; Lau, L.; Yin, W.J.; Liu, L.M. Tuning band gaps and optical absorption of BiOCl through doping and strain: Insight form DFT calculations. Phys. Chem. Chem. Phys. 2017, 19, 20968–20973. [Google Scholar] [CrossRef]
- Yu, Z.; Bo, S.; Li, L.; Lei, J. Coupling effects of strain on structural transformation and bandgap engineering in SnS monolayer. RSC Adv. 2017, 7, 30327–30333. [Google Scholar]
- Gao, C.; Zhang, Y.; Yang, H.; Liu, Y.; Liu, Y.; Du, J.; Ye, H.; Zhang, G. A DFT study of In doped Tl2O: A superior NO2 gas sensor with selective adsorption and distinct optical response. Appl. Surf. Sci. 2019, 494, 162–169. [Google Scholar] [CrossRef]
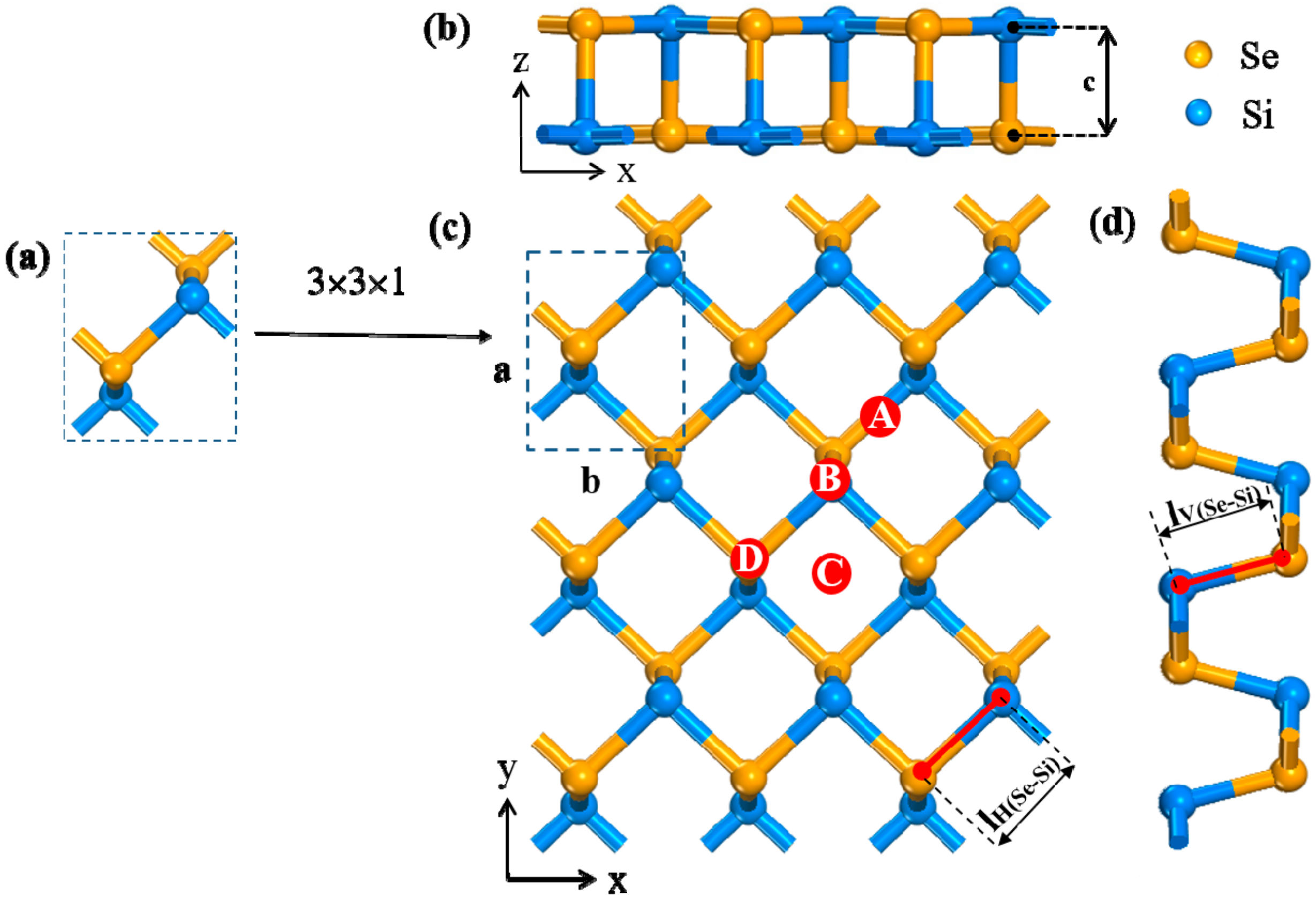
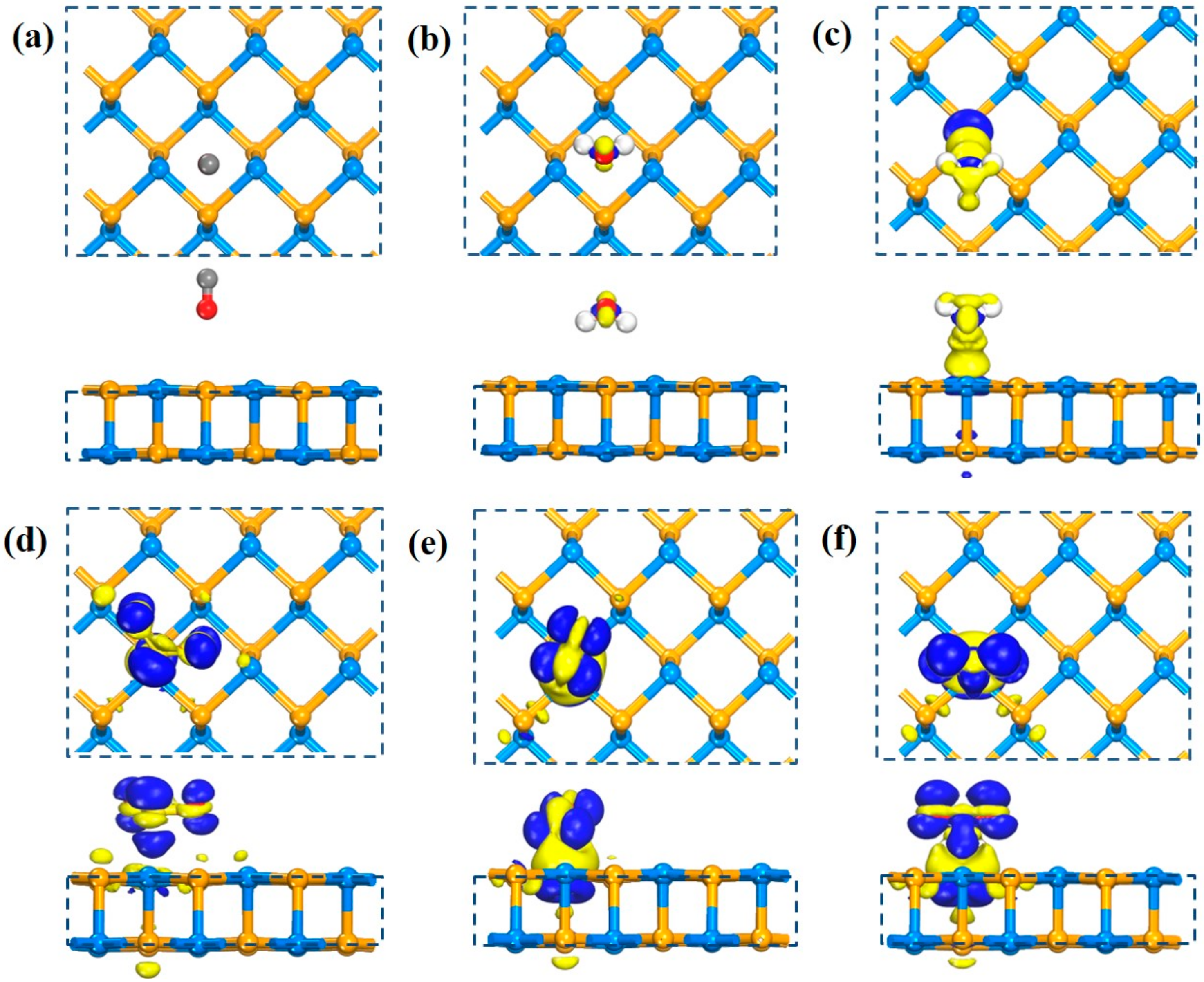
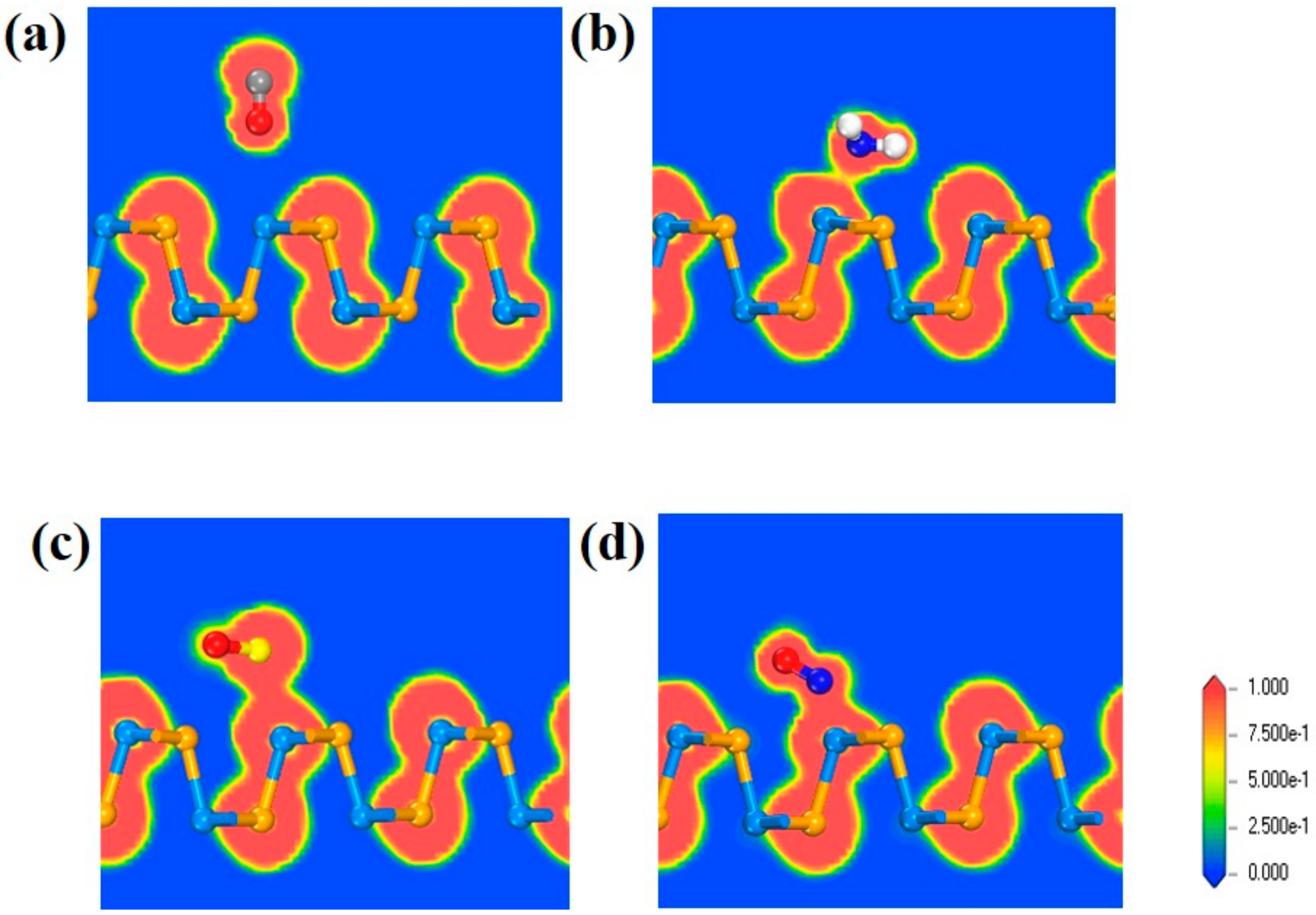
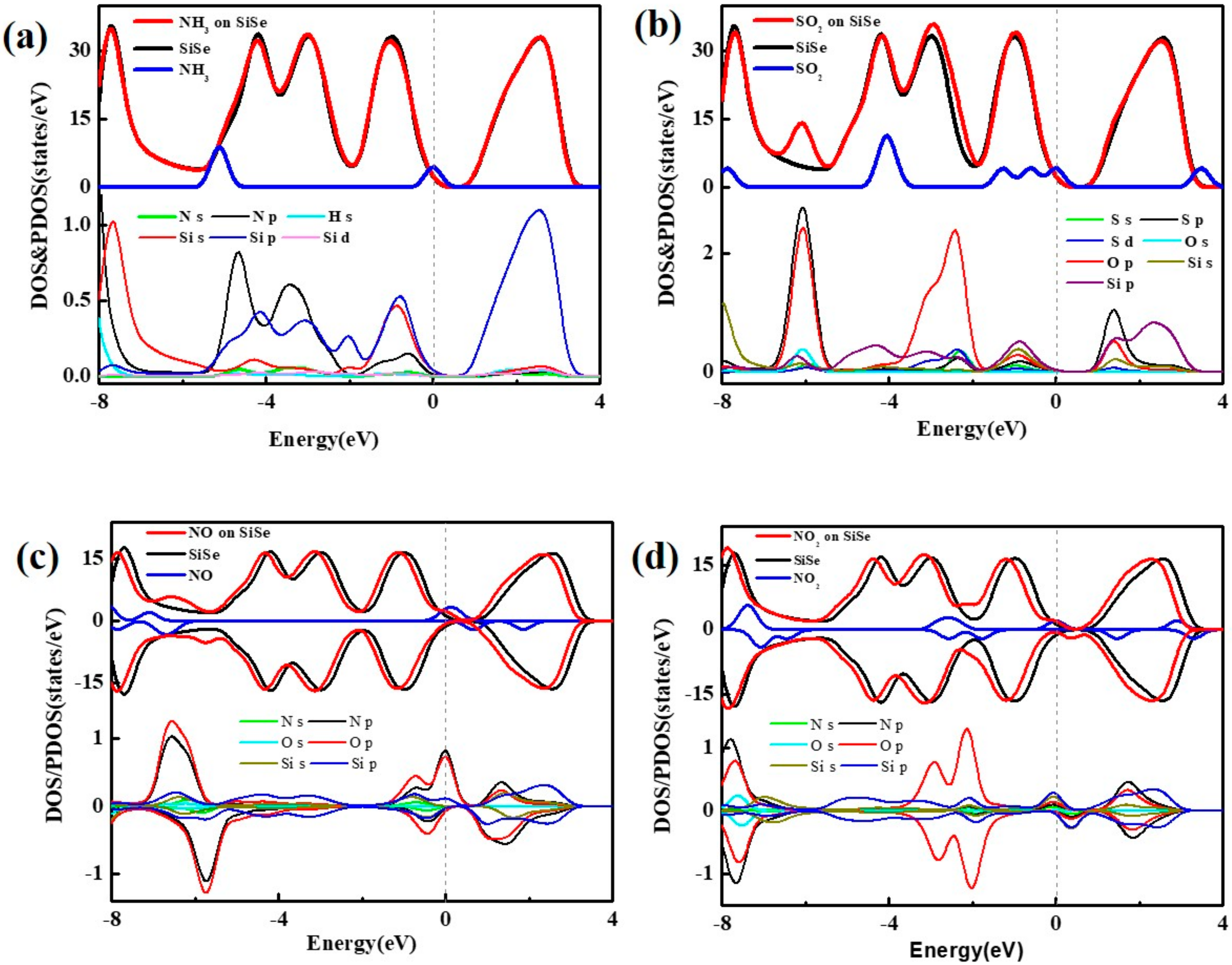
| Configuration | Site | Ead(eV) | d (Å) | ΔQ (e) | Si-Se(Å) |
|---|---|---|---|---|---|
| CO | C | −0.071 | 3.577 | 0.002/0.008 | 2.497/2.462 |
| O2 | B | −0.098 | 3.067 | −0.032/−0.037 | 2.492/2.467 |
| CO2 | D | −0.132 | 3.424 | −0.016/-0.016 | 2.495/2.469 |
| CH4 | A | −0.133 | 3.084 | −0.066/0.022 | 2.497/2.463 |
| H2S | A | −0.203 | 3.405 | 0.068/0.080 | 2.503/2.497 |
| H2O | C | −0.237 | 2.805 | −0.097/0.032 | 2.487/2.463 |
| NH3 | B | −0.414 | 2.471 | 0.153/0.178 | 2.503/2.658 |
| SO2 | A | −0.489 | 2.686 | −0.238/−0.197 | 2.448/2.441 |
| NO | B | −0.734 | 1.835 | −0.220/−0.355 | 2.417/2.528 |
| NO2 | B | −0.735 | 1.995 | −0.285/−0.386 | 2.409/2.608 |
| Configuration | Ead (eV) | d (Å) | ΔQ (e) | Si-Se(Å) |
|---|---|---|---|---|
| 0% NH3/SiSe | −0.414 | 2.471 | 0.178 | 2.503/2.658 |
| −2% NH3/SiSe | −0.426 | 2.443 | 0.184 | 2.482/2.669 |
| −2% to 0% NH3/SiSe | −0.413 | 2.454 | 0.180 | 2.505/2.661 |
| Parameter | 0% | −2% | −4% | −6% | −8% | |
|---|---|---|---|---|---|---|
| d (Å) | 2.471 | 2.384 | 2.351 | 2.273 | 2.226 | |
| ΔQ (e) | Si | 0.605 | 0.600 | 0.607 | 0.644 | 0.672 |
| Se | -0.618 | -0.602 | -0.598 | -0.578 | -0.568 | |
| Si-Se Bond(Å) | Horizontal | 2.502 | 2.494 | 2.472 | 2.460 | 2.444 |
| Vertical | 2.658 | 2.662 | 2.672 | 2.679 | 2.701 | |
| Parameter | 0% | -2% | -4% | -6% | -8% | |
|---|---|---|---|---|---|---|
| d (Å) | 2.471 | 2.503 | 2.518 | 2.479 | 2.465 | |
| ΔQ (e) | Si | 0.605 | 0.606 | 0.607 | 0.609 | 0.610 |
| Se | -0.618 | -0.619 | -0.626 | -0.624 | -0.627 | |
| Si-Se Bond(Å) | Horizontal | 2.502 | 2.527 | 2.551 | 2.572 | 2.596 |
| Vertical | 2.658 | 2.649 | 2.649 | 2.663 | 2.679 | |
| Configuration | Ead (eV) | ΔQ(NH3)(e) | ΔQ(SO2)(e) | d(NH3)(Å) | d(SO2)(Å) |
|---|---|---|---|---|---|
| SO2 and NH3/SiSe | −1.161 | 0.241↑ | -0.276↑ | 2.278↓ | 2.646↓ |
| NH3-SO2/SiSe | −0.752 | 0.243↑ | -0.278↑ | 2.226↓ | 2.639↓ |
| SO2-NH3/SiSe | −0.465 | 0.196↑ | -0.220↓ | 2.431↓ | 2.760↑ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Liu, L.; Liu, Q.; Wang, Z.; Gao, C.; Liu, Y.; Ye, H. Highly Selective Adsorption on SiSe Monolayer and Effect of Strain Engineering: A DFT Study. Sensors 2020, 20, 977. https://doi.org/10.3390/s20040977
Zhou Q, Liu L, Liu Q, Wang Z, Gao C, Liu Y, Ye H. Highly Selective Adsorption on SiSe Monolayer and Effect of Strain Engineering: A DFT Study. Sensors. 2020; 20(4):977. https://doi.org/10.3390/s20040977
Chicago/Turabian StyleZhou, Quan, Lian Liu, Qipeng Liu, Zeping Wang, Chenshan Gao, Yufei Liu, and Huaiyu Ye. 2020. "Highly Selective Adsorption on SiSe Monolayer and Effect of Strain Engineering: A DFT Study" Sensors 20, no. 4: 977. https://doi.org/10.3390/s20040977
APA StyleZhou, Q., Liu, L., Liu, Q., Wang, Z., Gao, C., Liu, Y., & Ye, H. (2020). Highly Selective Adsorption on SiSe Monolayer and Effect of Strain Engineering: A DFT Study. Sensors, 20(4), 977. https://doi.org/10.3390/s20040977





