Diurnal Patterns in Solute Concentrations Measured with In Situ UV-Vis Sensors: Natural Fluctuations or Artefacts?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Instrumentation
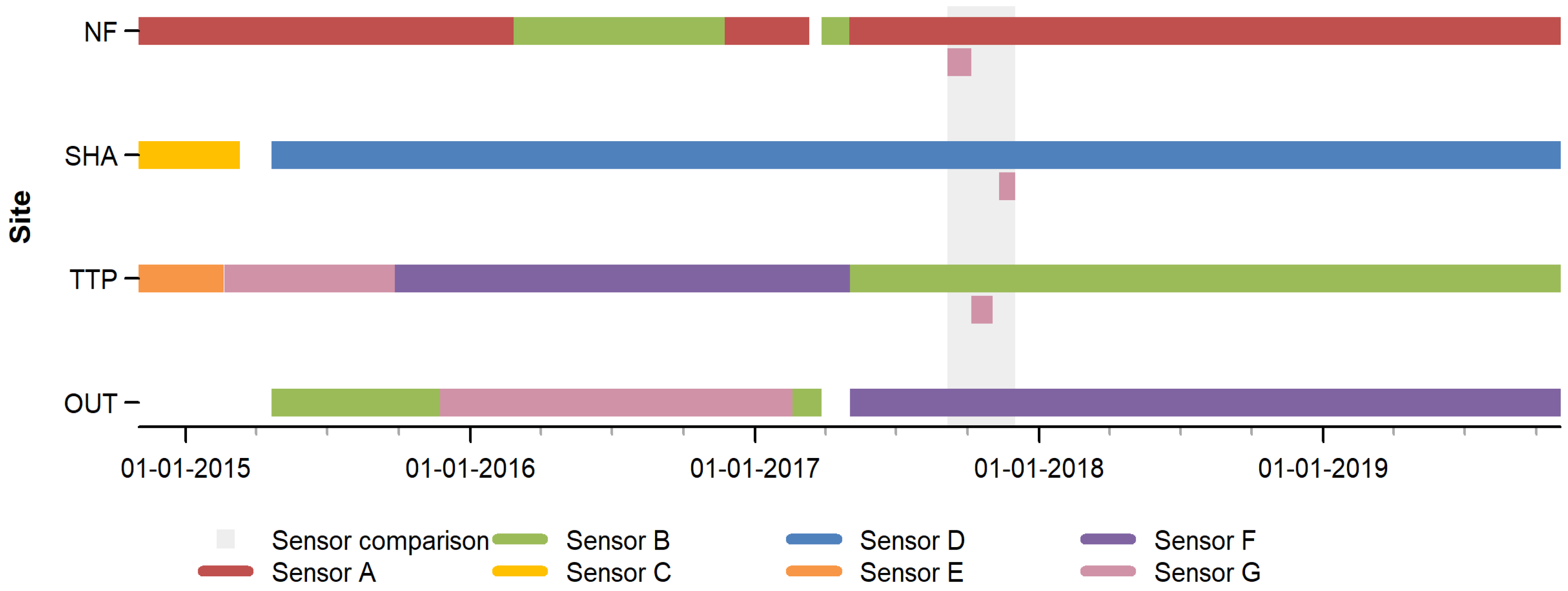
2.3. Experimental Set-Up
2.4. Data Processing
2.5. Data Analysis
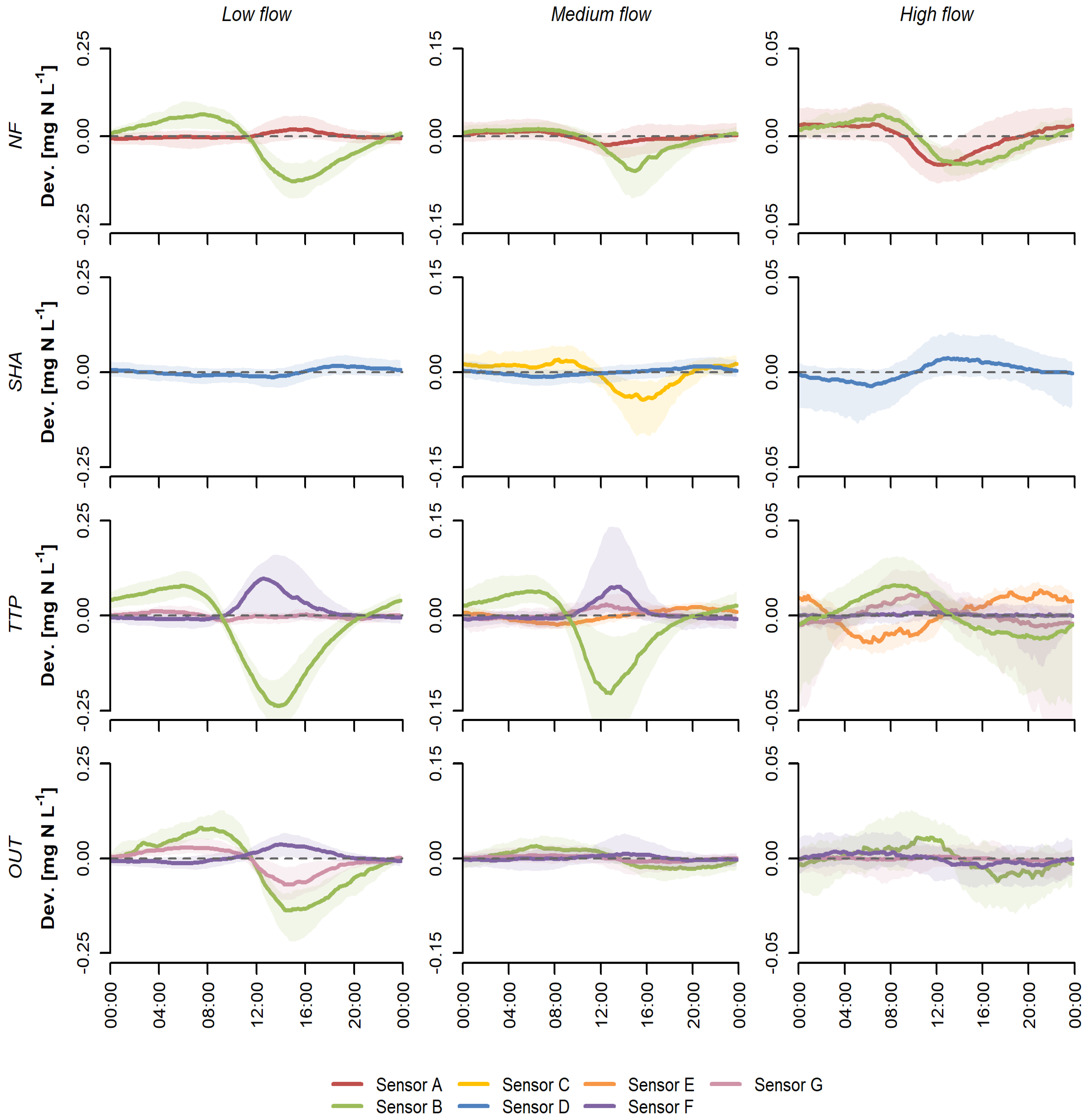
3. Results
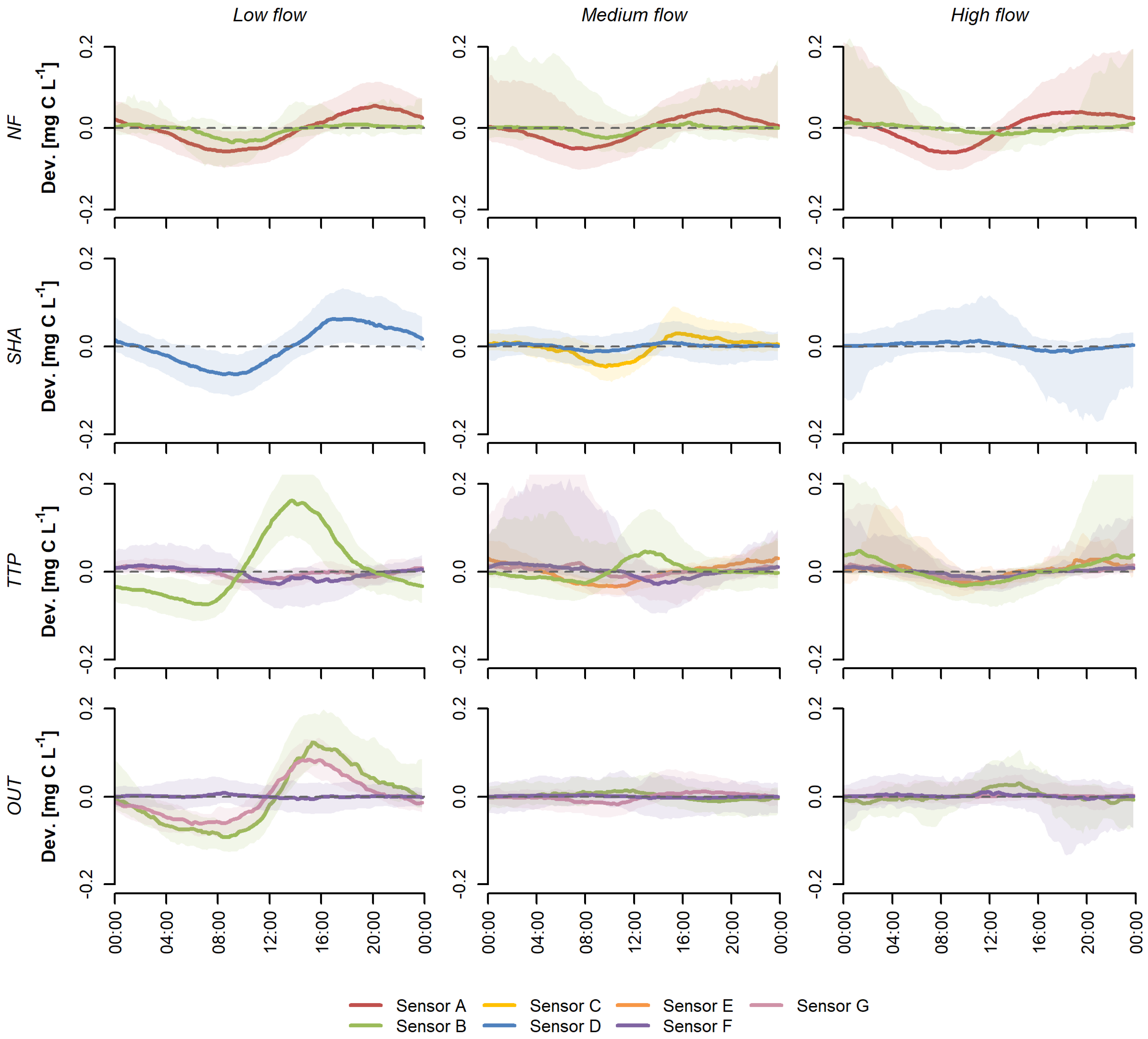
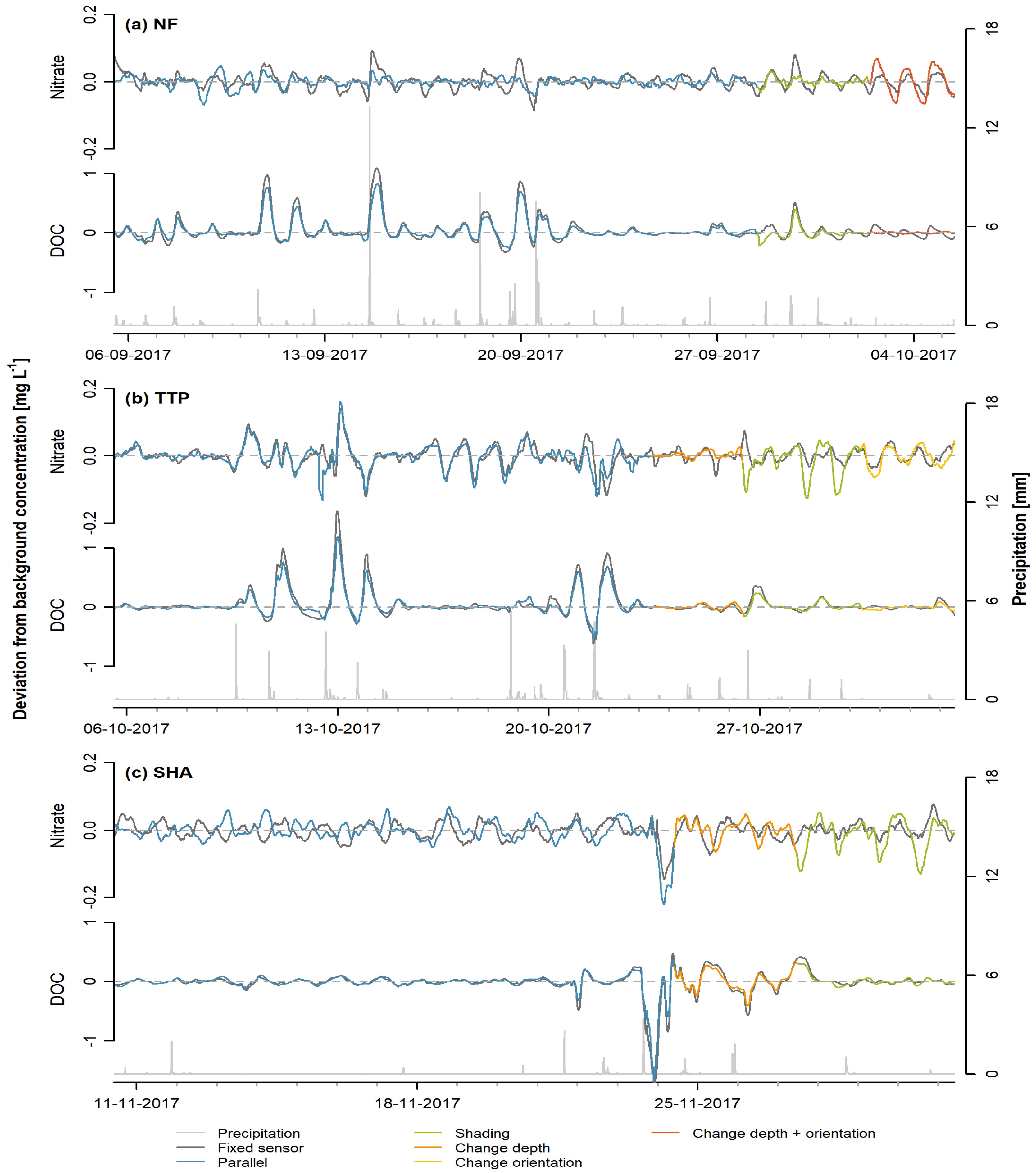
3.1. Sensor-Specific Patterns
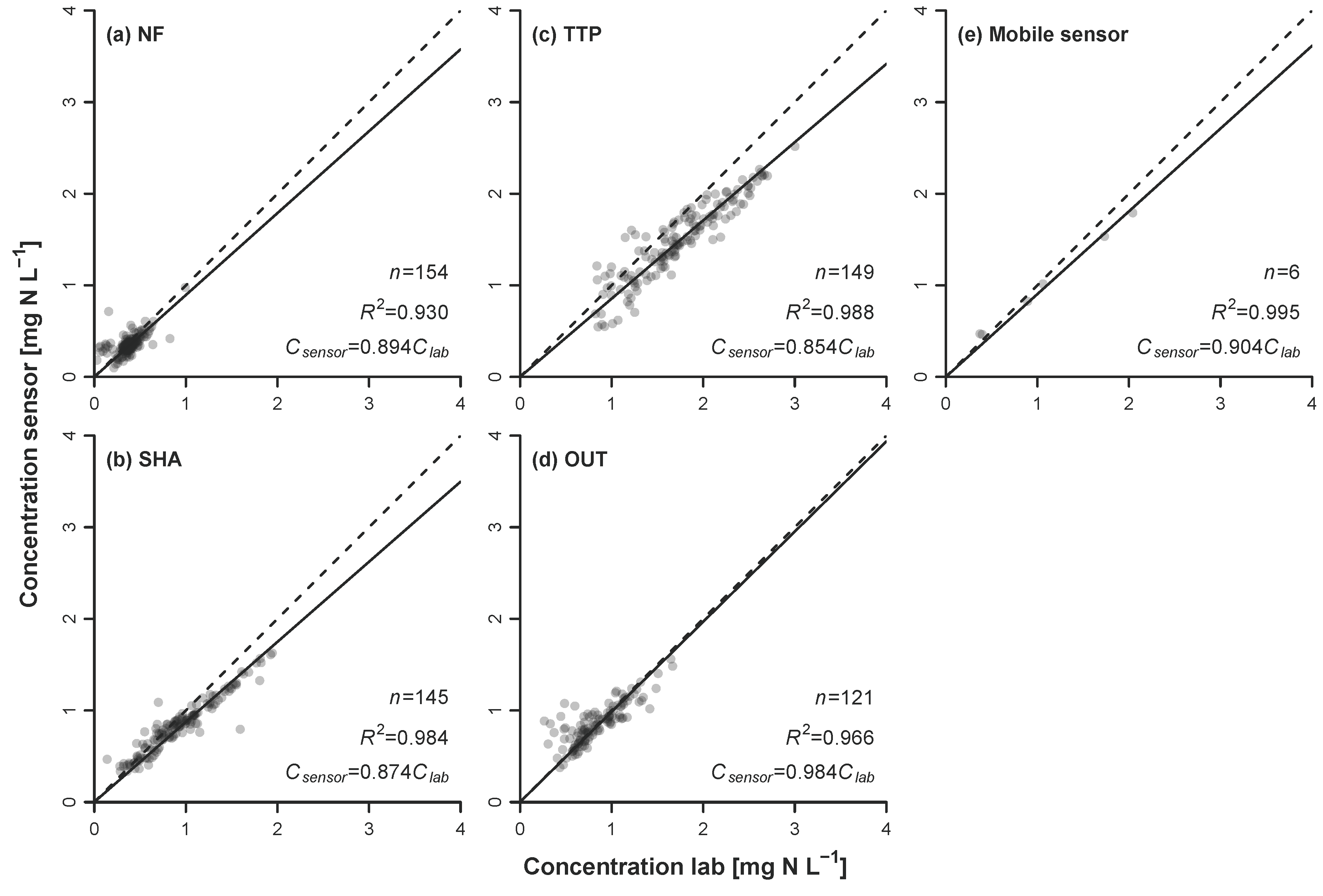
3.2. Sensor Comparison
4. Discussion
4.1. Variations in Diurnal Patterns
4.2. Explanatory Variables
4.3. Implications for the Use of In Situ UV-Vis Sensors
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
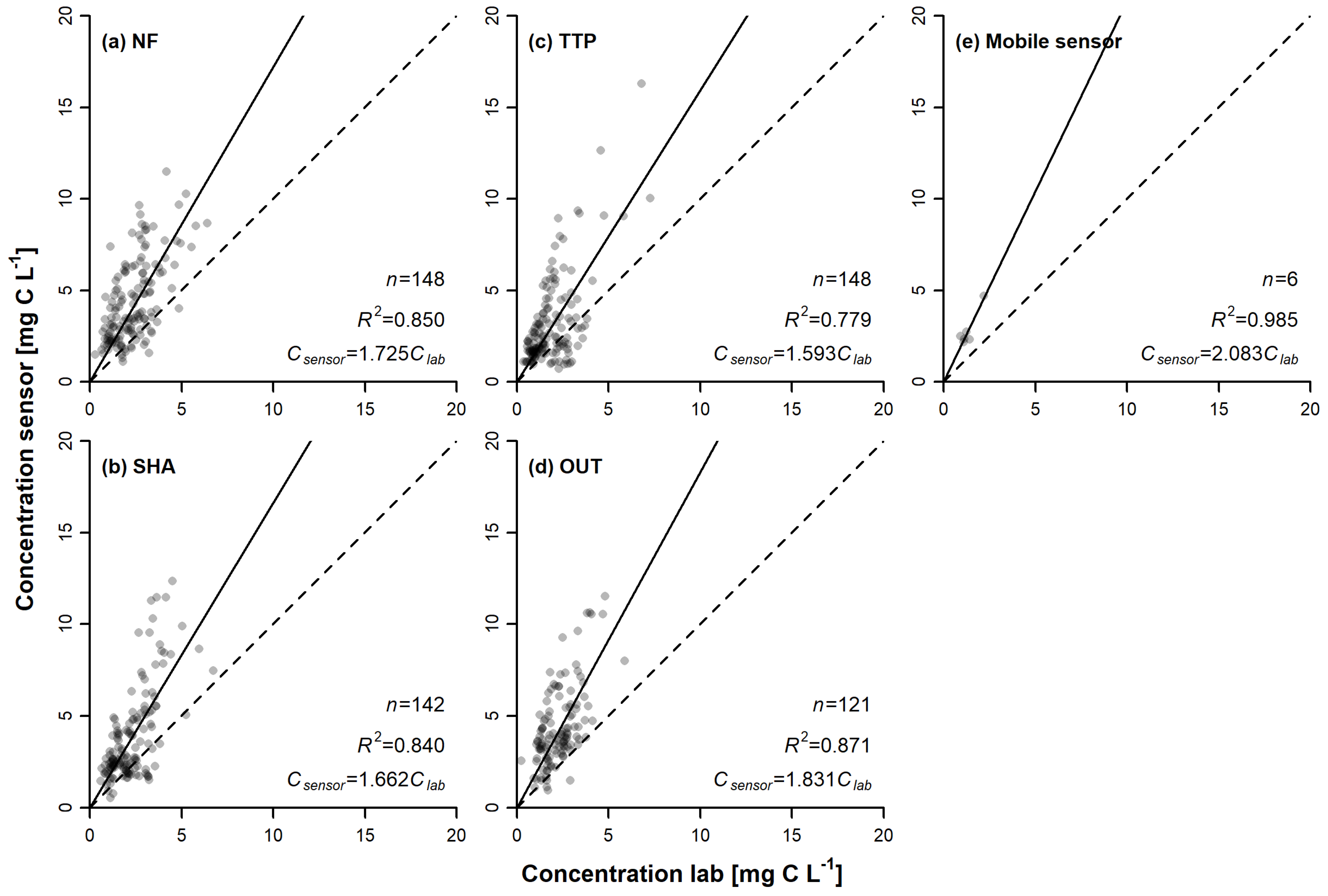
References
- Baulch, H.M.; Dillon, P.J.; Maranger, R.; Schiff, S.L. Diffusive and ebullitive transport of methane and nitrous oxide from streams: Are bubble-mediated fluxes important? J. Geophys. Res. 2011, 116, G04028. [Google Scholar] [CrossRef]
- Cohen, M.J.; Heffernan, J.B.; Albertin, A.; Martin, J.B. Inference of riverine nitrogen processing from longitudinal and diel variation in dual nitrate isotopes. J. Geophys. Res. 2012, 117, G01021. [Google Scholar] [CrossRef]
- Manny, B.A.; Wetzel, R.G. Diurnal changes in dissolved organic and inorganic carbon and nitrogen in a hardwater stream. Freshw. Biol. 1973, 3, 31–43. [Google Scholar] [CrossRef]
- Roberts, B.J.; Mulholland, P.J. In-stream biotic control on nutrient biogeochemistry in a forested stream, West Fork of Walker Branch. J. Geophys. Res. 2007, 112, G04002. [Google Scholar] [CrossRef]
- Viswanathan, V.C.; Molson, J.; Schirmer, M. Does river restoration affect diurnal and seasonal changes to surface water quality? A study along the Thur River, Switzerland. Sci. Total Environ. 2015, 532, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Volkmar, E.C.; Henson, S.S.; Dahlgren, R.A.; O’Green, A.T.; Van Nieuwenhuyse, E.E. Diel patterns of algae and water quality constituents in the San Joaquin River, California, USA. Chem. Geol. 2011, 283, 56–67. [Google Scholar] [CrossRef]
- Blaen, P.J.; Khamis, K.; Lloyd, C.E.M.; Comer-Warner, S.; Ciocca, F.; Thomas, R.M.; MacKenzie, A.R.; Krause, S. High-frequency monitoring of catchment nutrient exports reveals highly variable storm event responses and dynamic source zone activation. J. Geophys. Res. Biogeosci. 2017, 122, 2265–2281. [Google Scholar] [CrossRef]
- Sherson, L.R.; Van Horn, D.J.; Gomez-Velez, J.D.; Crossey, L.J.; Dahm, C.N. Nutrient dynamics in an alpine headwater stream: Use of continuous water quality sensors to examine responses to wildfire and precipitation events. Hydrol. Process. 2015, 29, 3193–3207. [Google Scholar] [CrossRef]
- Jacobs, S.R.; Weeser, B.; Guzha, A.C.; Rufino, M.C.; Butterbach-Bahl, K.; Windhorst, D.; Breuer, L. Using high-resolution data to assess land use impact on nitrate dynamics in East African tropical montane catchments. Water Resour. Res. 2018, 54, 1812–1830. [Google Scholar] [CrossRef]
- Vaughan, M.C.H.; Bowden, W.B.; Shanley, J.B.; Vermilyea, A.; Wemple, B.C.; Schroth, A.W. Using in situ UV-Visible spectrophotometer sensors to quantify riverine phosphorus partitioning and concentration at a high frequency. Limnol. Oceanogr. Methods 2018, 16, 840–855. [Google Scholar] [CrossRef]
- Zimmer, M.A.; Pellerin, B.A.; Burns, D.A.; Petrochenkov, G. Temporal Variability in Nitrate-Discharge Relationships in Large Rivers as Revealed by High-Frequency Data. Water Resour. Res. 2019, 55, 973–989. [Google Scholar] [CrossRef]
- McDowell, W.H. NEON and STREON: Opportunities and challenges for the aquatic sciences. Freshw. Sci. 2015, 34, 386–391. [Google Scholar] [CrossRef]
- Kirchner, J.W.; Feng, X.; Neal, C.; Robson, A.J. The fine structure of water-quality dynamics: The (high-frequency) wave of the future. Hydrol. Process. 2004, 18, 1353–1359. [Google Scholar] [CrossRef]
- Krause, S.; Lewandowski, J.; Dahm, C.N.; Tockner, K. Frontiers in real-time ecohydrology—A paradigm shift in understanding complex environmental systems. Ecohydrology 2015, 8, 529–537. [Google Scholar] [CrossRef]
- Rode, M.; Wade, A.J.; Cohen, M.J.; Hensley, R.T.; Bowes, M.J.; Kirchner, J.W.; Arhonditsis, G.B.; Jordan, P.; Kronvang, B.; Halliday, S.J.; et al. Sensors in the stream: The high-frequency wave of the present. Environ. Sci. Technol. 2016, 50, 10297–10307. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, E.S.; Heffernan, J.B.; Grimm, N.B.; Stanley, E.H.; Harvey, J.W.; Arroita, M.; Appling, A.P.; Cohen, J.; McDowell, W.H.; Hall, R.O., Jr.; et al. The metabolic regimes of flowing waters. Limnol. Oceanogr. 2018, 63, S99–S118. [Google Scholar] [CrossRef]
- Nimick, D.A.; Gammons, C.H.; Parker, S.R. Diel biogeochemical processes and their effect on the aqueous chemistry of streams: A review. Chem. Geol. 2011, 283, 3–17. [Google Scholar] [CrossRef]
- Scholefield, D.; Le Goff, T.; Braven, J.; Ebdon, L.; Long, T.; Butler, M. Concerted diurnal patterns in riverine nutrient concentrations and physical condiditon. Sci. Total Environ. 2005, 344, 201–210. [Google Scholar] [CrossRef]
- Schwab, M.P.; Klaus, J.; Pfister, L.; Weiler, M. Diel fluctuations of viscosity-driven riparian inflow affect streamflow DOC concentration. Biogeosciences 2018, 15, 2177–2188. [Google Scholar] [CrossRef]
- Bende-Michl, U.; Verburg, K.; Cresswell, H.P. High-frequency nutrient monitoring to infer seasonal patterns in catchment source availability, mobilisation and delivery. Environ. Monit. Assess. 2013, 185, 9191–9219. [Google Scholar] [CrossRef]
- Aubert, A.H.; Breuer, L. New seasonal shift in in-stream diurnal nitrate cycles identified by mining high-frequency data. PLoS ONE 2016, 11, e0153138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mulholland, P.J.; Thomas, S.A.; Valett, H.M.; Webster, J.R.; Beaulieu, J. Effects of light on NO3−uptake in small forested streams: Diurnal and day-to-day variations. J. North Am. Benthol. Soc. 2006, 25, 583–595. [Google Scholar] [CrossRef]
- Etheridge, J.R.; Birgand, F.; Burchell, M.R.; Smith, B.T. Addressing the Fouling of In Situ Ultraviolet-Visual Spectrometers Used to Continuously Monitor Water Quality in Brackish Tidal Marsh Waters. J. Environ. Qual. 2013, 42, 1896–1901. [Google Scholar] [CrossRef] [PubMed]
- Avagyan, A.; Runkle, B.R.K.; Kutzbach, L. Application of high-resolution spectral absorbance measurements to determine dissolved organic carbon concentration in remote areas. J. Hydrol. 2014, 517, 435–446. [Google Scholar] [CrossRef]
- Grayson, R.P.; Holden, J. Improved automation of dissolved organic carbon sampling for organic-rich surface waters. Sci. Total Environ. 2016, 543, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Ruhala, S.S.; Zarnetske, J.P. Using in-situ optical sensors to study dissolved organic carbon dynamics of streams and watersheds: A review. Sci. Total Environ. 2017, 575, 713–723. [Google Scholar] [CrossRef]
- Bieroza, M.Z.; Heathwaite, A.L. Unravelling organic matter and nutrient biogeochemistry in groundwater-fed rivers under baseflow conditions: Uncertainty in in situ high-frequency analysis. Sci. Total Environ. 2016, 572, 1520–1533. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Bartsch, S.; Fleckenstein, J.H.; Matzner, E.; Tenhunen, J.D.; Lee, S.D.; Park, S.K.; Park, J.-H. Differential storm responses of dissolved and particulate organic carbon in a mountainous headwater stream, investigated by high-frequency, in situ optical measurements. J. Geophys. Res. 2012, 117, G03013. [Google Scholar] [CrossRef]
- Pellerin, B.A.; Downing, B.D.; Kendall, C.; Dahlgreen, R.A.; Kraus, T.E.C.; Saraceno, J.; Spencer, R.G.M.; Bergamaschi, B.A. Assessing the sources and magnitude of diurnal nitrate variability in the San Joaquin River (California) with an in situ optical nitrate sensor and dual nitrate isotopes. Freshw. Biol. 2009, 54, 376–387. [Google Scholar] [CrossRef]
- Sandford, R.C.; Exenberger, A.; Worsfold, P.J. Nitrogen cycling in natural waters using in situ, reagentless UV spectrophotometry with simultaneous determination of nitrate and nitrite. Environ. Sci. Technol. 2007, 41, 8420–8425. [Google Scholar] [CrossRef]
- Wade, A.J.; Palmer-Felgate, E.J.; Halliday, S.J.; Skeffington, R.A.; Loewenthal, M.; Jarvie, H.P.; Bowes, M.J.; Greenway, G.M.; Haswell, S.J.; Bell, I.M.; et al. Hydrochemical processes in lowland rivers: Insights from in situ, high-resolution monitoring. Hydrol. Earth Syst. Sci. 2012, 16, 4323–4342. [Google Scholar] [CrossRef]
- ISRIC Soil and Terrain Database for Kenya, version 2.0, at scale 1:1 million (KENSOTER); ISRIC: Wageningen, The Netherlands, 2007.
- Jennings, D.J. Geology of the Molo Area; Ministry of Natural Resources, Geological Survey of Kenya: Nairobi, Kenya, 1971. [Google Scholar]
- Binge, F.W. Geology of the Kericho Area; Ministry of Commerce, Industry and Communications, Geological Survey of Kenya: Nairobi, Kenya, 1962. [Google Scholar]
- Jacobs, S.R.; Breuer, L.; Butterbach-Bahl, K.; Pelster, D.E.; Rufino, M.C. Land use affects total dissolved nitrogen and nitrate concentrations in tropical montane streams in Kenya. Sci. Total Environ. 2017, 603–604, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Leys, C.; Ley, C.; Klein, O.; Bernard, P.; Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviations around the median. J. Exp. Soc. Psychol. 2013, 49, 764–766. [Google Scholar] [CrossRef]
- Jacobs, S.; Weeser, B.; Rufino, M.; Breuer, L. Dataset for: Diurnal patterns in solute concentrations measured with in situ UV-Vis sensors: Natural fluctuations or artefacts? Available online: https://zenodo.org/record/3593769#.XjlS8fx7mUk (accessed on 29 December 2019). [CrossRef]
- Burns, D.A.; Miller, M.P.; Pellerin, B.A.; Capel, P.D. Patterns of diel variation in nitrate concentrations in the Potomac River. Freshw. Sci. 2016, 35, 1117–1132. [Google Scholar] [CrossRef]
- Lupon, A.; Martí, E.; Sabater, F.; Bernal, S. Green light: Gross primary production influences seasonal stream N export by controlling fine-scale N dynamics. Ecology 2016, 97, 133–144. [Google Scholar] [CrossRef]
- Moraetis, D.; Efstathiou, D.; Stamati, F.; Tzoraki, O.; Nikolaidis, N.P.; Schnoor, J.L.; Vozinakis, K. High-frequency monitoring for the identification of hydrological and bio-geochemical processes in a Mediterranean river basin. J. Hydrol. 2010, 389, 127–136. [Google Scholar] [CrossRef]
- Baulch, H.M.; Dillon, P.J.; Maranger, R.; Venkiteswaran, J.J.; Wilson, H.F.; Schiff, S.L. Night and day: Short-term variation in nitrogen chemistry and nitrous oxide emissions from streams. Freshw. Biol. 2012, 57, 509–525. [Google Scholar] [CrossRef]
- Hensley, R.T.; Cohen, M.J. On the emergence of diel solute signals in flowing waters. Water Resour. Res. 2016, 52, 759–772. [Google Scholar] [CrossRef]
- Spencer, R.G.M.; Pellerin, B.A.; Bergamaschi, B.A.; Downing, B.D.; Kraus, T.E.C.; Smart, D.R.; Dahlgren, R.A.; Hernes, P.J. Diurnal variability in riverine dissolved organic matter composition determined by in situ optical measurement in the San Joaquin River (California, USA). Hydrol. Process. 2007, 21, 3181–3189. [Google Scholar] [CrossRef]
- Duan, S.; Powell, R.T.; Bianchi, T.S. High frequency measurement of nitrate concentration in the Lower Mississippi River, USA. J. Hydrol. 2014, 519 Pt A, 376–386. [Google Scholar] [CrossRef]
- Huebsch, M.; Grimmeisen, F.; Zemann, M.; Fenton, O.; Richards, K.G.; Jordan, P.; Sawarieh, A.; Blum, P.; Goldscheider, N. Technical Note: Field experiences using UV/VIS sensors for high-resolution monitoring of nitrate in groundwater. Hydrol. Earth Syst. Sci. 2015, 19, 1589–1598. [Google Scholar] [CrossRef]
- Jones, T.D.; Chappell, N.A.; Tych, W. First Dynamic Model of Dissolved Organic Carbon Derived Directly from High-Frequency Observations through Contiguous Storms. Environ. Sci. Technol. 2014, 48, 13289–13297. [Google Scholar] [CrossRef] [PubMed]
- Jollymore, A.; Johnson, M.S.; Hawthorne, I. Submersible UV-Vis spectroscopy for quantifying streamwater organic carbon dynamics: Implementation and challenges before and after forest harvest in a headwater stream. Sensors 2012, 12, 3798–3813. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.S.; Kim, S.; Wilton, T.F.; Schilling, K.E.; Davis, C.A. Nitrate uptake in an agricultural stream estimated from high-frequency, in-situ sensors. Environ. Monit. Assess. 2018, 190, 226. [Google Scholar] [CrossRef]
- Bende-Michl, U.; Hairsine, P.B. A systematic approach to choosing an automated nutrient analyser for river monitoring. J. Environ. Monit. 2010, 12, 127–134. [Google Scholar] [CrossRef]
- Etheridge, J.R.; Birgand, F.; Osborne, J.A.; Osburn, C.L.; Burchell, M.R.; Irving, J. Using in situ ultraviolet-visual spectroscopy to measure nitrogen, carbon, phosphorus, and suspended solids concentrations at a high frequency in a brackish tidal marsh. Limnol. Oceanogr. Methods 2014, 12, 10–22. [Google Scholar] [CrossRef]
- Pellerin, B.A.; Saraceno, J.F.; Shanley, J.B.; Sebestyen, S.D.; Aiken, G.R.; Wollheim, W.M.; Bergamaschi, B.A. Taking the pulse of snowmelt: In situ sensors reveal seasonal, event and diurnal patterns of nitrate and dissolved organic matter variability in an upland forest stream. Biogeochemistry 2012, 108, 183–198. [Google Scholar] [CrossRef]
- Lee, E.-J.; Yoo, G.-Y.; Jeong, Y.; Kim, K.-U.; Park, J.-H.; Oh, N.-H. Comparison of UV–VIS and FDOM sensors for in situ monitoring of stream DOC concentrations. Biogeosciences 2015, 12, 3109–3118. [Google Scholar] [CrossRef]
- Koehler, A.-K.; Murphy, K.; Kiely, G.; Sottocornola, M. Seasonal variation of DOC concentration and annual loss of DOC from an Atlantic blanket bog in South Western Ireland. Biogeochemistry 2009, 95, 231–242. [Google Scholar] [CrossRef]
- Kämäri, M.; Tattari, S.; Lotsari, E.; Koskiaho, J.; Lloyd, C.E.M. High-frequency monitoring reveals seasonal and event-scale water quality variation in a temporally frozen river. J. Hydrol. 2018, 564, 619–639. [Google Scholar] [CrossRef]
- Waterloo, M.J.; Oliveira, S.M.; Drucker, D.P.; Nobre, A.D.; Cuartas, L.A.; Hodnett, M.G.; Langedijk, I.; Jans, W.W.P.; Tomasella, J.; de Araújo, A.C.; et al. Export of organic carbon in run-off from an Amazonian rainforest blackwater catchment. Hydrol. Process. 2006, 20, 2581–2597. [Google Scholar] [CrossRef]
- Lloyd, C.E.M.; Freer, J.E.; Johnes, P.J.; Collins, A.L. Using hysteresis analysis of high-resolution water quality monitoring data, including uncertainty, to infer controls on nutrient and sediment transfer in catchments. Sci. Total Environ. 2016, 543, 388–404. [Google Scholar] [CrossRef] [PubMed]
| Site | Land Use | Coordinates 1 | Area [km2] | Elevation [m a.s.l.] | Mean Annual Precipitation [mm y−1] | Median (min., max.) Discharge [m3 s−1] |
|---|---|---|---|---|---|---|
| NF | Natural forest | 0°27′47.591″ S, 35°18′32.046″ E | 35.9 | 1954–2385 | 1894 | 0.52 (0.082, 5.79) |
| SHA | Smallholder agriculture | 0°24′4.024″ S, 35°28′31.733″ E | 27.2 | 2380–2691 | 1568 | 0.22 (0.014, 3.55) |
| TTP | Tea and tree plantations | 0°28′34.917″ S, 35°13′17.220″ E | 33.3 | 1786–2141 | 1810 | 0.37 (0.056, 3.82) |
| OUT | Mixed | 0°28′59.548″ S, 35°10′54.557″ E | 1021.3 | 1715–2932 | 1769 | 12.1 (1.36, 66.6) |
| Site | Start Time | End Time | Treatment |
|---|---|---|---|
| NF | 05-09-2017 11:20 | 28-09-2017 10:00 | Parallel |
| 28-09-2017 11:10 | 02-10-2017 08:40 | Shading | |
| 02-10-2017 09:20 | 05-10-2017 11:00 | Change depth + orientation | |
| TTP | 05-10-2017 13:30 | 23-10-2017 09:30 | Parallel |
| 23-10-2017 09:40 | 26-10-2017 10:00 | Change depth | |
| 26-10-2017 10:20 | 30-10-2017 08:50 | Shading | |
| 30-10-2017 09:10 | 02-11-2017 11:20 | Change orientation | |
| SHA | 10-11-2017 10:10 | 24-11-2017 09:40 | Parallel |
| 24-11-2017 10:00 | 27-11-2017 11:00 | Change depth | |
| 27-11-2017 11:10 | 01-12-2017 10:00 | Shading |
| Site | Q70 [m3 s−1] | Q30 [m3 s−1] | Nitrate [mg N L−1] | Dissolved Organic Carbon [mg C L−1] | ||||
|---|---|---|---|---|---|---|---|---|
| Low Flow | Medium Flow | High Flow | Low Flow | Medium Flow | High Flow | |||
| NF | 0.32 | 0.84 | 0.36 ± 0.13 | 0.42 ± 0.08 | 0.42 ± 0.09 | 2.58 ± 1.19 | 2.86 ± 1.45 | 2.93 ± 1.26 |
| SHA | 0.08 | 0.46 | 0.52 ± 0.12 | 0.89 ± 0.16 | 1.30 ± 0.26 | 4.56 ± 1.70 | 2.18 ± 0.92 | 1.39 ± 0.36 |
| TTP | 0.24 | 0.64 | 1.17 ± 0.28 | 1.74 ± 0.21 | 2.21 ± 0.29 | 3.16 ± 1.60 | 1.80 ± 0.96 | 1.29 ± 0.75 |
| OUT | 6.41 | 19.7 | 0.65 ± 0.19 | 0.84 ± 0.19 | 0.99 ± 0.19 | 3.51 ± 1.31 | 2.32 ± 1.10 | 2.02 ± 0.65 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, S.R.; Weeser, B.; Rufino, M.C.; Breuer, L. Diurnal Patterns in Solute Concentrations Measured with In Situ UV-Vis Sensors: Natural Fluctuations or Artefacts? Sensors 2020, 20, 859. https://doi.org/10.3390/s20030859
Jacobs SR, Weeser B, Rufino MC, Breuer L. Diurnal Patterns in Solute Concentrations Measured with In Situ UV-Vis Sensors: Natural Fluctuations or Artefacts? Sensors. 2020; 20(3):859. https://doi.org/10.3390/s20030859
Chicago/Turabian StyleJacobs, Suzanne R., Björn Weeser, Mariana C. Rufino, and Lutz Breuer. 2020. "Diurnal Patterns in Solute Concentrations Measured with In Situ UV-Vis Sensors: Natural Fluctuations or Artefacts?" Sensors 20, no. 3: 859. https://doi.org/10.3390/s20030859
APA StyleJacobs, S. R., Weeser, B., Rufino, M. C., & Breuer, L. (2020). Diurnal Patterns in Solute Concentrations Measured with In Situ UV-Vis Sensors: Natural Fluctuations or Artefacts? Sensors, 20(3), 859. https://doi.org/10.3390/s20030859






