Derivation of Respiratory Metrics in Health and Asthma
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Breathing Data Collection
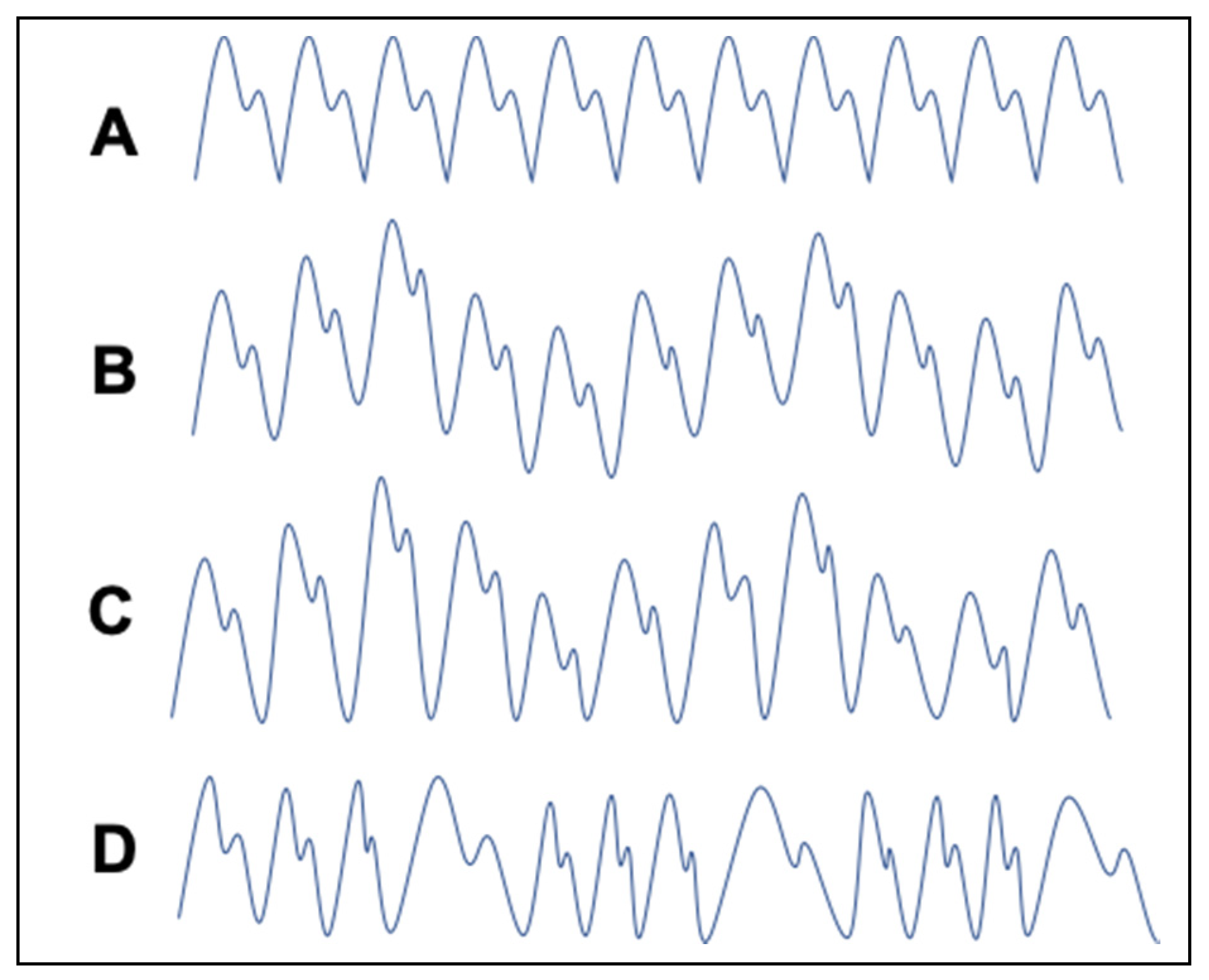
2.3. Principle of Breathing Signal Extraction from the PPG Signal
2.4. Machine Learning Models Used
2.5. Extraction of Key Breathing Metrics from Generated Volume Trace
- inspiration period (Tinsp): the period in seconds between a trough and a peak within the TVW signal.
- expiration period (Texp): the period in seconds between a peak and a trough within the TVW signal.
- I:E ratio: the ratio between consecutive inspiration time and expiration period. Derived values for Tinsp and Texp are used for this calculation.
- inter-breath interval (IBI): the period in seconds between two consecutive peaks within the TVW signal.
- breathing rate (BR): the amount of breaths per minute (derived independently of IBI).
2.6. Performance in Extracting Breathing Traces and Metrics
2.7. Statistical Analysis
3. Results
3.1. Participant Population
3.2. Datasets
Training Time
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Frey, U.; Brodbeck, T.; Majumdar, A.; Taylor, D.R.; Town, G.I.; Silverman, M.J.; Suki, B. Risk of severe asthma episodes predicted from fluctuation analysis of airway function. Nat. Cell Biol. 2005, 438, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Thamrin, C.; Zindel, J.; Nydegger, R.; Reddel, H.K.; Chanez, P.; Wenzel, S.E.; Fitzpatrick, S.; Watt, R.A.; Suki, B.; Frey, U. Predicting future risk of asthma exacerbations using individual conditional probabilities. J. Allergy Clin. Immunol. 2011, 127, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Calverley, P.M.A.; Pauwels, R.; Löfdahl, C.-G.; Svensson, K.; Higenbottam, T.; Carlsson, L.-G.; Ståhl, E. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. Eur. Respir. J. 2005, 26, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Yañez, A.M.; Guerrero, D.; De Alejo, R.P.; Garcia-Rio, F.; Alvarez-Sala, J.L.; Calle-Rubio, M.; De Molina, R.M.; Falcones, M.V.; Ussetti, P.; Sauleda, J.; et al. Monitoring Breathing Rate at Home Allows Early Identification of COPD Exacerbations. Chest 2012, 142, 1524–1529. [Google Scholar] [CrossRef] [PubMed]
- Channa, A.; Popescu, N.; Malik, N.U.R. Managing COVID-19 Global Pandemic with High-Tech Consumer Wearables: A Comprehensive Review. In 2020 12th International Congress on Ultra Modern Telecommunications and Control Systems and Workshops (ICUMT); Institute of Electrical and Electronics Engineers (IEEE): New York, NY, USA, 2020; pp. 222–228. [Google Scholar]
- Ravichandran, V.; Murugesan, B.; Balakarthikeyan, V.; Ram, K.; Preejith, S.; Joseph, J.; Sivaprakasam, M. RespNet: A deep learning model for extraction of respiration from photoplethysmogram. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Messe, Berlin, 23–27 July 2019; pp. 5556–5559. [Google Scholar]
- Prinable, J.B.; Jones, P.W.; Thamrin, C.; McEwan, A. Using a recurrent neural network to derive tidal volume from a photoplethsmograph. In Proceedings of the 2017 IEEE Life Sciences Conference (LSC), Sydney, Australia, 13–15 December 2017; pp. 218–221. [Google Scholar]
- Prinable, J.; Jones, P.; Boland, D.; Thamrin, C.; McEwan, A. Derivation of breathing metrics from a photoplethysmograph at rest. Machine Learning Methodology. JMIR mHealth uHealth 2020, 8, e13737. [Google Scholar] [CrossRef] [PubMed]
- Addison, P.; Watson, J. Secondary wavelet feature decoupling (SWFD) and its use in detecting patient respiration from the photoplethysmogram. In Proceedings of the 25th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (IEEE Cat. No.03CH37439), Cancun, Mexico, 17–21 September 2003; pp. 2602–2605. [Google Scholar]
- Charlton, P.H.; Bonnici, T.; Tarassenko, L.; Clifton, D.A.; Beale, R.; Watkinson, P.J. An assessment of algorithms to estimate respiratory rate from the electrocardiogram and photoplethysmogram. Physiol. Meas. 2016, 37, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Karlen, W.; Raman, S.; Ansermino, J.M.; Dumont, G.A. Multiparameter Respiratory Rate Estimation from the Photoplethysmogram. IEEE Trans. Biomed. Eng. 2013, 60, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Addison, P.S. Respiratory effort from the photoplethysmogram. Med Eng. Phys. 2017, 41, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Juniper, E.F.; O’Byrne, P.M.; Roberts, J. Measuring asthma control in group studies: Do we need airway calibre and rescue β2-agonist use? Respir. Med. 2001, 95, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Prinable, J.; Foster, J.; McEwan, A.; Young, P.M.; Tovey, E.R.; Thamrin, C. Motivations and Key Features for a Wearable Device for Continuous Monitoring of Breathing: A Web-Based Survey. JMIR Biomed. Eng. 2017, 2. [Google Scholar] [CrossRef]
- Li, Q.; Clifford, G.D. Dynamic time warping and machine learning for signal quality assessment of pulsatile signals. Physiol. Meas. 2012, 33, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, P.; Garde, A.; Molavi, B.; Ansermino, J.M.; Dumont, G.A. Extracting Instantaneous Respiratory Rate From Multiple Photoplethysmogram Respiratory-Induced Variations. Front. Physiol. 2018, 9, 948. [Google Scholar] [CrossRef] [PubMed]
- L’Her, E.; N’Guyen, Q.-T.; Pateau, V.; Bodenes, L.; Lellouche, F. Photoplethysmographic determination of the respiratory rate in acutely ill patients: Validation of a new algorithm and implementation into a biomedical device. Ann. Intensive Care 2019, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, V.; Liu, H.; Chen, F.; Hong, W.; Hughes, S.; Zheng, D. Toward Accurate Extraction of Respiratory Frequency From the Photoplethysmogram: Effect of Measurement Site. Front. Physiol. 2019, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Jarchi, D.; Salvi, D.; Tarassenko, L.; Clifton, D. Validation of Instantaneous Respiratory Rate Using Reflectance PPG from Different Body Positions. Sensors 2018, 18, 3705. [Google Scholar] [CrossRef] [PubMed]
- Hillman, D.R.; Prentice, L.; Finucane, K.E. The pattern of breathing in acute severe asthma. Am. Rev. Respir. Dis. 1986, 133, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Raoufy, M.R.; Ghafari, T.; Darooei, R.; Nazari, M.; Mahdaviani, S.A.; Eslaminejad, A.R.; Almasnia, M.; Gharibzadeh, S.; Mani, A.R.; Hajizadeh, S. Classification of Asthma Based on Nonlinear Analysis of Breathing Pattern. PLoS ONE 2016, 11, e0147976. [Google Scholar] [CrossRef] [PubMed]





| Status | ||
|---|---|---|
| Characteristic | No Asthma (n = 11) | Asthma (n = 11) |
| Sex: Male n (%) | 6 (55) | 4 (36) |
| Age, mean (SD) years | 30.1 (7.3) | 55.9 (16.3) |
| BMI, mean (SD) kg/m2 | 25.1 (4.8) | 26.7 (5.0) |
| ACQ5, mean (SD) | 1.04 (0.94) | |
| %predFEV1, mean (SD) | 84.6 (22.1) | |
| %predFVC, mean (SD) | 102.8 (15.9) | |
| FEV1/FVC, mean (SD)% | 68.3 (15.3) | |
| Metric | Reference | LSTM n = 550 | U-Net n = 550 | ||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | p-Value | Mean (SD) | p-Value | |
| Tinsp (s) | 3.50 (1.47) | 3.51 (1.38) | p = 0.87 | 3.48 (1.33) | p = 0.83 |
| Texp (s) | 3.28 (1.19) | 3.09 (0.88) | p < 0.05 | 3.04 (0.83) | p = 0.001 |
| I:E ratio (unitless) | 1.11 (0.62) | 0.97 (0.20) | p < 0.001 | 0.96 (0.19) | p = 2.63 |
| BR (BPM) | 9.99 (2.81) | 10.21 (2.53) | p = 0.17 | 10.28 (2.52) | p = 0.07 |
| IBI (s) | 6.77 (2.15) | 6.59 (2.05) | p = 0.14 | 6.52 (1.99) | p < 0.05 |
| Method | r2 | p | Absolute Bias | 95% LoA | Relative Bias (%) | 95% LoA |
|---|---|---|---|---|---|---|
| Tinsp (seconds) | ||||||
| LSTM | 0.66 | p < 0.001 | 0.01 | −2.31 to 2.34 | 1.89 | −52.95 to 56.74 |
| U-Net | 0.69 | p < 0.001 | −0.02 | −2.19 to 2.16 | 1.30 | −52.15 to 54.74 |
| Texp (seconds) | ||||||
| LSTM | 0.46 | p < 0.001 | −0.19 | −2.35 to 1.98 | −3.70 | −55.21 to 47.80 |
| U-Net | 0.47 | p < 0.001 | −0.24 | −2.36 to 1.89 | −4.97 | −56.84 to 46.89 |
| I:E ratio | ||||||
| LSTM | −0.04 | 0.39 | −0.14 | −1.43 to 1.16 | −4.65 | −87.18 to 77.88 |
| U-Net | 0.01 | 0.89 | −0.14 | −1.42 to 1.13 | −5.30 | −87.07 to 76.47 |
| IBI (seconds) | ||||||
| LSTM | 0.81 | p < 0.001 | −0.19 | −2.73 to 2.35 | −2.39 | −32.76 to 27.97 |
| U-Net | 0.81 | p < 0.001 | −0.25 | −2.76 to 2.26 | −3.16 | −33.69 to 27.36 |
| BR (BPM) | ||||||
| LSTM | 0.87 | p < 0.001 | 0.22 | −2.51 to 2.96 | 2.99 | −27.04 to 33.02 |
| U-Net | 0.86 | p < 0.001 | 0.29 | −2.54 to 3.11 | 3.69 | −27.17 to 34.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prinable, J.; Jones, P.; Boland, D.; McEwan, A.; Thamrin, C. Derivation of Respiratory Metrics in Health and Asthma. Sensors 2020, 20, 7134. https://doi.org/10.3390/s20247134
Prinable J, Jones P, Boland D, McEwan A, Thamrin C. Derivation of Respiratory Metrics in Health and Asthma. Sensors. 2020; 20(24):7134. https://doi.org/10.3390/s20247134
Chicago/Turabian StylePrinable, Joseph, Peter Jones, David Boland, Alistair McEwan, and Cindy Thamrin. 2020. "Derivation of Respiratory Metrics in Health and Asthma" Sensors 20, no. 24: 7134. https://doi.org/10.3390/s20247134
APA StylePrinable, J., Jones, P., Boland, D., McEwan, A., & Thamrin, C. (2020). Derivation of Respiratory Metrics in Health and Asthma. Sensors, 20(24), 7134. https://doi.org/10.3390/s20247134






