Silicon Microcantilever Sensors to Detect the Reversible Conformational Change of a Molecular Switch, Spiropyan
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Spiropyran Dithiolane Derivative Synthesis
2.3. UV-Vis Studies
2.4. Microcantilever Functionalization
2.5. Microcantilever Deflection Measurements
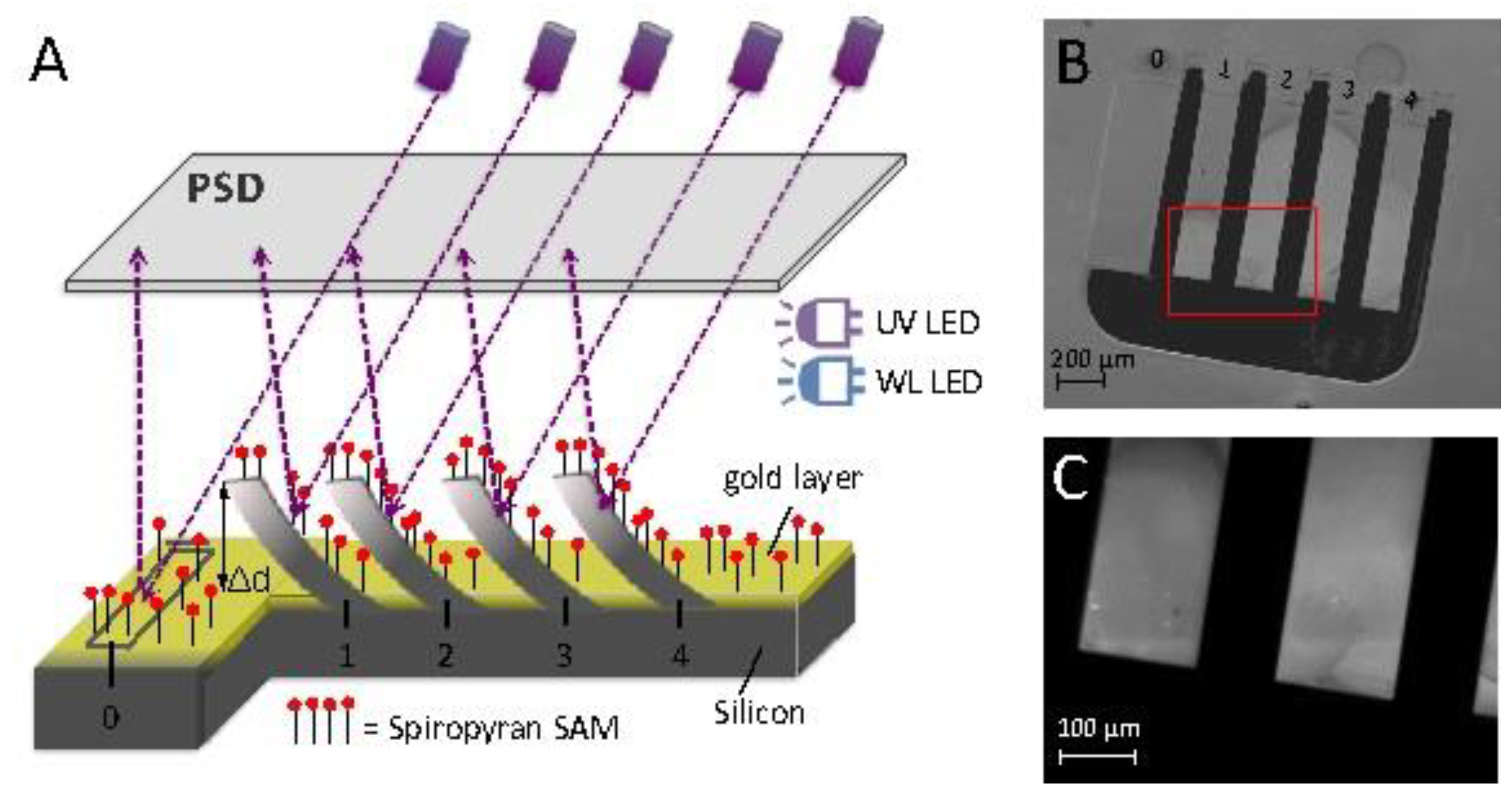
2.6. Detection of Surface Stress
3. Results and Discussion
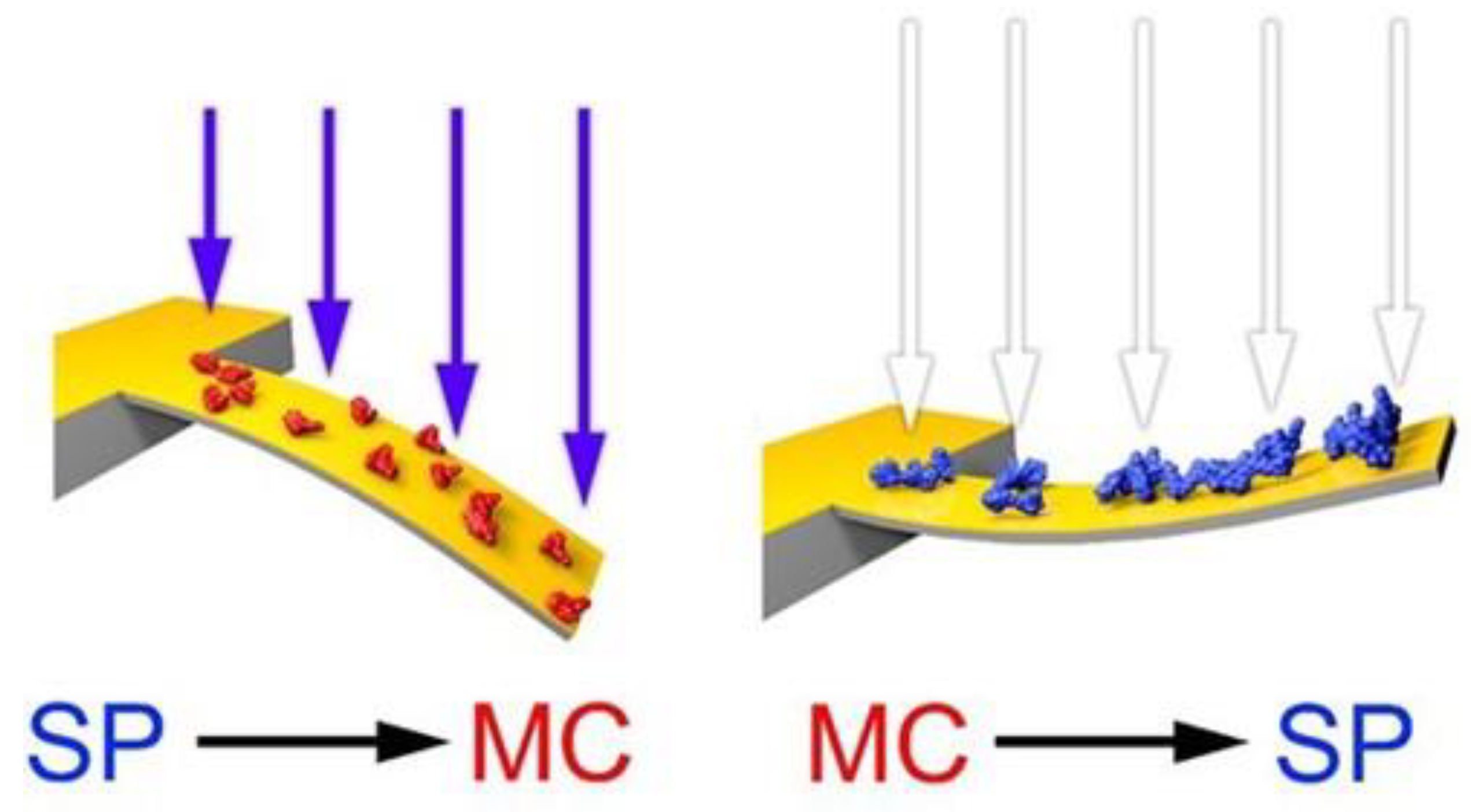
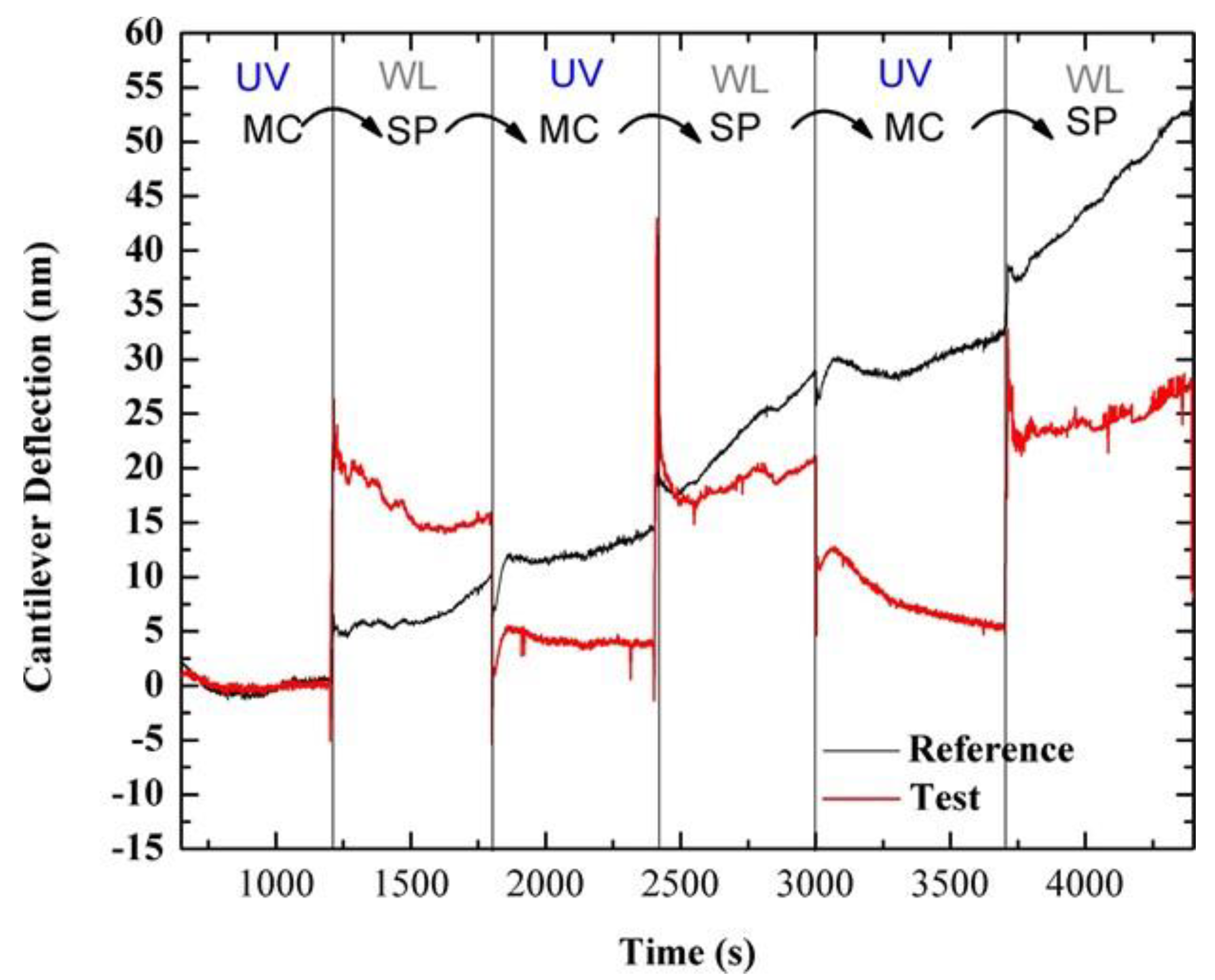
3.1. Photo-Induced Microcantilever Deflections
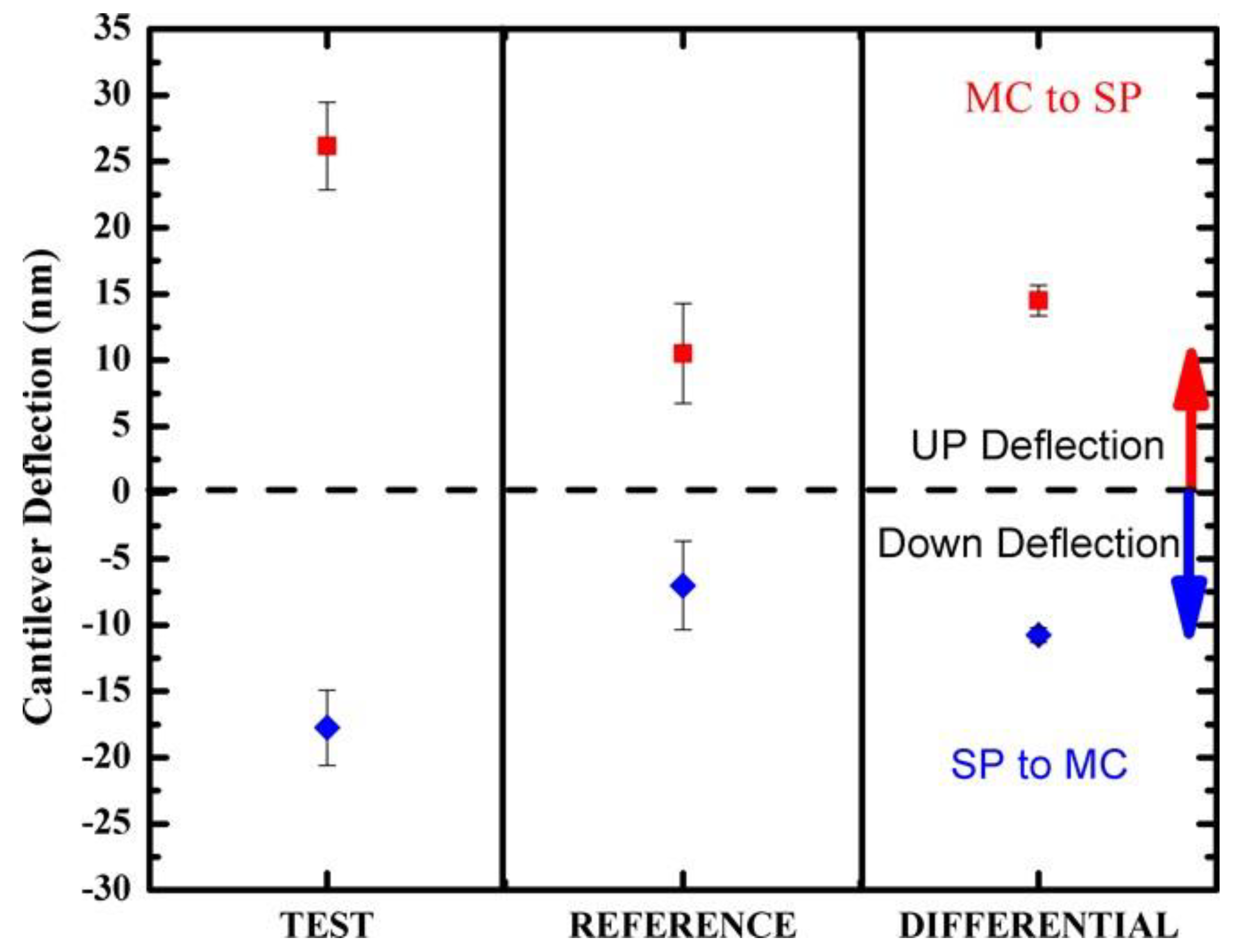
3.2. Photo-Induced Surface Stress
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carrascosa, L.G.; Moreno, M.; Álvarez, M.; Lechuga, L.M. Nanomechanical biosensors: A new sensing tool. TrAC Trends Anal. Chem. 2006, 25, 196–206. [Google Scholar] [CrossRef]
- Boisen, A.A.; Dohn, S.; Keller, S.S.; Schmid, S.; Tenje, M. Cantilever-like micromechanical sensors. Reports Prog. Phys. 2012, 74, 36101. [Google Scholar] [CrossRef]
- Lang, H.P.; Hegner, M.; Gerber, C. Nanomechanical Cantilever Array Sensors. In Springer Handbook of Nanotechnology; Bhushan, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 427–452. [Google Scholar]
- Fritz, J. Cantilever biosensors. Analyst 2008, 133, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, J.T.; Putrino, G.; Jeffery, R.D.; Silva, K.K.M.B.D.; Martyniuk, M.; Keating, A.; Faraone, L. MEMS based hydrogen sensing with parts-per-billion resolution. Sens. Actuators B Chem. 2019, 281, 335–342. [Google Scholar] [CrossRef]
- Thundat, T.; Warmack, R.; Chen, G.; Allison, D. Thermal and ambient induced deflections of scanning force microscope cantilevers. Appl. Phys. Lett. 1994, 64, 2894–2896. [Google Scholar] [CrossRef]
- Lang, H.P.; Ballerab, M.K.; Berger, R.; Gerber, C.; Gimzewski, J.K.; Battiston, F.M.; Fornaro, P.; Ramseyer, J.P.; Meyer, E.; Güntherod, H.J. An artificial nose based on a micromechanical cantilever array. Anal. Chim. Acta 1999, 393, 59–65. [Google Scholar] [CrossRef]
- Dammer, U.; Hegner, M.; Anselmetti, D.; Wagner, P.; Dreier, M.; Huber, W.; Güntherodt, H.J. Specific antigen/antibody interactions measured by force microscopy. Biophys. J. 1996, 70, 2437–2441. [Google Scholar] [CrossRef]
- Zhang, N.; Tan, Z.; Li, J.; Meng, W.; Xu, L.; Zhang, N.H.; Tan, Z.Q.; Li, J.J.; Meng, W.L.; Xu, L.W. Interactions of Single stranded DNA on microcantielvers. Curr. Opin. Colloid Interfacr. Sci. 2011, 16, 592–596. [Google Scholar] [CrossRef]
- Huber, F.; Lang, H.P.; Backmann, N.; Rimoldi, D.; Gerber, C. Direct detection of a BRAF mutation in total RNA from melanoma cells using cantilever arrays. Nat. Nanotechnol. 2013, 8, 125–129. [Google Scholar] [CrossRef]
- Jensen, J.; Maloney, N.; Hegner, M. A multi-mode platform for cantilever arrays operated in liquid. Sens. Actuators B Chem. 2013, 183, 388–394. [Google Scholar] [CrossRef]
- Bache, M.; Taboryski, R.; Schmid, S.; Aamand, J.; Jakobse, M.J. Investigations on antibody binding to a micro-cantilever coated with a BAM pesticide residue. Nanoscale Res. Lett. 2011, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Xu, P.; Cai, S.; Yu, H.; Li, X. Detection of volatile-organic-compounds (VOCs) in solution using cantilever-based gas sensors. Talanta 2018, 182, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Hegner, M.; Gerber, C.; Arntz, Y.; Zhang, J.; Bertoncinis, P.; Husale, S.; Lang, H.P.; Grange, W. Chapter 11–Biological Single Molecule Applications and Advanced Biosensing. J. Chromatogr. Libr. 2003, 68, 241–263. [Google Scholar]
- Chen, T.; Chang, D.; Liu, T.; Desikan, R.; Datar, R.; Thundat, T.; Berger, R.; Zauscher, S. Glucose-responsive polymer brushes for microcantilever sensing. J. Mater. Chem. 2010, 20, 3391–3395. [Google Scholar] [CrossRef]
- Le, X.; Peng, L.; Pang, J.; Xu, Z.; Gao, C.; Xie, J. Humidity sensors based on AlN microcantilevers excited at high-order resonant modes and sensing layers of uniform graphene oxide. Sens. Actuators B Chem. 2019, 283, 198–206. [Google Scholar] [CrossRef]
- Mala Serene, I.; RajasekharaBabu, M.; Alex, Z.C. A study and analysis of Microcantilever materials for disease detection. Mater. Today Proc. 2018, 5, 1219–1225. [Google Scholar] [CrossRef]
- Chen, C.H.; Hwang, R.Z.; Huang, L.S.; Lin, S.M.; Chen, H.C.; Yang, Y.C.; Lin, Y.T.; Yu, S.A.; Lin, Y.S.; Wang, Y.H.; et al. A Wireless Bio-MEMS Sensor for C-Reactive Protein Detection Based on Nanomechanics. IEEE Trans. Biomed. Eng. 2009, 56, 462–470. [Google Scholar] [CrossRef]
- Kim, J.Y.; Oyunbaatar, N.-E.; Lee, D.-W. Fully automated high-throughput cardiac toxicity screening platform using interlocking-structured 192 SU-8 cantilever arrays. Sens. Actuators B Chem. 2019, 285, 129–136. [Google Scholar] [CrossRef]
- Vashist, S.K.; Holthöfer, H. Microcantilevers for Sensing Applications. Meas. Control. 2010, 43, 84–88. [Google Scholar] [CrossRef]
- Ma, R.-H.; Ho, M.-C.; Lee, C.-Y.; Wang, Y.-H.; Fu, L.-M. Micromachined silicon cantilever paddle for high-flow-rate sensing. Sensors Mater. 2006, 18, 405–417. [Google Scholar]
- Zhao, L.; Huang, L.; Luo, G.; Wang, J.; Wang, H.; Wu, Y.; Li, Z.; Zhou, X.; Jiang, Z. An immersive resonant sensor with microcantilever for pressure measurement. Sens. Actuators A Phys. 2019, 111686. [Google Scholar] [CrossRef]
- Florea, L.; Diamond, D.; Benito-Lopez, F. Photo-Responsive Polymeric Structures Based on Spiropyran. Macromol. Mater. Eng. 2012, 297, 1148–1159. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Huang, L.; O’Brien, S.; Kim, P.; Nuckolls, C. Directing and Sensing Changes in Molecular Conformation on Individual Carbon Nanotube Field Effect Transistors. J. Phys. Chem. C 2005, 127, 15045–15047. [Google Scholar] [CrossRef] [PubMed]
- Levitus, M.; Glasser, G.; Neher, D.; Aramendía, P.F. Direct measurement of the dipole moment of a metastable merocyanine by electromechanical interferometry. Chem. Phys. Lett. 1997, 277, 118–124. [Google Scholar]
- Bletz, M.; Pfeifer-Fukumura, U.; Kolb, U.; Baumann, W. Ground- and First-Excited-Singlet-State Electric Dipole Moments of Some Photochromic Spirobenzopyrans in Their Spiropyran and Merocyanine Form. J. Phys. Chem. A 2002, 106, 2232–2236. [Google Scholar] [CrossRef]
- Gruler, H.; Vilanove, R.; Rondelez, F. Reversible photochemical strain in langmuir monolayers. Phys. Rev. Lett. 1980, 44, 590. [Google Scholar] [CrossRef]
- Gruler, H.; Vilanove, R.; Rondelez, F. Photochromism of monolayers of poly(methylmethacrylate) having spirobenzopyran side groups. Macromolecules 1983, 16, 825–831. [Google Scholar]
- Panaiotov, I.; Taneva, S.; Bois, A.; Rondelez, F. Photoinduced dilatational motion in monolayers of poly(methyl methacrylate) having benzospiropyran side groups. Macromolecules 1991, 24, 4250–4254. [Google Scholar] [CrossRef]
- Florea, L.; McKeon, A.; Diamond, D.; Benito-Lopez, F. Spiropyran polymeric microcapillary coatings for photodetection of solvent polarity. Langmuir 2013, 29, 2790–2797. [Google Scholar] [CrossRef][Green Version]
- Francis, W.; Dunne, A.; Delaney, C.; Florea, L.; Diamond, D. Spiropyran based hydrogels actuators—Walking in the light. Sens. Actuators B Chem. 2017, 250, 608–616. [Google Scholar] [CrossRef]
- Stumpel, J.E.; Ziółkowski, B.; Florea, L.; Diamond, D.; Broer, D.J.; Schenning, A.P.H.J. Photoswitchable ratchet surface topographies based on self-protonating spiropyran-NIPAAM hydrogels. ACS Appl. Mater. Interfaces 2014, 6, 7268–7274. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowski, B.; Florea, L.; Theobald, J.; Benito-Lopez, F.; Diamond, D. Self-protonating spiropyran-co-NIPAM-co-acrylic acid hydrogel photoactuators. Soft Matter 2013, 9, 8754–8760. [Google Scholar] [CrossRef]
- Rosario, R.; Gust, D.; Hayes, M.; Jahnke, F.; Springer, J.; Garcia, A.A. Photon-modulated wettability changes on spiropyran coated surfaces. Langmuir 2002, 18, 8062–8069. [Google Scholar] [CrossRef]
- Benito-Lopez, F.; Scarmagnani, S.; Walsh, Z.; Paull, B.; Macka, M.; Diamond, D. Spiropyran modified micro-fluidic chip channels as photonically controlled self-indicating system for metal ion accumulation and release. Sens. Actuators B Chem. 2009, 140, 295–303. [Google Scholar] [CrossRef]
- Fries, K.H.; Driskell, J.D.; Sheppard, G.R.; Locklin, J. Fabrication of spiropyran-containing thin film sensors used for the simultaneous identification of multiple metal ions. Langmuir 2011, 27, 12253–12260. [Google Scholar] [CrossRef]
- Natali, M.; Giordani, S. Interaction studies between photochromic spiropyrans and transition metal cations: The curious case of copper. Org. Biomol. Chem. 2012, 10, 1162–1171. [Google Scholar] [CrossRef]
- Dunne, A.; Delaney, C.; McKeon, A.; Nesterenko, P.; Paull, B.; Benito-Lopez, F.; Diamond, D.; Florea, L. Micro-Capillary Coatings Based on Spiropyran Polymeric Brushes for Metal Ion Binding, Detection, and Release in Continuous Flow. Sensors 2018, 18, 1083. [Google Scholar] [CrossRef]
- Gelmi, A.; Zanoni, M.; Higgins, M.J.; Gambhir, S.; Officer, D.L.; Diamond, D.; Wallace, G.G. Optical switching of protein interactions on photosensitive–electroactive polymers measured by atomic force microscopy. J. Mater. Chem. B 2013, 1, 2162–2168. [Google Scholar] [CrossRef]
- Chung, D.-J.; Ito, Y.; Imanishi, Y. Preparation of porous membranes grafted with poly(spiropyran-containing methacrylate) and photocontrol of permeability. J. Appl. Polym. Sci. 2018, 51, 2027–2033. [Google Scholar] [CrossRef]
- Davis, D.A.; Hamilton, A.; Yang, J.; Cremar, L.D.; Van Gough, D.; Potisek, S.L.; Ong, M.T.; Braun, P.V.; Martínez, T.J.; White, S.R.; et al. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature 2009, 459, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Potisek, S.L.; Davis, D.A.; Sottos, N.R.; White, S.R.; Moore, J.S. Mechanophore-Linked Addition Polymers. J. Am. Chem. Soc. 2007, 129, 13808–13809. [Google Scholar] [CrossRef] [PubMed]
- Grogan, C.; Florea, L.; Koprivica, S.; Scarmagnani, S.; O’Neill, L.; Lyng, F.; Pedreschi, F.; Benito-Lopez, F.; Raiteri, R. Microcantilever arrays functionalised with spiropyran photoactive moieties as systems to measure photo-induced surface stress changes. Sens. Actuators B Chem. 2016, 237, 479–486. [Google Scholar] [CrossRef]
- Lotze, C.; Luo, Y.; Corso, M.; Franke, K.J.; Haag, R.; Pascual, J.I. Reversible electron-induced cis–trans isomerization mediated by intermolecular interactions. J. Phys. Condens. Matter. 2012, 24, 394016. [Google Scholar] [CrossRef]
- Ivashenko, O.; van Herpt, J.T.; Feringa, B.L.; Rudolf, P.; Browne, W.R. UV/Vis and NIR Light-Responsive Spiropyran Self-Assembled Monolayers. Langmuir 2013, 29, 4290–4297. [Google Scholar] [CrossRef]
- Zhang, R.; Best, A.; Berger, R.; Cherian, S.; Lorenzoni, S.; Macis, E. Multiwell micromechanical cantilever array reader for biotechnology. Rev. Sci. Instrum. 2007, 78, 084103. [Google Scholar] [CrossRef]
- Braun, T.; Huber, F.; Ghatkesar, M.K.; Backmann, N.; Lang, H.P.; Gerber, C.; Hegner, M. Processing of kinetic microarray signals. Sens. Actuators B Chem. 2007, 128, 75–82. [Google Scholar] [CrossRef]
- Braun, T.; Backmann, N.; Vögtli, M.; Bietsch, A.; Engel, A.; Lang, H.-P.; Gerber, C.; Hegner, M. Conformational Change of Bacteriorhodopsin Quantitatively Monitored by Microcantilever Sensors. Biophys. J. 2006, 90, 2970–2977. [Google Scholar] [CrossRef][Green Version]
- Wagner, P.; Theato, P. Light induced wettability changes on polymer surfaces. Polymer 2014, 55, 3436–3453. [Google Scholar] [CrossRef]
- Huber, F.; Hegner, M.; Gerber, C.; Güntherodt, H.-J.; Lang, H.P. Label free analysis of transcription factors using microcantilever arrays. Biosens. Bioelectron. 2006, 21, 1599–1605. [Google Scholar] [CrossRef]
- Bai, X.; Hou, H.; Zhang, B.; Tang, J. Label-free detection of kanamycin using aptamer-based cantilever array sensor. Biosens. Bioelectron. 2014, 56, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Livan Alonso-Sarduy, Paolo De Los Rios, Fabrizio Benedetti, Dusan Vobornik, Giovanni Dietler, Sandor Kasas, Giovanni Longo Real-Time Monitoring of Protein Conformational Changes Using a Nano-Mechanical Sensor. PLoS ONE 2014, 9, e103674.
- Schulze, G.; Franke, K.J.; Pascual, J.I. Induction of a Photostationary Ring-Opening–Ring-Closing State of Spiropyran Monolayers on the Semimetallic Bi(110) Surface. Phys. Rev. Lett. 2012, 109, 26102. [Google Scholar] [CrossRef] [PubMed]
- Walther, M.; Fleming, P.M.; Padovani, F.; Hegner, M. An optimized measurement chamber for cantilever array measurements in liquid incorporating an automated sample handling system. EPJ Tech. Instrum. 2015, 2, 7. [Google Scholar] [CrossRef]

| State Transition | Surface Stress Induced (N m−1) | Type of Stress |
|---|---|---|
| SP → MC | −1.3 ± 0.1 × 10−3 | Compressive |
| MC → SP | +1.8 ± 0.1 × 10−3 | Tensile |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grogan, C.; Amarandei, G.; Lawless, S.; Pedreschi, F.; Lyng, F.; Benito-Lopez, F.; Raiteri, R.; Florea, L. Silicon Microcantilever Sensors to Detect the Reversible Conformational Change of a Molecular Switch, Spiropyan. Sensors 2020, 20, 854. https://doi.org/10.3390/s20030854
Grogan C, Amarandei G, Lawless S, Pedreschi F, Lyng F, Benito-Lopez F, Raiteri R, Florea L. Silicon Microcantilever Sensors to Detect the Reversible Conformational Change of a Molecular Switch, Spiropyan. Sensors. 2020; 20(3):854. https://doi.org/10.3390/s20030854
Chicago/Turabian StyleGrogan, Catherine, George Amarandei, Shauna Lawless, Fran Pedreschi, Fiona Lyng, Fernando Benito-Lopez, Roberto Raiteri, and Larisa Florea. 2020. "Silicon Microcantilever Sensors to Detect the Reversible Conformational Change of a Molecular Switch, Spiropyan" Sensors 20, no. 3: 854. https://doi.org/10.3390/s20030854
APA StyleGrogan, C., Amarandei, G., Lawless, S., Pedreschi, F., Lyng, F., Benito-Lopez, F., Raiteri, R., & Florea, L. (2020). Silicon Microcantilever Sensors to Detect the Reversible Conformational Change of a Molecular Switch, Spiropyan. Sensors, 20(3), 854. https://doi.org/10.3390/s20030854










