Chiral Plasmonics and Their Potential for Point-of-Care Biosensing Applications
Abstract
1. Introduction
2. Plasmonics for Enhanced Chiroptical Effects
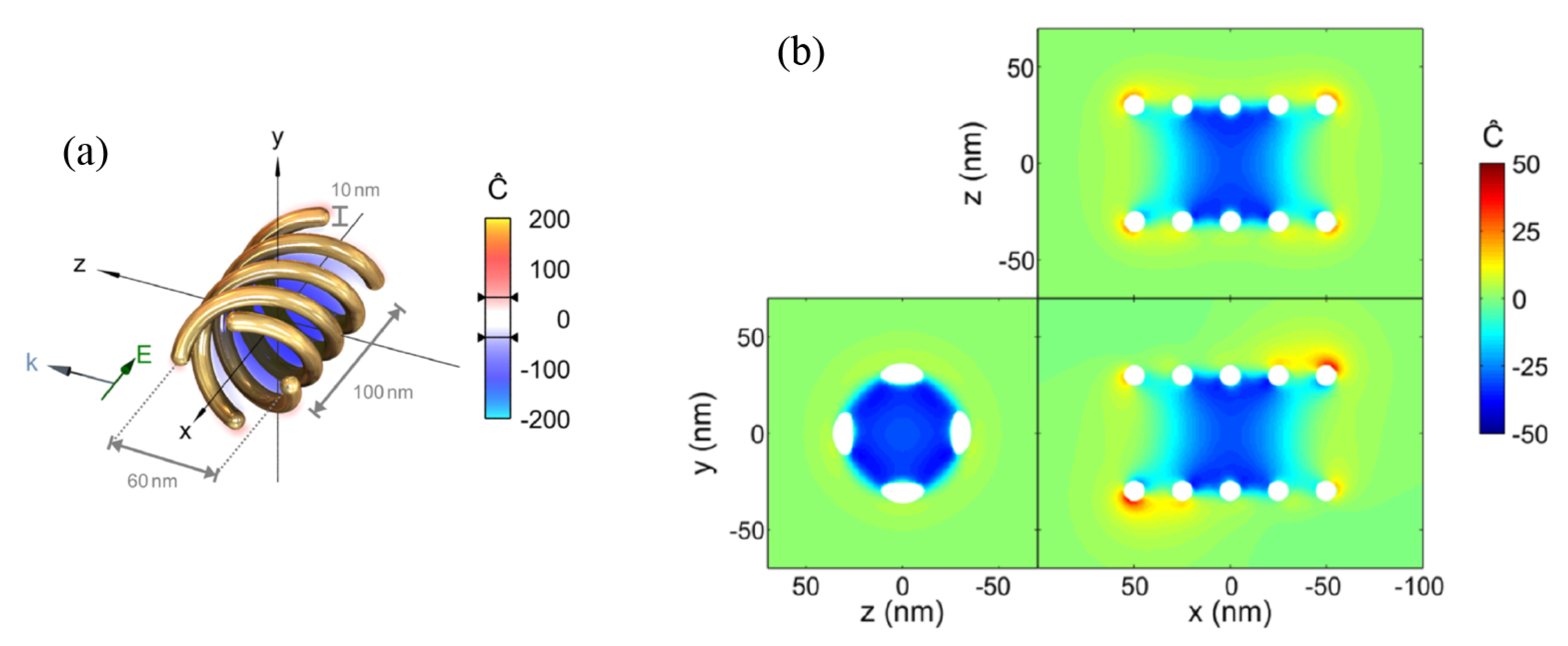
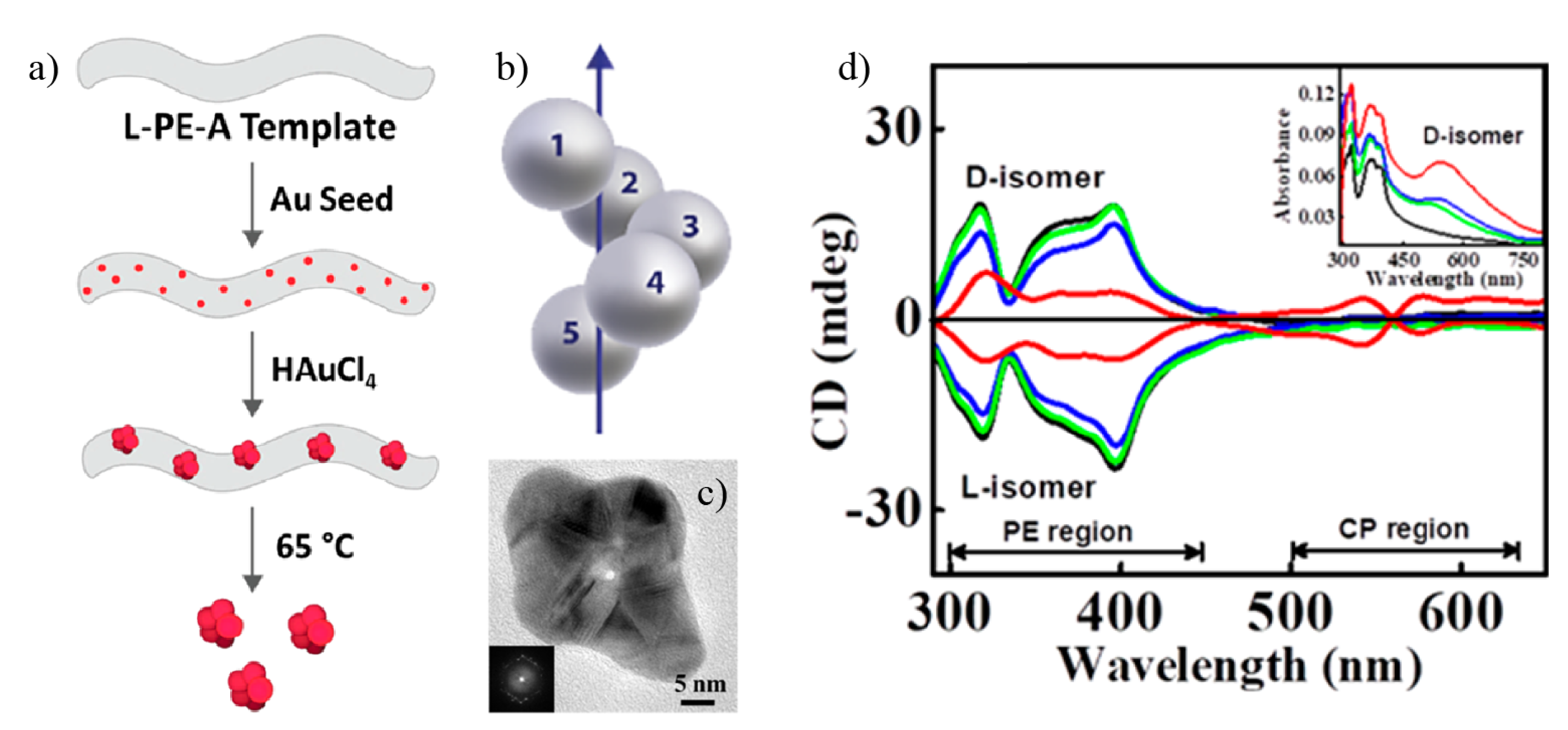
3. Chiral Plasmonic Nanoparticles
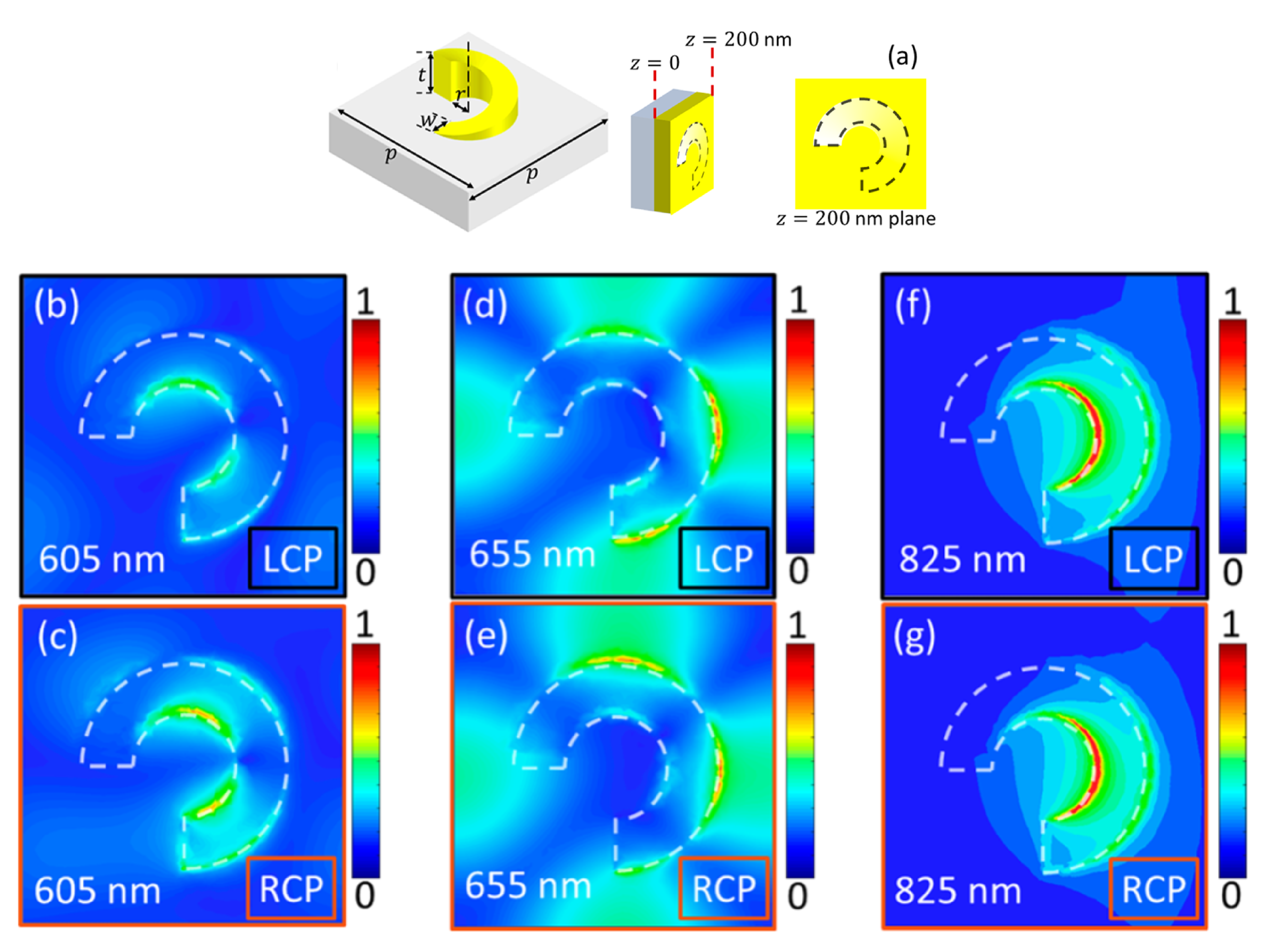
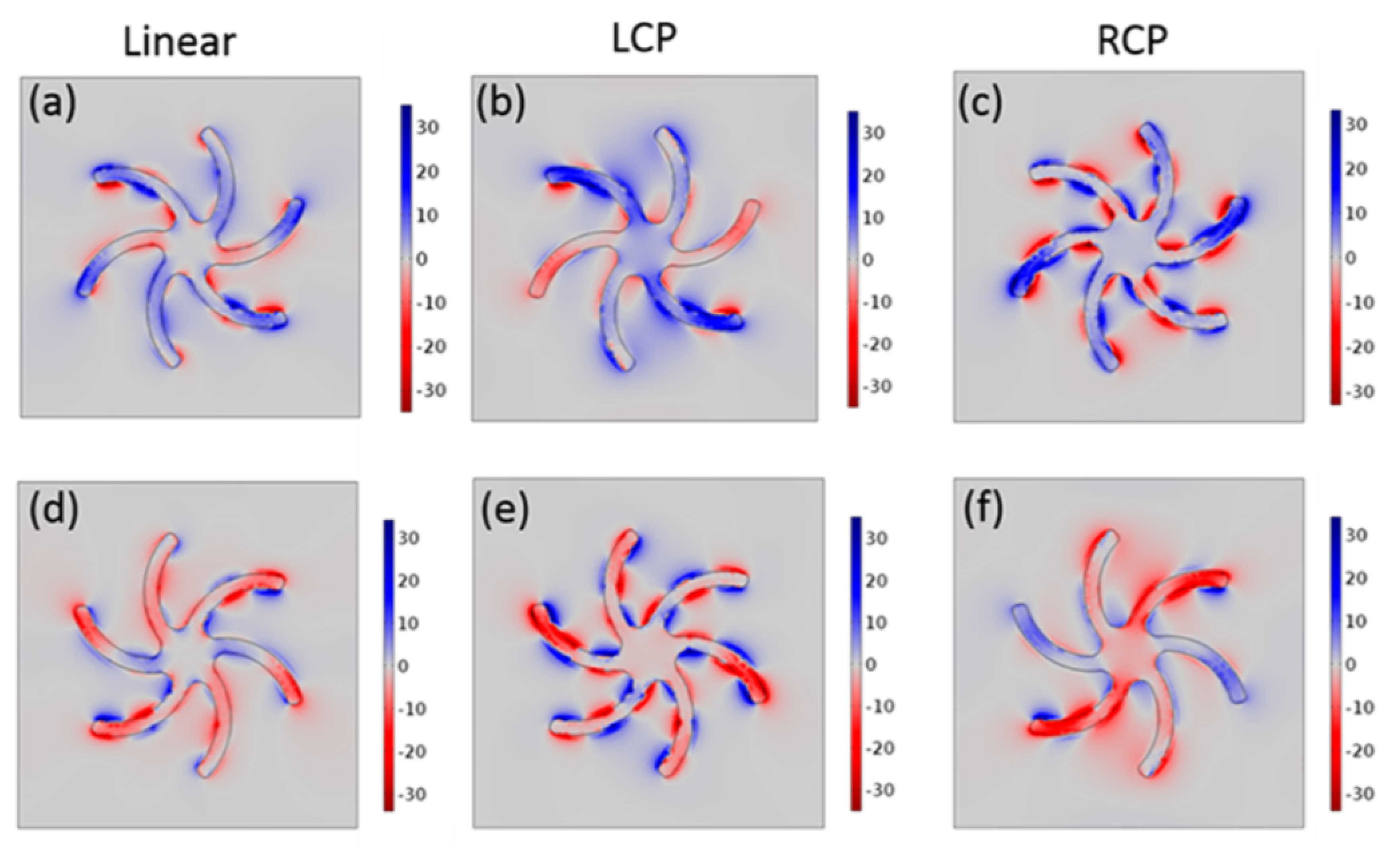
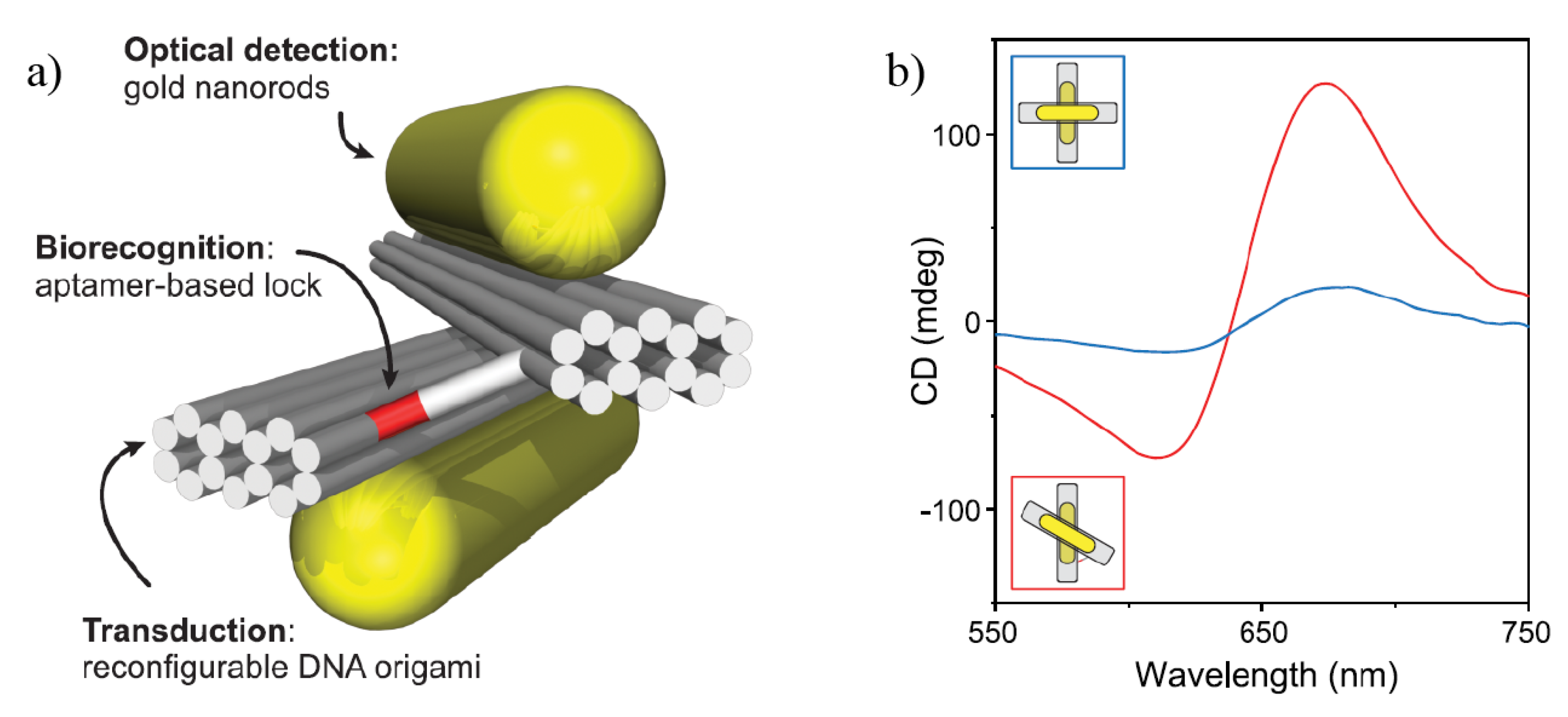
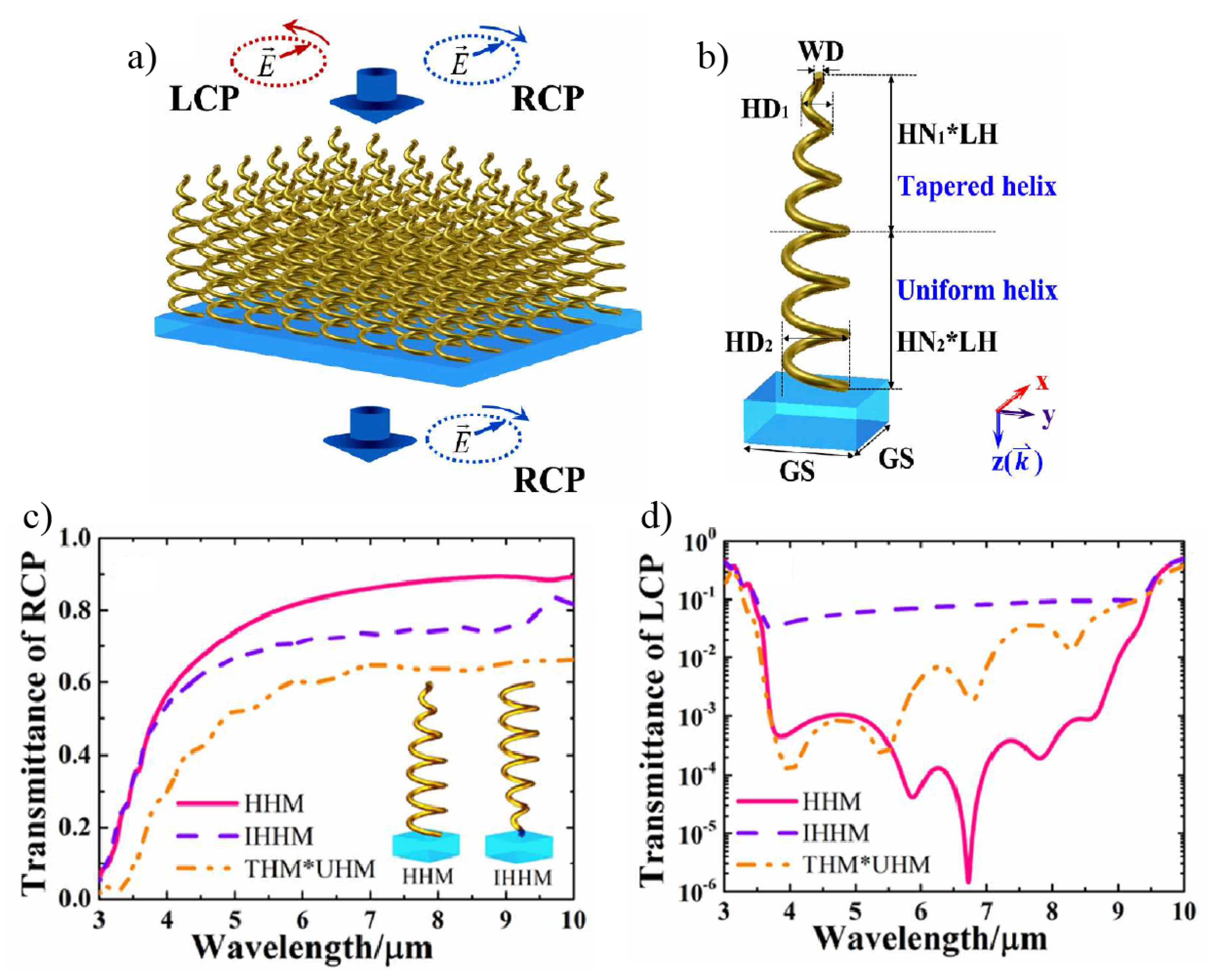
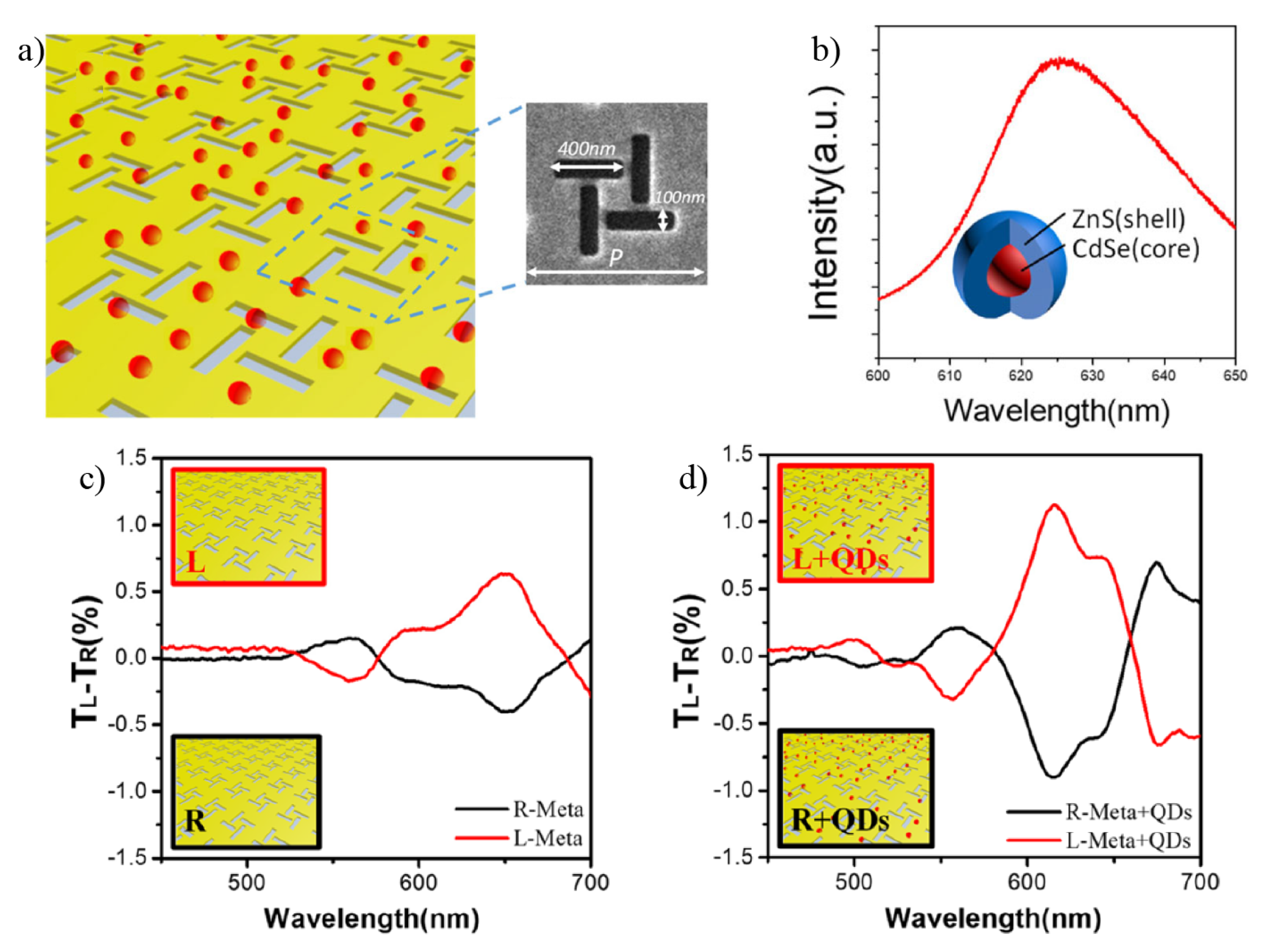
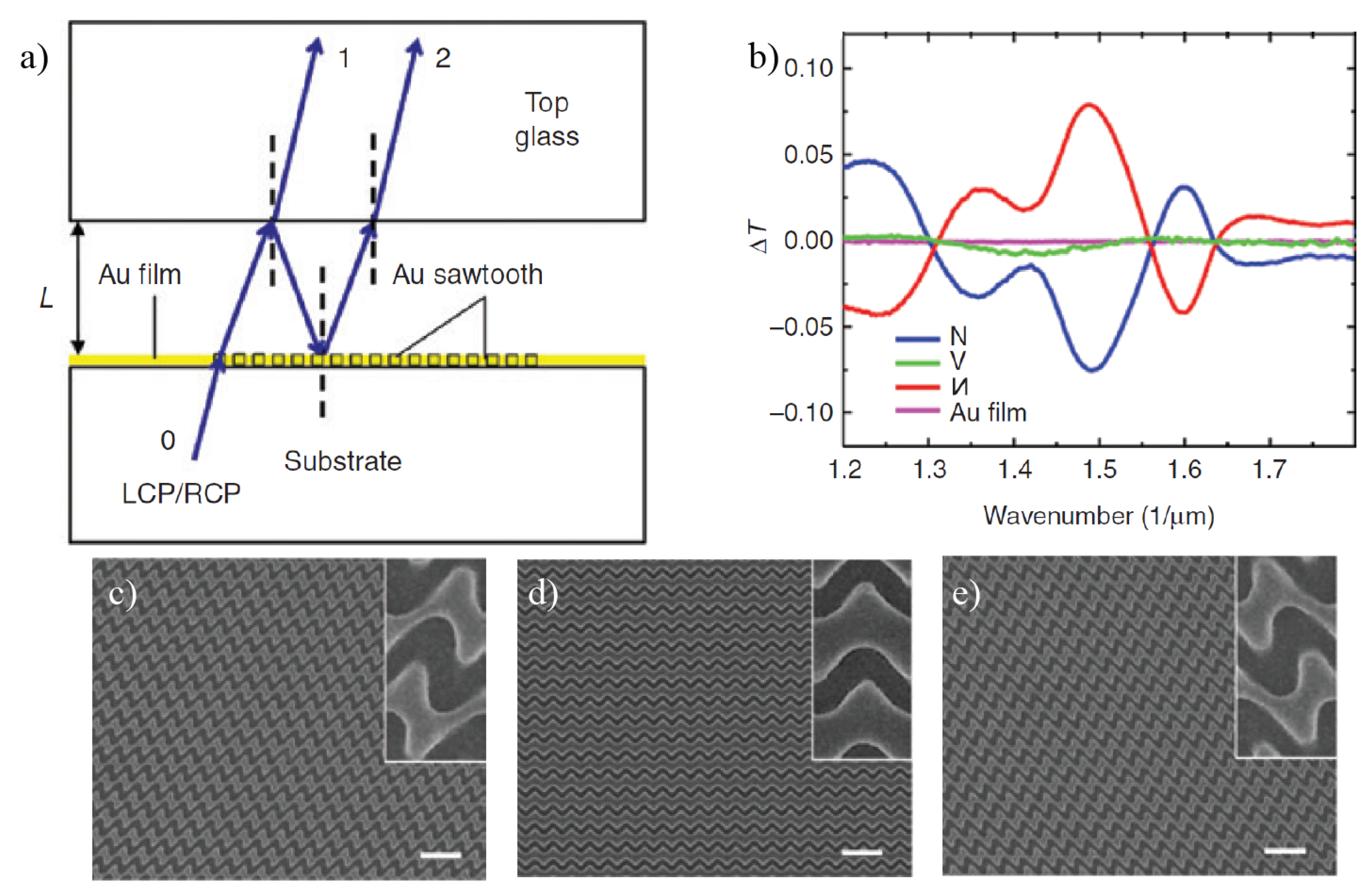
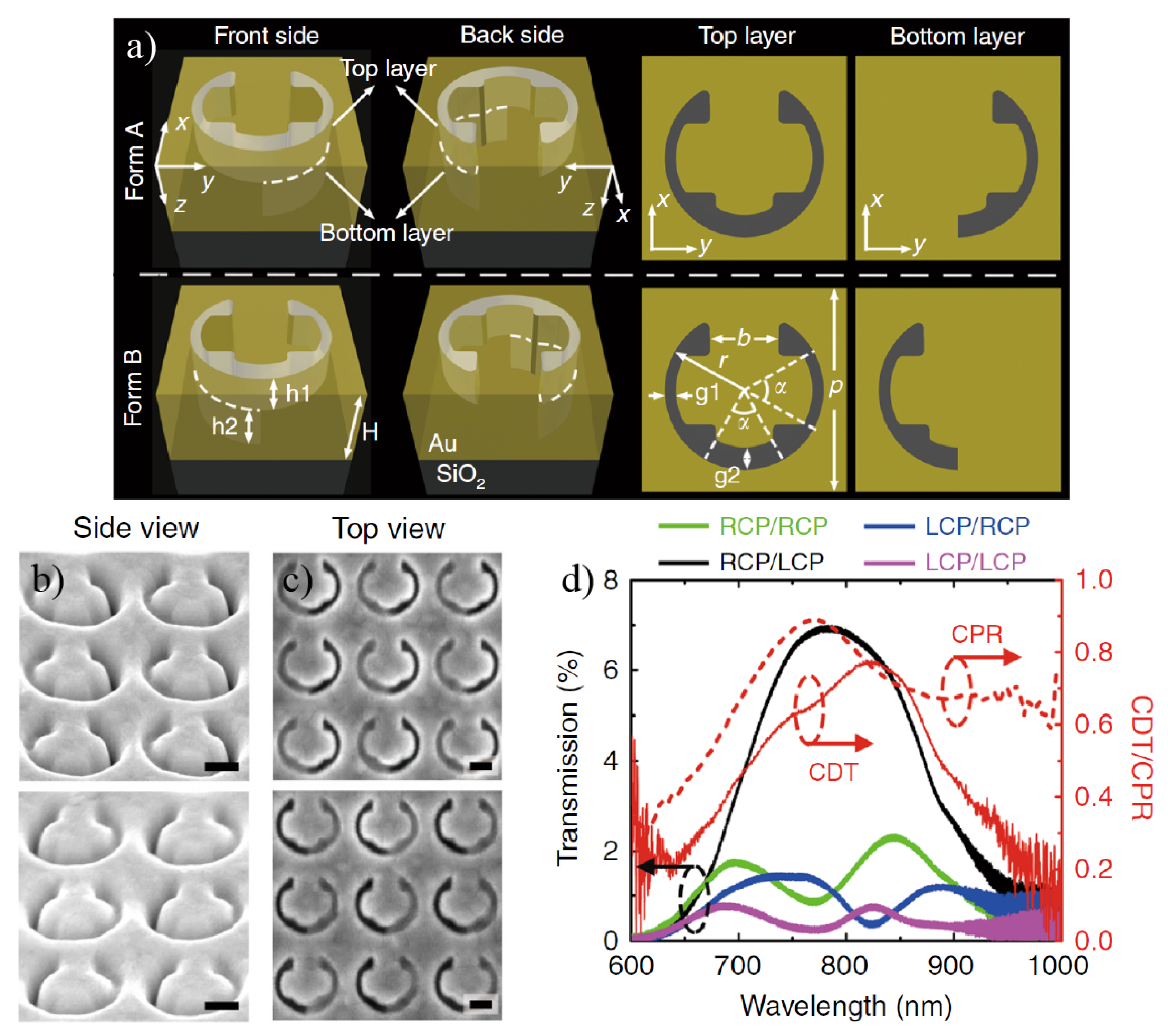
4. Chiral Plasmonic Metasurfaces
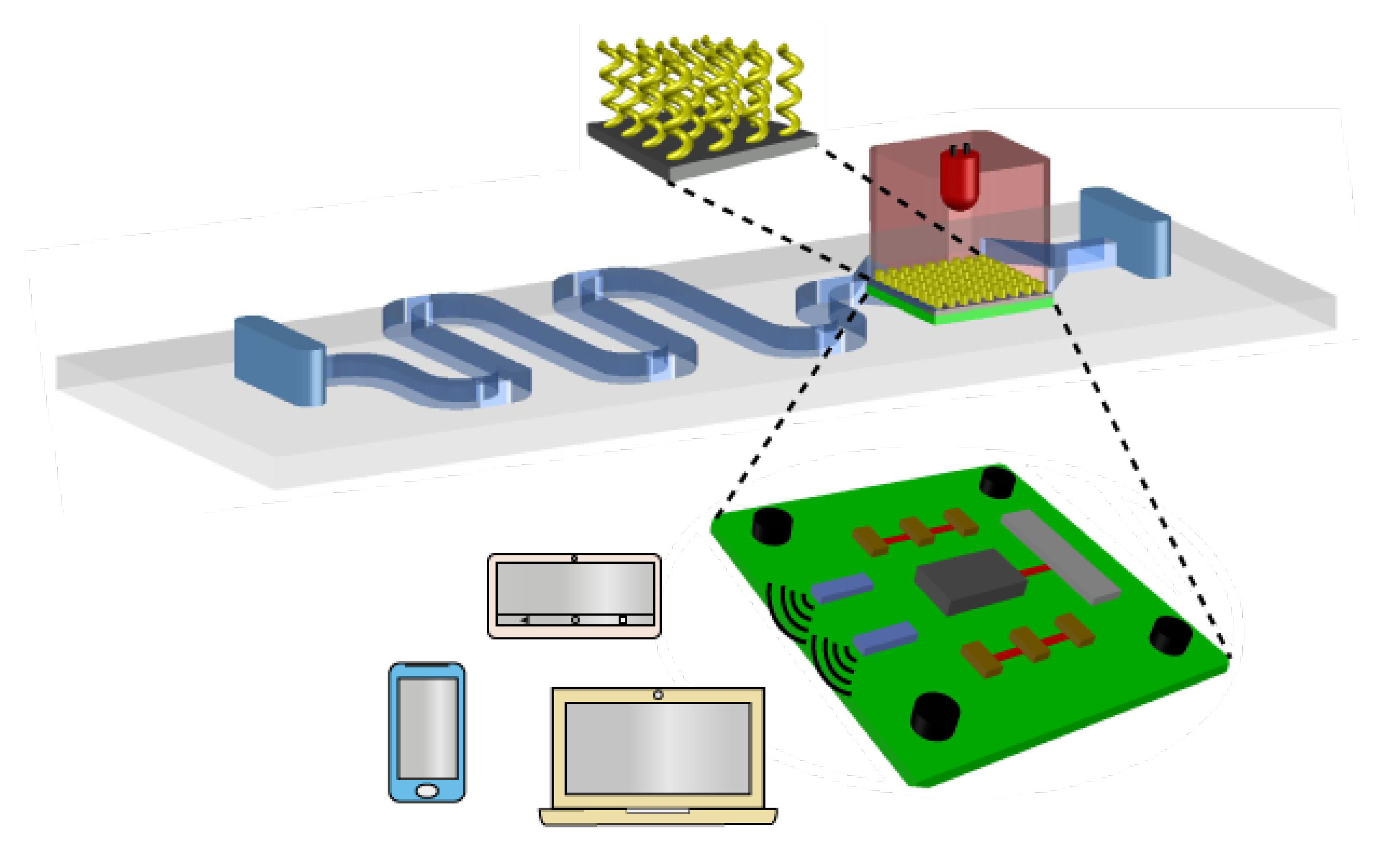
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- McConathy, J.; Owens, M.J. Stereochemistry in Drug Action. Primary Care Companion J. Clin. Psychiatry 2013, 5, 70–73. [Google Scholar] [CrossRef]
- Singh, K.; Shakya, P.; Kumar, A.; Alok, S.; Kamal, M.; Singh, S.P. Stereochemistry and its role in drug design. Int. J. Pharm. Sci. Res. 2014, 5, 4644–4659. [Google Scholar]
- Singh, R.; Nalwa, H.S. Medical Applications of Nanoparticles in Biological Imaging, Cell Labeling, Antimicrobial Agents and Anticancer Nanodrugs. J. Biomed. Nanotechnol. 2011, 7, 489–503. [Google Scholar] [CrossRef]
- Patching, S.G. Surface plasmon resonance spectroscopy for characterization of membrane protein-ligand interactions and its potential for drug discovery. Biochim. Biophys. Acta 2014, 1839, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zhou, J.; Wang, J.; Zhou, R.; Xu, B. Chirality Controls Reaction-Diffusion of Nanoparticles for Inhibiting Cancer Cells. Chem. Nano Mat. 2016, 3, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Jazayeria, M.H.; Aghaieb, T.; Avanc, A.; Vatankhahd, A.; Ghaffarid, M.R.S. Colorimetric detection based on gold nano particles (GNPs): An easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion). Sens. Bio-Sens. Res. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Nguyen, L.A.; He, H.; Pham-Hyu, C. Chiral Drugs: An Overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar]
- Smith, S.W. Chiral Toxicology: It’s the Same Thing...Only Different. Toxicol. Sci. 2009, 110, 4–30. [Google Scholar] [CrossRef]
- Tokunaga, E.; Yamamoto, T.; Ito, E.; Shibata, N. Understanding the Thalidomide Chirality in Biological Processes by the Self-disproportionation of Enantiomers. Sci. Rep. 2018, 8, 17131. [Google Scholar] [CrossRef]
- Dobson, C.M. Protein Folding and Misfolding. Nature 2003, 426, 884–890. [Google Scholar] [CrossRef]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to Study Proteins by Circular Dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar]
- Lieberman, I.; Shemer, G.; Fried, T.; Kosower, E.M.; Markovich, G. Plasmon-Resonance-Enhanced Absorption and Circular Dichroism. Angew. Chem. Int. Ed. 2008, 47, 4855–4857. [Google Scholar] [CrossRef] [PubMed]
- Hendry, E.; Carpy, T.; Johnston, J.; Popland, M.; Mikhaylovskiy, R.V.; Lapthorn, A.J.; Kelly, S.M.; Barron, L.D.; Gadegaard, N.; Kadodwala, M. Ultrasensitive detection and characterization of biomolecules using superchiral fields. Nat. Nanotechnol. 2010, 5, 783. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.G.; Gibbs, J.G.; Lee, T.; Fischer, P. Hybrid nanocolloids with programmed three-dimensional shape and material composition. Nat. Mater. 2013, 12, 802–807. [Google Scholar] [CrossRef]
- Esposito, M.; Tasco, V.; Cucuna, M.; Todisco, F.; Benedetti, A.; Tarantini, I.; Giorgi, M.; Sanvitto, D.; Passaseo, A. Nanoscale 3D Chiral Plasmonic Helices with Circular Dichroism at Visible Frequencies. ACS Photonics 2014, 2, 105–114. [Google Scholar] [CrossRef]
- Nair, G.; Singh, H.J.; Paria, D.; Venkatapathi, M.; Ghosh, A. Plasmonic Interactions at Close Proximity in Chiral Geometries: Route toward Broadband Chiroptical Response and Giant Enantiomeric Sensitivity. J. Phys. Chem. C 2014, 118, 4991–4997. [Google Scholar] [CrossRef]
- Gutsche, P.; Mäusle, R.; Burger, S. Locally Enhanced and Tunable Optical Chirality in Helical Metamaterials. Photonics 2016, 3, 60. [Google Scholar] [CrossRef]
- Zu, S.; Bao, Y.; Fang, Z. Planar plasmonic chiral nanostructures. Nanoscale 2016, 8, 3900–3905. [Google Scholar] [CrossRef]
- Schäferling, M.; Yin, X.; Engheta, N.; Giessen, H. Helical Plasmonic Nanostructures as Prototypical Chiral Near-Field Sources. ACS Photonics 2014, 1, 530–537. [Google Scholar] [CrossRef]
- Schäferling, M.; Engheta, N.; Giessen, H.; Weiss, T. Reducing the Complexity: Enantioselective Chiral Near-Fields by Diagonal Slit and Mirror Configuration. ACS Photonics 2016, 3, 1076–1084. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Y.; Pan, L.; Fang, Y. Analyzing Intrinsic Plasmonic Chirality by Tracking the Interplay of Electric and Magnetic Dipole Modes. Sci. Rep. 2017, 7, 11151. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Salazar, J.R.; Oliveira, O.N., Jr. Plasmonic Biosensing. Chem. Rev. 2018, 118, 10617–10625. [Google Scholar] [CrossRef] [PubMed]
- Bochenkov, V.E.; Shabatina, T.I. Chiral Plasmonic Biosensors. Biosensors 2018, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xu, L.; Wang, L.; Xu, C.; Kuang, H. Chirality-Based Biosensors. Adv. Funct. Mater. 2019, 29, 1805512. [Google Scholar] [CrossRef]
- Pal, S.; Deng, Z.; Ding, B.; Yan, H.; Liu, Y. DNA-Origami-Direct Self-Assembly of Discrete Silver-Nanoparticle Architectures. Angew. Chem. Int. 2010, 49, 2700–2704. [Google Scholar] [CrossRef] [PubMed]
- Frank, B.; Yin, X.; Schäferling, M.; Zhao, J.; Hein, S.M.; Braun, P.V.; Giessen, H. Large-Area 3D Chiral Plasmonic Structures. ACS Nano 2013, 7, 6321–6329. [Google Scholar] [CrossRef]
- Kühler, P.; Roller, E.M.; Schreiber, R.; Liedl, T.; Lohmüller, F.J. Plasmonic DNA-Origami Nanonantennas for Surface-Enhanced Raman Spectroscopy. Nano Lett. 2014, 14, 2914–2919. [Google Scholar] [CrossRef]
- Song, J.C.W.; Rudner, M.S. Chiral plasmons without magnetic field. Proc. Natl. Acad. Sci. USA 2016, 113, 4658–4663. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, H.; Yao, K.; Cai, W.; Chen, H.; Liu, Y. Circular Dichroism Metamirrors with Near-Perfect Extinction. ACS Photonics 2016, 3, 2096–2101. [Google Scholar] [CrossRef]
- Schnell, M.; Sarriugarte, P.; Neuman, T.; Khanikaev, A.B.; Shvets, G.; Aizpurua, J.; Hillenbrand, R. Real-Space Mapping of the Chiral Near-Field Distributions in Spiral Antennas and Planar Metasurfaces. Nano Lett. 2016, 16, 663–670. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, J.; Wang, G.; Fu, T.; Qu, Y.; Zhang, Z. Plasmonic chirality of L-shaped nanostructure composed of two slices with different thickness. Opt. Express 2016, 24, 2307–2317. [Google Scholar] [CrossRef]
- Li, Z.; Wang, W.; Rosenmann, D.; Czaplewski, D.A.; Yang, X.; Gao, J. All-metal structural color printing based on aluminum plasmonic metasurfaces. Opt. Express 2016, 24, 20472–20480. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chi, C.; Jiang, M.; Li, R.; Zu, S.; Li, Y.; Fang, Z. Plasmonic Chiral Nanostructures: Chiroptical Effects and Applications. Adv. Opt. Mater. 2017, 5, 1700040. [Google Scholar] [CrossRef]
- Zhao, Y.; Askarpour, A.N.; Sun, L.; Shi, J.; Li, X.; Alú, A. Chirality detection of enantiomers using twisted optical metamaterials. Nat. Commun. 2017, 8, 14180. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, E.; Tsakmakidis, K.L.; Askarpour, A.N.; Dehkhoda, P.; Tavakoli, A.; Altug, H. Nanophotonic Platforms for Enhanced Chiral Sensing. ACS Photonics 2018, 5, 2669–2675. [Google Scholar] [CrossRef]
- Woźniak, P.; De Leon, I.; Höflich, K.; Haverkamp, C.; Christiansen, S.; Leuchs, G.; Banzer, P. Chiroptical response of a single plasmonic nanohelix. Opt. Express 2018, 15, 19275–19293. [Google Scholar] [CrossRef]
- Kong, X.T.; Khorashad, L.K.; Wnag, Z. Photothermal Circular Dichroism Induced by Plasmon Resonances in Chiral Metamaterial Absorbers and Bolometers. Nano Lett. 2018, 18, 2001–2008. [Google Scholar] [CrossRef]
- Kaniewska, M.; Trojanowicz, M. Chiral Biosensors and Immunosensors. In Biosensors: Emerging Materials and Applications; In Tech: Rijeka, Croatia, 2011; Volume 7. [Google Scholar]
- Murugkar, S.; Leon, I.D.; Horton, M.; Qassin, H.; Leach, J.; Boyd, R.W. Planar chiral metamaterials for biosensing applications. Plasmonics Biol. Med. X 2013, 8597, 85970Y. [Google Scholar]
- Wu, T.; Ren, J.; Wang, R.; Zhang, X. Competition of Chiroptical Effect Caused by Nanostructures and Chiral Molecules. J. Phys. Chem. C 2014, 118, 20529–20537. [Google Scholar] [CrossRef]
- Kailasa, S.K.; Koduru, J.R.; Desai, M.L.; Park, T.J.; Singhal, R.K.; Basu, H. Recent progress on surface chemistry of plasmonic metal nanoparticles for colorimetric assay of drugs in pharmaceutical and biological samples. TrAC Trends Anal. Chem. 2018, 105, 106–120. [Google Scholar] [CrossRef]
- Xu, L.L.; Zhang, H.F.; Li, M.; Ng, S.W.; Feng, J.H.; Mao, G.G. Chiroptical Activity from an Achiral Biological Meta-Organic Framework. J. Am. Chem. Soc. 2018, 140, 11569–11572. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Cohen, A.E. Optical Chirality and Its Interaction with Matter. Phys. Rev. Lett. 2010, 104, 163901. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A. On the Optical Rotary Dispersion of Polymers. J. Chem. Phys. 1965, 43, 959. [Google Scholar] [CrossRef]
- Lipkin, D.M. Existence of a New Conservation Law in Electromagnetic Theory. J. Math. Phys. 1964, 5, 696. [Google Scholar] [CrossRef]
- Poulikakos, L.V.; Thureja, P.; Stollmann, A.; de Leo, E.; Norris, D.J. Chiral Light Design and Detection Inspired by Optical Antenna Theory. Nano Lett. 2018, 18, 4633–4640. [Google Scholar] [CrossRef]
- Pham, A.; Zhao, A.; Genet, C.; Drezet, A. Optical Chirality Density and Flux Measured in the Local Density of States of Spiral Plasmonic Structures. Phys. Rev. A 2018, 98, 013837. [Google Scholar] [CrossRef]
- Aba, T.; Qu, Y.; Abudukelimu, A.; Ullah, H.; Zhang, Z. Chiral Response of a Metasurface Composed of Nanoholes and Tilted Nanorods. Appl. Opt. 2019, 58, 5936–5941. [Google Scholar] [CrossRef]
- Rajaei, M.; Zeng, J.; Albooyeh, M.; Kamandi, M.; Hanifeh, M.; Capolino, F.; Wickramasinghe, H.K. Giant Circular Dichroism at Visible Frequencies Enabled by Plasmonic Ramp-Shaped Nanostructures. ACS Photonics 2019, 6, 924–931. [Google Scholar] [CrossRef]
- Gilroy, C.; Hashiyada, S.; Endo, K.; Karimullah, A.S.; Barron, L.D.; Okamoto, H.; Togawa, Y.; Kadodwala, M. Roles of Superchirality and Interference in Chiral Plasmonic Biodetection. J. Phys. Chem. C 2019, 123, 15195–15203. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, J.; Yang, X. Chiral Grayscale Imaging with Plasmonic Metasurfaces of Stepped Nanoapertures. Adv. Opt. Mater. 2019, 7, 1801467. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Rong, X.; Luo, Y.; Chen, H.; Zheng, L.; Lin, F.; Shen, B.; Gong, Y.; Zhang, S.; et al. Tailoring MoS2 Valley-Polarized Photoluminescence with Super Chiral Near-Field. Adv. Mater. 2018, 30, 1801908. [Google Scholar] [CrossRef] [PubMed]
- Tseng, M.L.; Lin, Z.-H.; Kuo, H.Y.; Huang, T.-T.; Huang, Y.T.; Chung, T.L.; Chu, C.H.; Huang, J.-S.; Tsai, D.P. Stress-Induced 3D Chiral Fractal Metasurface for Enhanced and Stabilized Broadband Near-Field Optical Chirality. Adv. Opt. Mater. 2019, 7, 1900617. [Google Scholar] [CrossRef]
- Champi, H.A.A.; Bustamante, R.H.; Salcedo, W.J. Optical Enantioseparation of Chiral Molecules Using Asymmetric Plasmonic Nanoapertures. Opt. Mater. Exp. 2019, 9, 1763. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Adamo, G.; Teh, B.H.; Wu, Q.Y.S.; Teng, J.; Sun, H. A Novel Chiral Metasurface with Controllable Circular Dichroism Induced by Coupling Localized and Propagating Modes. Adv. Opt. Mater. 2016, 4, 883–888. [Google Scholar] [CrossRef]
- Qu, Y.; Huang, L.; Wang, L.; Zhang, Z. Giant circular dichroism induced by tunable resonance in twisted Z-shaped nanostructure. Opt. Exp. 2017, 25, 5480. [Google Scholar] [CrossRef]
- Kelly, C.; Khorashad, L.K.; Gadegaard, N.; Barron, L.D.; Govorov, A.O.; Karimullah, A.S.; Kadodwala, M. Controlling Metamaterial Transparency with Superchiral Fields. ACS Photonics 2018, 5, 535–543. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, Y.; Qv, L.; Fang, Y. Electromagnetic Energy Redistribution in Coupled Chiral Particle Chain-Film System. Nanoscale Res. Lett. 2018, 13, 194. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Gao, J. Spin-Controlled Wavefront Shaping with Plasmonic Chiral Geometric Metasurfaces. Light Sci. Appl. 2018, 7, 84. [Google Scholar] [CrossRef]
- Lu, F.; Zhang, W.; Zhang, J.; Zhang, L.; Xue, T.; Liu, M.; Meng, C.; Mao, D.; Mei, T. Dynamic manipulation of optical chirality for gammadion nanostructures. Appl. Phys. Exp. 2019, 12, 072015. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, S.; Wang, Y.; Xiao, L. Tunable atom-trapping based on a plasmonic chiral metamaterial. Nanophotonics 2019, 8, 1739–1745. [Google Scholar] [CrossRef]
- Yin, S.; Ji, W.; Xiao, D.; Li, Y.; Li, K.; Yin, Z.; Jiang, S.; Shao, L.; Luo, D.; Liu, Y.J. Intrinsically or extrinsically reconfigurable chirality in plasmonic chiral metasurfaces. Opt. Commun. 2019, 448, 10–14. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Gao, J. 3D Janus Plasmonic Helical Nanoapertures for Polarization-Encrypted Data Storage. Light Sci. Appl. 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zheng, J.; Feng, J.; Qian, Q.; Zhou, Y. Reversible modulation of plasmonic chiral signals of achiral gold nanorods using a chiral supramolecular template. Chem. Commun. 2019, 55, 11378–11381. [Google Scholar] [CrossRef] [PubMed]
- Hashiyada, S.; Narushima, T.; Okamoto, H. Active Control of Chiral Optical near Fields on a Single Metal Nanorod. ACS Photonics 2019, 6, 677–683. [Google Scholar] [CrossRef]
- Horrer, A.; Zhang, Y.; Gérard, D.; Béal, J.; Kociak, M.; Plain, J.; Bachelot, R. Local Optical Chirality Induced by Near-Field Mode Interference in Achiral Plasmonic Metamolecules. Nano Lett. 2020, 20, 509–516. [Google Scholar] [CrossRef]
- Reyntjens, S.; Puers, R. Focused ion beam induced deposition: Fabrication of three-dimensional microstructures and Young’s modulus of the deposited material. J. Micromech. Microeng. 2000, 10, 181–188. [Google Scholar] [CrossRef]
- Huth, M.; Porrati, F.; Dobrovolskiy, O.V. Focused electron beam induced deposition meets materials science. Microelectron. Eng. 2018, 185, 9–28. [Google Scholar] [CrossRef]
- Lee, H.E.; Ahn, H.Y.; Mun, J.; Lee, Y.Y.; Kim, M.; Cho, N.H.; Chang, K. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 2018, 556, 360–365. [Google Scholar] [CrossRef]
- Schneider, F.; Möritz, N.; Dietz, H. The sequence of events during folding of a DNA origami. Sci. Adv. 2019, 5, eaaw1412. [Google Scholar] [CrossRef]
- Zhou, C.; Duan, X.; Liu, N. A plasmonic nanorod that walks on DNA origami. Nat. Commun. 2015, 6, 8102. [Google Scholar] [CrossRef]
- Huang, Y.; Nguyen, M.; Natarajan, A.K.; Nguyen, V.H. A DNA Origami-Based Chiral Plasmonic Sensing Device. Appl. Mater. Interfaces 2018, 10, 44221–44225. [Google Scholar] [CrossRef]
- Shemer, G.; Krichevski, O.; Markovich, G.; Molotsky, T.; Lubitz, T.; Kotyar, A.B. Chirality of Silver Nanoparticles Synthesized on DNA. J. Am. Chem. Soc. 2006, 128, 11006–11007. [Google Scholar] [CrossRef]
- Swasey, S.M.; Karimova, M.; Aikens, C.M.; Schultz, D.E.; Simon, A.J.; Gwinn, E.G. Chiral Electronic Transitions in Fluorescent Silver Cluster Stabilized by DNA. ACS Nano 2014, 8, 6883–6892. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Luong, N.; Fan, Z.; Kuzyk, A.; Nickels, P.C.; Zhang, T.; Smith, D.M.; Yurke, B.; Kuang, W.; Govorov, A.O.; et al. Chiral plasmonic DNA nanostructures with switchable circular dichroism. Nat. Commun. 2013, 4, 2948. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Govorov, A.O. Helical Metal Nanoparticle Assemblies with Defects: Plasmonic Chirality and Circular Dichroism. J. Phys. Chem. 2011, 115, 13254–13261. [Google Scholar] [CrossRef]
- Kuzyk, A.; Schreiber, R.; Fan, Z.; Pardatscher, G.; Roller, E.M.; Högele, A.; Simmel, F.C.; Govorov, A.O.; Liedl, T. DNA-based self-assembly of chiral plasmonic nanostructures with tailored optical response. Nature 2012, 483, 311–314. [Google Scholar] [CrossRef]
- George, J.; Kar, S.; Anupriya, E.S.; Somasundaran, S.M.; Das, A.D.; Sissa, C.; Painelli, A.; Thomas, K.G. Chiral Plasmons: Au Nanoparticle Assemblies on Thermoresponsive Organic Template. ACS Nano 2019, 13, 4392–4401. [Google Scholar] [CrossRef]
- Kumar, J.; Eraña, H.; López-Martínez, E.; Claes, N.; Martín, V.F.; Solís, D.M.; Bals, S.; Cortajarena, A.L.; Castilla, J.; Liz-Marzán, L.M. Detection of Amyloid Fibrils in Parkinson’s Disease Using Plasmonic Chirality. Proc. Natl. Acad. Sci. USA 2018, 115, 3225–3230. [Google Scholar] [CrossRef]
- Saito, K.; Tatsuma, T. Chiral Plasmonic Nanostructures Fabricated by Circularly Polarized Light. Nano Lett. 2018, 18, 3209–3212. [Google Scholar] [CrossRef]
- Hentschel, M.; Schäferling, M.; Duan, X.; Giessen, H.; Liu, N. Chiral plasmonics. Sci. Adv. 2017, 3, e1602735. [Google Scholar] [CrossRef]
- Schäferling, M.; Dregely, D.; Hentschel, M.; Giessen, H. Tailoring Enhanced Optical Chirality: Design Principles for Chiral Plasmonic Nanostructure. Phys. Rev. X 2012, 2, 031010. [Google Scholar] [CrossRef]
- Zhao, Y.; Saleh, A.A.E.; Dionne, J.A. Enantioselective Optical Trapping of Chiral Nanoparticles with Plasmonic Tweezers. ACS Photonics 2016, 3, 304–309. [Google Scholar] [CrossRef]
- Chen, H.T.; Taylor, A.J.; Yu, N. A review of metasurfaces: Physics and applications. Rep. Prog. Phys. 2016, 79, 076401. [Google Scholar] [CrossRef] [PubMed]
- Glybovski, S.B.; Tretyakov, S.A.; Belov, P.A.; Kivshar, Y.S.; Simovski, C.R. Metasurfaces: From Microwaves to Visible. Phys. Rep. 2016, 634, 1–72. [Google Scholar] [CrossRef]
- Ouyang, L.; Wang, W.; Rosenmann, D.; Czaplewski, D.A.; Gao, J.; Yang, X. Near-infrared chiral plasmonic metasurface absorbers. Opt. Express 2018, 26, 31484–31489. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yao, Z.; Wang, Q.; Hu, F.; Xu, X. Coupling Tai Chi Chiral Metamaterials with Strong Optical Activity in Terahertz Region. Plasmonics 2015, 10, 1005–1011. [Google Scholar] [CrossRef]
- Sezen, M. Focused Ion Beam (FIB)—Novel Methodologies and Recent Applications for Multidisciplinary Sciences. Mod. Electron Microsc. Phys. Life Sci. 2016, 18, 121–140. [Google Scholar]
- Chen, Y.; Gao, J.; Yang, X. Chiral Metamaterials of Plasmonic Slanted Nanoapertures with Symmetry Breaking. Nano Lett. 2018, 18, 520–527. [Google Scholar] [CrossRef]
- Ndao, A.; Belkhir, A.; Salut, R.; Baida, F.I. Slanted annular aperture arrays as enhanced-transmission metamaterials: Excitation of the plasmonic transverse electromagnetic guided mode. Appl. Phys. Lett. 2013, 103, 211901. [Google Scholar] [CrossRef]
- Lv, J.; Khoo, E.H.; Leong, E.S.P.; Hu, L.; Jiang, X.; Li, Y.; Luo, D.; Si, G.; Liu, Y.J. Maskless fabrication of slanted annular aperture arrays. Nanotechnology 2017, 28, 225302. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Q.; Wang, X.; Gao, W.; Li, J.; Tam, W.Y. Measuring circular phase-dichroism of chiral metasurface. Nanophotonics 2019, 8, 909–920. [Google Scholar] [CrossRef]
- Ji, R.; Wang, S.-W.; Liu, X.; Guo, H.; Lu, W. Hybrid helical metamaterials for giant and ultra-wide circular dichroism. ACS Photonics 2016, 12, 2368–2374. [Google Scholar] [CrossRef]
- Wang, Z.; Wnag, Y.; Adamo, G.; Teng, J.; Sun, H. Induced Optical Chirality and Circularly Polarized Emission from Achiral CdSe/ZnS Quantum Dots via Resonantly Coupling with Plasmonic Chiral Metasurfaces. Laser Photonics Rev. 2019, 13, 1800276. [Google Scholar] [CrossRef]
- Fang, Y.; Verre, R.; Shao, L.; Nordlander, P.; Käll, M. Hot Electron Generation and Cathodoluminescence Nanoscopy of Chiral Split Ring Resonators. Nano Lett. 2016, 16, 5183–5190. [Google Scholar] [CrossRef]
- Liu, H.; Shang, Z.; Wu, X.; Dou, C.; Zhang, J. Tunable circular dichroism of bilayer b-type chiral nanostructure. Opt. Commun. 2019, 448, 76–81. [Google Scholar] [CrossRef]
- Rifat, A.A.; Rahmani, M.; Xu, L.; Miroshnichenko, E. Hybrid Metasurface Based Tunable Near-Perfect Absorber and Plasmonic Sensor. Materials 2018, 11, 1091. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, H.; Bao, K.; Håkanson, U.; Halas, N.J.; Nordlander, P.; Xu, H. Chiral Surface Plasmon Polaritons on Metallic Nanowires. Phys. Rev. Lett. 2011, 107, 096801. [Google Scholar] [CrossRef]
- Wang, W.; Rosenmann, D.; Czaplewski, D.A.; Yang, X.; Gao, J. Realizing structural color generation with aluminum plasmonic V-groove metasurface. Opt. Express 2017, 25, 20454–20465. [Google Scholar] [CrossRef]
- St. John, A.; Price, C.P. Existing and Emerging Technologies for Point-of-Care Testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar]
- Shaw, L.V. Practical challenges related to point of care testing. Pract. Lab. Med. 2016, 4, 22–29. [Google Scholar] [CrossRef]
- Zarei, M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. Trends Anal. Chem. 2017, 91, 26–41. [Google Scholar] [CrossRef]
- Taylor, A.B.; Zijlstra, P. Single-Molecule plasmon Sensing: Current Status and Future Prospects. ACS Sens. 2017, 2, 1103–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Z.; Cheng, F.; Zhang, Y.; Chen, L. Highly sensitive on-site detection of glucose in human urine with naked eye based on enzymatic-like reaction mediated etching of gold nanorods. Biosens. Bioelectron. 2017, 89, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Yip, P.M.; Venner, A.A.; Shea, J.; Fuezery, A.; Huang, Y.; Massicote, L.; Tetreault, N.; Tomalty, C.; Shaw, J.L.V. Point-of-care testing: A position statement from the Canadian Society of Clinical Chemists. Clin. Biochem. 2018, 53, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Rafique, R.; Baek, S.H.; Nguyen, T.P.; Park, K.Y.; Kim, E.B.; Kim, J.G.; Park, J.P.; Kailasa, S.K.; Kim, H.J.; Chung, C.; et al. Gold-copper nanoshell dot-blot immunoassay for naked-eye sensitive detection of tuberculosis specific CFP-10 antigen. Biosens. Bioelectron. 2018, 121, 111–117. [Google Scholar]
- Goel, S. From waste to watts in micro-devices: Review on development of Membranedand Membraneless Microfluidic Microbial Fuel Cell. Appl. Mater. Today 2018, 11, 270–279. [Google Scholar] [CrossRef]
- Mazzotta, F.; Höök, F.; Jonsson, M.P. High throughput fabrication of plasmonic nanostructures in nanofluidic pores for biosensing applications. Nanotechnology 2012, 23, 415304. [Google Scholar] [CrossRef]
- Gomez, F.A. The future of microfluidic point-of-care diagnostic devices. Bioanalysis 2013, 5, 1–3. [Google Scholar] [CrossRef]
- Oh, B.R.; Huang, N.T.; Chen, W.; Seo, J.H.; Chen, P.; Cornell, T.T.; Shanley, T.P.; Fu, J.; Kurabayashi, K. Integrated Nanoplasmonic Sensing for Cellular Functional Immunoanalysis Using Human Blood. ACS Nano 2014, 8, 2667–2676. [Google Scholar] [CrossRef]
- Cappi, G.; Spiga, F.M.; Moncada, Y.; Ferretti, A.; Beyeler, M.; Bianchessi, M.; Decosters, L.; Buclin, T.; Guiducci, C. Label-Free Detection of Tobramycin in Serum by Transmission-Localized Surface Plasmon Resonance. Anal. Chem. 2015, 87, 5278–5285. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Li, J. Construction of Plasmonic Nano-Biosensor-Based Devices for Point-of-Care Testing. Small Methods 2017, 1, 1700197. [Google Scholar] [CrossRef]
- Wang, L.J.; Chang, Y.C.; Sun, R.; Li, L. A multichannel smartphone optical biosensor for high-throughput point-of-care diagnostics. Biosens. Bioelectron. 2017, 87, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Brolo, A.G. Plasmonics for future biosensors. Nat. Photonics 2012, 6, 709–713. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Werable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed Point-of-Care Testing-xPOCT. Trends Biotechnol. 2017, 35, 728–742. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef]
- Vilela, P.H.; Rodrigues, J.J.P.C.; Solic, P.S.; Saleem, K.; Furtado, V. Performance evaluation of a Fog-assisted IoT solution for e-Health applications. Future Gener. Comput. Syst. 2019, 97, 379–386. [Google Scholar] [CrossRef]
- Sacha, G.M.; Varona, P. Artificial intelligence in nanotechnology. Nanotechnology 2013, 24, 452002. [Google Scholar] [CrossRef]
- Ma, W.; Cheng, F.; Liu, Y. Deep-Learning-Enable On-Demand Design of Chiral Metamaterials. ACS Nano 2018, 12, 6326–6334. [Google Scholar] [CrossRef]
- Pasluosta, C.F.; Gassner, H.; Winkler, J.; Klucken, J.; Eskofier, B.M. An Emerging Era in the Management of Parkinson’s Disease: Wearable Technologies and the Internet of Things. IEEE J. Biomed. Health Inform. 2015, 19, 1873–1881. [Google Scholar] [CrossRef]
- Klímová, B.; Kucča, K. Internet of Things in the Assesment, Diagnostics and Treatment of Parkinson’s Disease. Health Technol. 2019, 9, 87–91. [Google Scholar] [CrossRef]
- Vashist, S.K. Point-of-Care Diagnostics: Recent Advances and Trends. Biosensors 2018, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.; Woolley, T. Point of Care Testing. Surgery 2016, 34, 91–93. [Google Scholar] [CrossRef]
- Zarei, M. Advances in point-of-care technologies for molecular diagnostics. Biosens. Bioelectron. 2017, 98, 494–506. [Google Scholar] [CrossRef] [PubMed]
- King, K.R.; Grazette, L.P.; Paltoo, D.N.; MCDevitt, J.T.; Sia, S.K.; Barrett, P.M.; Apple, F.S.; Gurbel, P.A.; Weissleder, R.; Leeds, H.; et al. Point-of-Care Technologies for Precision Cardiovascular Care and Clinical Research. JACC Basic Transl. Sci. 2016, 1, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Sridhara, A.; Melo, R.; Richer, L.; Chee, N.H.; Kim, J.; Linder, V.; Steinmiller, D.; Sia, S.K.; Gomes-Solecki, M. Microfluidics-based point-of-care test for serodiagnosis of Lyme Disease. Sci. Rep. 2016, 6, 35069. [Google Scholar] [CrossRef]
- Darwish, N.T.; Sekaran, S.D.; Khor, S.M. Point-of-care tests: A review of advances in the emerging diagnostic tools for dengue virus infection. Sens. Actuators B 2018, 255, 3316–3331. [Google Scholar] [CrossRef]
- Loubier, S.; Moatti, J.P. Economic evaluation of point-of-care diagnostic technologies for infectious diseases. Clin. Microbiol. Infect. 2010, 16, 1070–1076. [Google Scholar] [CrossRef]
- Garcia, P.J.; You, P.; Fridley, G.; Mabey, D.; Peeling, R. Point-of-care diagnostic tests for low-resource settings. The Lancet Glob. Health 2015, 3, 257–258. [Google Scholar] [CrossRef]
- Scott, S.A. Clinical Pharmacogenomics: Opportunities and Challenges at Point of Care. Clin. Pharmacol. Ther. 2013, 93, 33–35. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, X. A portable paper-based microfluidic platform for multiplexed electrochemical detection of human immunodeficiency virus and hepatitis C virus antibodies in serum. Microfluidics 2016, 10, 024119. [Google Scholar] [CrossRef] [PubMed]
- Rebouda, J.; Xub, G.; Garretta, A.; Adrikoc, M.; Yanga, Z.; Tukahebwac, E.M.; Rowellc, C.; Coopera, M. Paper-based microfluidics for DNA diagnostics of malaria in low resource underserved rural communities. Proc. Natl. Acad. Sci. USA 2019, 116, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.G.; Materon, E.M.; Melendez, M.E.; Carvalho, A.L.; Faria, R.C. Disposable Microfluidic Immunoarray Device for Sensitive Breast Cancer Biomarker Detection. ACS Appl. Mater. Interfaces 2017, 9, 27433–27440. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Huang, X.; Guo, J.; Ma, X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018, 110, 78–88. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
A. Paiva-Marques, W.; Reyes Gómez, F.; N. Oliveira, O., Jr.; Mejía-Salazar, J.R. Chiral Plasmonics and Their Potential for Point-of-Care Biosensing Applications. Sensors 2020, 20, 944. https://doi.org/10.3390/s20030944
A. Paiva-Marques W, Reyes Gómez F, N. Oliveira O Jr., Mejía-Salazar JR. Chiral Plasmonics and Their Potential for Point-of-Care Biosensing Applications. Sensors. 2020; 20(3):944. https://doi.org/10.3390/s20030944
Chicago/Turabian StyleA. Paiva-Marques, Willian, Faustino Reyes Gómez, Osvaldo N. Oliveira, Jr., and J. Ricardo Mejía-Salazar. 2020. "Chiral Plasmonics and Their Potential for Point-of-Care Biosensing Applications" Sensors 20, no. 3: 944. https://doi.org/10.3390/s20030944
APA StyleA. Paiva-Marques, W., Reyes Gómez, F., N. Oliveira, O., Jr., & Mejía-Salazar, J. R. (2020). Chiral Plasmonics and Their Potential for Point-of-Care Biosensing Applications. Sensors, 20(3), 944. https://doi.org/10.3390/s20030944





