An Electrochemical Approach for the Selective Detection of Cancer Metabolic Creatine Biomarker with Porous Nano-Formulated CMNO Materials Decorated Glassy Carbon Electrode
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of CMNO Nanomaterials
2.3. Fabrication of GCE by CMNO Nanomaterials
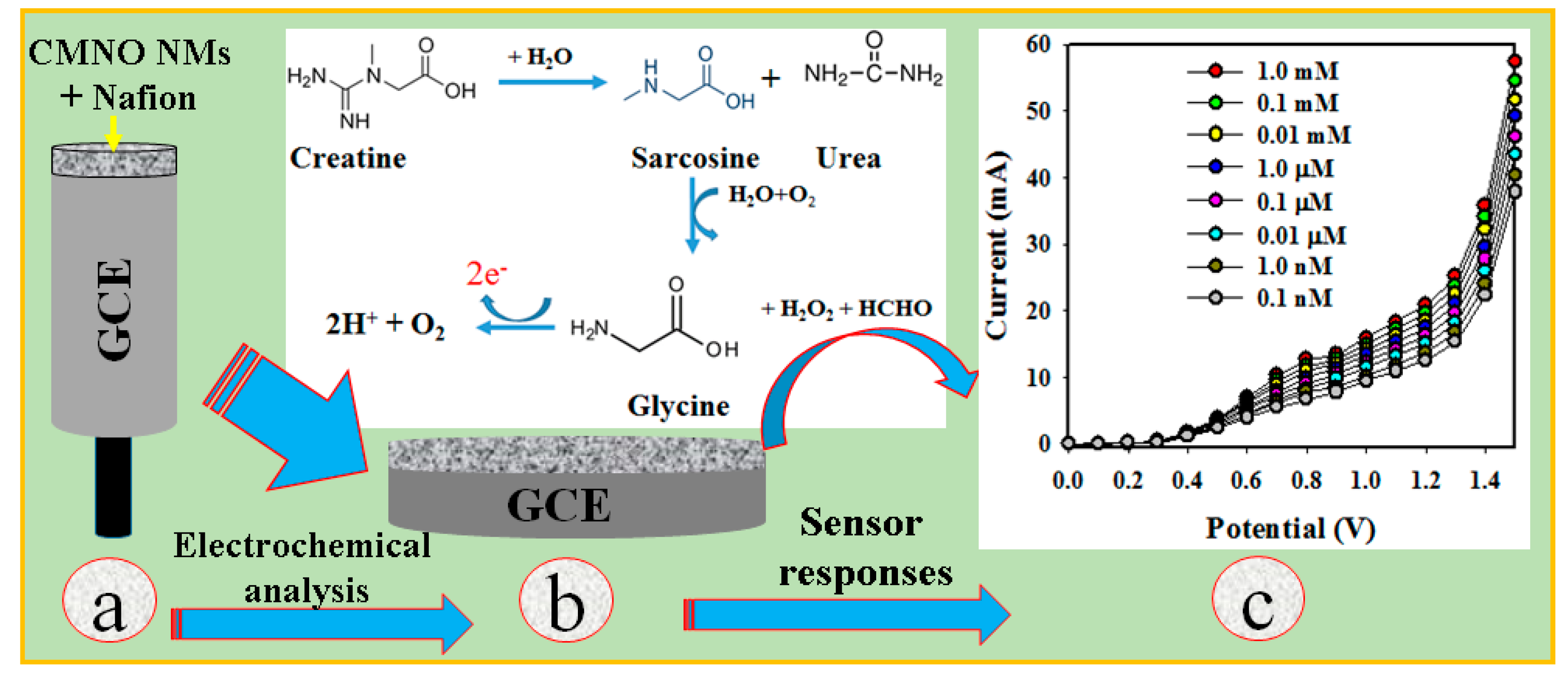
3. Results and Discussions
3.1. Characterization of CMNO Nanomaterials
3.2. Application of CA Sensor with CMNO NMs/GCE
3.3. Real Samples Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, R.; Naclerio, F.; Allgrove, J.; Jimenez, A. Creatine supplementation with specific view to exercise/sports performance: An update. J. Int. Soc. Sports. Nutr. 2012, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Deminice, R.; da Silva, R.P.; Lamarre, S.G.; Brown, C.; Furey, G.N.; McCarter, S.A.; Jordao, A.A.; Kelly, K.B.; King-Jones, K.; Jacobs, R.L.; et al. Creatine supplementation prevents the accumulation of fat in the livers of rats fed a high-fat diet. J. Nutr. 2011, 141, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.B. Creatine: Biosynthesis, regulation, and function. Adv. Enzymol. Relat. Areas Mol. Biol. 1979, 50, 177–242. [Google Scholar] [PubMed]
- Braissant, O.; Henry, H. AGAT, GAMT and SLC6A8 distribution in the central nervous system, in relation to creatine deficiency syndromes: A review. J. Inherit. Metab. Dis. 2008, 31, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Braissant, O.; Henry, H.; Beard, E.; Uldry, J. Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids 2011, 40, 1315–1324. [Google Scholar] [CrossRef]
- Schulze, A. Creatine deficiency syndromes. Mol. Cell. Biochem. 2003, 244, 143–150. [Google Scholar] [CrossRef]
- Masaru, S.; Mitsutaka, Y. A new enzymatic serum creatinine measurement based on an endogenous creatine-eliminating system. Clin. Chim. Acta 1984, 143, 147–155. [Google Scholar] [CrossRef]
- Beyer, C.; Hoenderdos, A.; Mairuhu, W.M.; Statius, L.W. Evaluative and comparative study of an enzymatic method using creatine kinase for the determination of urinary creatine. Clin. Chim. Acta 1984, 136, 263–270. [Google Scholar] [CrossRef]
- Chen, S.P.; Kuan, S.S.; Guilbault, G.G. Fluorometric enzymatic determination ofserum creatinine on the surface of silicone-rubber pads. Clin. Chim. Acta 1980, 100, 21–31. [Google Scholar]
- Patel, D.P.; Pauly, G.T.; Tada, T.; Parker, A.L.; Toulabi, L.; Kanke, Y.; Oike, T.; Krausz, K.W.; Gonzalez, F.J.; Harris, C.C. Improved detection and precise relative quantification of the urinary cancer metabolite biomarkers—Creatine riboside, creatinine riboside, creatine and creatinine by UPLC-ESI-MS/MS: Application to the NCI-Maryland cohort population controls and lung cancer cases. J. Pharm. Biomed. Anal. 2020, 191, 113596. [Google Scholar] [PubMed]
- Rahman, M.M.; Awual, M.R.; Asiri, A.M. Preparation and evaluation of composite hybrid nanomaterials for rare-earth elements separation and recovery. Sep. Purif. Technol. 2020, 253, 117515. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M. Sensitive 1,2-dichlorobenzene chemi-sensor development based on solvothermally prepared FeO/CdO nanocubes for environmental safety. J. Ind. Eng. Chem. 2018, 62, 392–400. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M. 2-Nitrophenol sensor-based wet-chemically prepared binary doped Co3O4/Al2O3 nanosheets by an electrochemical approach. RSC Adv. 2018, 8, 960–970. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M.; Opo, F.A.D.M. Fabrication of selective and sensitive chemical sensor probe based on ternary nano-formulated CuO/MnO2/Gd2O3 spikes by hydrothermal approach. Sci. Rep. 2020, 10, 20248. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Rahman, M.M.; Marwani, H.M.; Hasnat, M.A. Insights of temperature dependent catalysis and kinetics of electro-oxidation of nitrite ions on a glassy carbon electrode. Electrochim. Acta 2020, 362, 137102. [Google Scholar] [CrossRef]
- Hussain, M.M.; Asiri, A.M.; Rahman, M.M. Non-enzymatic simultaneous detection of acetylcholine and ascorbic acid using ZnO.CuO nanoleaves: Real sample analysis. Microchem. J. 2020, 159, 105534. [Google Scholar] [CrossRef]
- Rahman, M.M.; Adeosun, W.A.; Asiri, A.M. Fabrication of selective and sensitive chemical sensor development based on flower-flake La2ZnO4 nanocomposite for effective non-enzymatic Sensing of hydrogen peroxide by electrochemical method. Microchem. J. 2020, 159, 105536. [Google Scholar] [CrossRef]
- Asiri, A.M.; Adeosun, W.A.; Rahman, M.M. Development of highly efficient non-enzymatic nitrite sensor using La2CuO4 nanoparticles. Microchem. J. 2020, 159, 105527. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Baghayeri, M. Determination of Trace Tl(I) by Differential Pulse Anodic Stripping Voltammetry Using a Novel Modified Carbon Paste Electrode. J. Electrochem. Soc. 2020, 167, 066508. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A.; Fayazi, M.; Baghayeri, M.; Hosseinifar, A. Non-Enzymatic Amperometric Sensing of Hydrogen Peroxide Based on Vanadium Pentoxide Nanostructures. J. Electrochem. Soc. 2019, 166, B367–B372. [Google Scholar] [CrossRef]
- Rouhi, M.; Lakouraj, M.M.; Baghayeri, M. Low Band Gap Conductive Copolymer of Thiophene with p-Phenylenediamine and Its Magnetic Nanocomposite: Synthesis, Characterization and Biosensing Activity. Polym. Compos. 2019, 40, 1034–1042. [Google Scholar] [CrossRef]
- Nodehi, M.; Baghayeri, M.; Ansari, R.; Veisi, H. Electrochemical quantification of 17α—Ethinylestradiol in biological samples using a Au/Fe3O4@TA/MWNT/GCE sensor. Mater. Chem. Phys. 2020, 244, 122687. [Google Scholar] [CrossRef]
- Baghayeri, M.; Ghanei-Motlagh, M.; Tayebee, R.; Fayazi, M.; Narenji, F. Application of graphene/zinc-based metal-organic framework nanocomposite for electrochemical sensing of As(III) in water resources. Anal. Chim. Acta 2020, 1099, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Baghayeri, M.; Ansari, R.; Nodehi, M.; Razavipanah, I.; Veisi, H. Label-free Electrochemical Bisphenol A Aptasensor Based on Designing and Fabrication of a Magnetic Gold Nanocomposite. Electroanalysis 2018, 30, 2160–2166. [Google Scholar] [CrossRef]
- Baghayeri, M.; Ansari, R.; Nodehi, M.; Razavipanah, I.; Veisi, H. Voltammetric aptasensor for bisphenol A based on the use of a MWCNT/Fe3O4@gold nanocomposite. Microchim. Acta 2018, 185, 320. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M.; Awual, M.R. Fabrication of 4-aminophenol sensor based on hydrothermally prepared ZnO/Yb2O3 nanosheets. New J. Chem. 2017, 41, 9159–9169. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M.; Islam, M.A. Ethanol sensor development based on ternarydoped metal oxides (CdO/ZnO/Yb2O3) nanosheets for environmental safety. RSC Adv. 2017, 7, 22627–22639. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M.; Islam, M.A. Fabrication of selective chemical sensor with ternary ZnO/SnO2/Yb2O3 nanoparticles. Talanta 2017, 170, 215–223. [Google Scholar] [CrossRef]
- Zoolfakar, A.S.; Rani, R.A.; Morfa, A.J.; O’Mullaned, A.P.; Kalantar-zadeh, K. Nanostructured Copper Oxide Semiconductors: A Perspective on Materials, Synthesis methods and Applications. J. Mater. Chem. C 2014, 2, 5247–5270. [Google Scholar] [CrossRef]
- Grigore, M.E.; Biscu, E.R.; Holban, A.M.; Gestal, M.C.; Grumezescu, A.M. Methods of Synthesis, Properties and Biomedical Applications of CuO Nanoparticles. Pharmaceuticals 2016, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.A.; Rahman, M.M.; Asiri, A.M.; Marwani, H.M. Sensitive 3-chlorophenol sensor development based on facile Er2O3/CuO nanomaterials for environmental safety. New J. Chem. 2018, 42, 3936–3946. [Google Scholar] [CrossRef]
- Rahman, M.M.; Asiri, A.M. Development of selective and sensitive bicarbonate chemical sensor based on wet-chemically prepared CuO-ZnO nanorods. Sens. Actuators B Chem. 2015, 214, 82–91. [Google Scholar] [CrossRef]
- ul Haque, S.; Nasar, A.; Rahman, M.M. Applications of chitosan (CHI)-reduced graphene oxide (rGO)-polyaniline (PAni) conducting composite electrode for energy generation in glucose biofuel cell. Sci. Rep. 2020, 10, 10428. [Google Scholar] [CrossRef] [PubMed]
- Sharma1, S.; Chauhan, P.; Husain, S. Structural and optical properties of Mn2O3 nanoparticles & its gas sensing applications. Adv. Mater. Process. 2016, 1, 220–225. [Google Scholar]
- Sharrouf, M.; Awad, R.; Roumié, M.; Marhaba, S. Structural, Optical and Room Temperature Magnetic Study of Mn2O3 Nanoparticles. Mater. Sci. Appl. 2015, 6, 850–859. [Google Scholar]
- Sone, B.T.; Fuku, X.G.; Maaza, M. Physical & Electrochemical Properties of Green Synthesized Bunsenite NiO Nanoparticles via Callistemon Viminalis’ Extracts. Int. J. Electrochem. Sci. 2016, 11, 8204–8220. [Google Scholar]
- Amor, M.B.; Hamzaoui, N.; Boukhachem, A.; Mrabet, C.; Ghamnia, M.; Yumak, A.; Boubaker, K.; Petkova, P.; Amlouk, M. Optical, Physical, Chemical and Electrical Properties of Nickel Oxide Sprayed Thin Films under Tin Doping Effects. Adv. Ceram. Sci. Eng. (ACSE) 2015, 24, 72–76. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, C.; Qi, K.; Cui, X. Photo-reduced Cu/CuO nanoclusters on TiO2 nanotube arrays as highly efficient and reusable catalyst. Sci. Rep. 2017, 7, 39695. [Google Scholar] [CrossRef]
- Chen, R.X.; Zhu, S.L.; Mao, J.; Cui, Z.D.; Yang, X.J.; Liang, Y.Q.; Li, Z.Y. Synthesis of CuO/Co3O4 Coaxial Hetero-structures for Efficient and Recycling Photo degradation. Int. J. Photoenergy 2015, 1, 1–11. [Google Scholar]
- Rahman, M.M.; Gruner, G.; Al-Ghamdi, M.S.; Daous, M.A.; Khan, S.B.; Asiri, A.M. Chemo-sensors development based on low-dimensional codoped Mn2O3-ZnO nanoparticles using flat-silver electrodes. Chem. Cent. J. 2013, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Khan, S.B.; Marwani, H.M.; Asiri, A.M.; Alamry, K.A. Selective Iron (III) ion uptake using CuO-TiO2 nanostructure by inductively coupled plasma-optical emission spectrometry. Chem. Cent. J. 2012, 6, 158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhu, D.; Guo, C.; Liu, J.; Wang, L.; Dub, Y.; Qiao, S.Z. Two-dimensional metal–organic frameworks with high oxidation states for efficient electrocatalytic urea oxidation. Chem. Commun. 2017, 53, 10906–10909. [Google Scholar] [CrossRef] [PubMed]
- Kalaiyarasan, G.; Aswathi, K.; Joseph, J. Formation of nanoporous NiS films from electrochemically modified GC surface with Nickel Hexacyanoferrate film and its performance for the hydrogen evolution reaction. Int. J. Hydrogen Energy 2017, 42, 22866–22876. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.; Li, F.; Wang, Z.; Hu, N.; Wen, Z.; Liu, Q. Merging of Kirkendall Growth and Ostwald Ripening: CuO@MnO2 Core-shell Architectures for Asymmetric Supercapacitors. Sci. Rep. 2016, 4, 4518. [Google Scholar] [CrossRef]
- Wang, X.; Wu, W.; Chen, Z.; Wang, R. Bauxite-supported Transition Metal Oxides: Promising Low-temperature and SO2-tolerant Catalysts for Selective Catalytic Reduction of NOx. Sci. Rep. 2015, 5, 9766. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, D.; Luo, Z.; Yu, Y.; Shi, X.; Yuan, G.; Xie, J. Hierarchically Structured Co3O4@Pt@MnO2 Nanowire Arrays for High-Performance Supercapacitors. Sci. Rep. 2013, 3, 2978. [Google Scholar] [CrossRef]
- Ethiraj, A.S.; Kang, D.J. Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res. Lett. 2012, 7, 70. [Google Scholar] [CrossRef]
- Suleiman, M.; Mousa, M.; Hussein, A.I.A. Wastewater Disinfection by Synthesized Copper Oxide Nanoparticles Stabilized with Surfactant. J. Mater. Environ. Sci. 2015, 6, 1924–1937. [Google Scholar]
- Li, Z.Y.; Akhtar, M.S.; Bui, P.T.M.; Yang, O.B. Predominance of two dimensional (2D) Mn2O3 nanowalls thin film for high performance electrochemical supercapacitors. Chem. Eng. J. 2017, 330, 1240–1247. [Google Scholar] [CrossRef]
- Ashouri, F.; Zare, M.; Bagherzadeh, M. Manganese and cobalt-terephthalate metal-organic frameworks as a precursor for synthesis ofMn2O3, Mn3O4 and Co3O4 nanoparticles: Active catalysts for olefin heterogeneous oxidation. Inorg. Chem. Commun. 2015, 61, 73–76. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, D.; Xu, J.; Lu, Y.; Liu, Y.; Qiu, K.; Zhang, Y.; Luo, Y. Solution growth of NiO nanosheets supported on Ni foam as high-performance electrodes for supercapacitors. Nanoscale Res. Lett. 2014, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Yung, T.Y.; Huang, L.Y.; Chan, T.Y.; Wang, K.S.; Liu, T.Y.; Chen, P.T.; Chao, C.Y.; Liu, L.K. Synthesis and characterizations of Ni-NiO nanoparticles on PDDA-modified graphene for oxygen reduction reaction. Nanoscale Res. Lett. 2014, 9, 444. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, C.; Wu, S.; Liu, G.; Zhang, F.; Wang, T. Lotus-root-like NiO nanosheets and flower-like NiO microspheres: Synthesis and magnetic properties. Cryst. Eng. Comm. 2011, 13, 4930–4934. [Google Scholar] [CrossRef]
- Mohan, S.; Srivastava, P.; Maheshwari, S.N.; Sundar, S.; Prakash, R. Nano-structured nickel oxide based DNA biosensor for detection of visceral leishmaniasis (Kala-azar). Analyst 2011, 136, 2845–2851. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Z.; Liu, Y.; Tang, S.; Han, X.; Chen, M. Controlled synthesis of Mn3O4 nanocrystallites and MnOOH nanorods by a solvothermal method. J. Cryst. Growth 2004, 263, 394–399. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hussain, M.M.; Asiri, A.M.; Alamry, K.A.; Hasnat, M.A. An enzyme free detection of L-Glutamic acid using deposited CuO.GdO nanospikes on a flat glassy carbon electrode. Surfaces Interfaces 2020, 20, 100617. [Google Scholar] [CrossRef]
- George, G.; Anandhan, S. Electrospun nickel oxide nanofiber webs for thermistor applications. Int. J. Plast. Technol. 2014, 18, 374–382. [Google Scholar] [CrossRef]
- Ren, S.; Li, C.; Zhao, X.; Wu, Z.; Wang, S.; Sun, G.; Xin, Q.; Yang, X. Surface modification of sulfonated poly(ether ether ketone) membranes using nafion solution for direct methanol fuel cells. J. Membrane Sci. 2005, 247, 59–63. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zhang, L.; Wang, H. Electrochemical detection of trace cadmium in soilusing a Nafion/stannum film-modified molecular wire carbon paste electrodes. Ionics 2013, 19, 1687–1693. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Uddin, M.T.; Islam, M.A.; Rahman, M.M. Wet-chemically prepared low-dimensional ZnO/Al2O3/Cr2O3 nanoparticles for xanthine sensor development using an electrochemical method. RSC Adv. 2018, 8, 12562–12572. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ahmed, J.; Asiri, A.M. Thiourea sensor development based on hydrothermally prepared CMO nanoparticles for environmental safety. Biosens. Bioelectron. 2018, 99, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hussain, M.M.; Asiri, A.M. Bilirubin sensor based on CuO-CdO composites deposited in a nafion/glassy carbon electrode matrixes. Prog. Nat. Sci.Mater. Int. 2017, 27, 566–573. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hussain, M.M.; Asiri, A.M. Ultrasensitive and label-free detection of creatine based on CdO nanoparticles: A real sample approach. New J. Chem. 2017, 41, 6667–6677. [Google Scholar] [CrossRef]
- Rahmana, M.M.; Ahmed, J.; Asiri, A.M. Development of Creatine sensor based on antimony-doped tin oxide (ATO) nanoparticles. Sens. Actuators B 2017, 242, 167–175. [Google Scholar] [CrossRef]
- Alcantara, R.; Lavela, P.; Tirado, J.L. Structure and Electrochemical Properties of Boron-Doped LiCoO2. J. Solid State Chem. 1997, 134, 265–273. [Google Scholar] [CrossRef]
- Zhao, L.; Su, G.; Liu, W.; Cao, L.n.; Wang, J.; Dong, Z.; Song, M. Optical and electrochemical properties of Cu-doped NiO films prepared by electrochemical deposition. Appl. Surf. Sci. 2011, 257, 3974–3979. [Google Scholar] [CrossRef]
- Cheng, H.; Li, M.L.; Su, C.Y.; Li, N.; Liu, Z.Q. Cu-Co Bimetallic Oxide Quantum Dot Decorated Nitrogen-Doped Carbon Nanotubes: A High-Efficiency Bifunctional Oxygen Electrode for Zn–Air Batteries. Adv. Funct. Mater. 2017, 27, 1701833. [Google Scholar] [CrossRef]
- Malkhandi, S.; Yang, B.; Manohar, A.K.; Manivannan, A.; Prakash, G.K.S.; Narayanan, S.R. Electrocatalytic Properties of Nanocrystalline Calcium-Doped Lanthanum Cobalt Oxide for Bifunctional Oxygen Electrodes. J. Phys. Chem. Lett. 2012, 3, 967–972. [Google Scholar] [CrossRef]
- Marshall, A.T.; Haverkamp, R.G. Electrocatalytic activity of IrO2–RuO2 supported on Sb-doped SnO2 nanoparticles. Electrochim. Acta 2010, 55, 1978–1984. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Uddin, M.T.; Islam, M.A.; Awual, M.R.; Rahman, M.M. Detection of uric acid based on doped ZnO/Ag2O/Co3O4 nanoparticles fabricated glassy carbon electrode. New J. Chem. 2019, 43, 8651–8659. [Google Scholar] [CrossRef]
- Hussain, M.M.; Rahman, M.M.; Asiri, A.M.; Awual, M.R. Non-enzymatic simultaneous detection of L-glutamic acid and uric acid using mesoporous Co3O4 nanosheets. RSC Adv. 2016, 6, 80511–80521. [Google Scholar] [CrossRef]
- Abu-Zied, B.; Alam, M.M.; Asiri, A.M.; Ahmed, J.; Rahman, M.M. Efficient hydroquinone sensor development based on Co3O4 nanoparticle. Microchemical J. 2020, 157, 104972. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Uddin, M.T.; Islam, M.A.; Awual, M.R.; Rahman, M.M. One-step wet-chemical synthesis of ternary ZnO/CuO/Co3O4 nanoparticles for sensitive and selective melamine sensor development. New J. Chem. 2019, 43, 4849–4858. [Google Scholar] [CrossRef]
- Rahman, M.M.; Alam, M.M.; Asiri, A.M. Development of an efficient phenolic sensor based on facile Ag2O/Sb2O3 nanoparticles for environmental remediation. Nanoscale Adv. 2019, 1, 696–705. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Uddin, M.T.; Islam, M.A.; Rahman, M.M. In-situ glycine sensor development based ZnO/Al2O3/Cr2O3 nanoparticles. ChemistrySelect 2018, 3, 11460–11468. [Google Scholar] [CrossRef]
- Akhter, H.; Murshed, J.; Rashed, M.A.; Oshima, Y.; Nagao, Y.; Rahman, M.M.; Asiri, A.M.; Hasnat, M.A.; Uddin, M.N.; Siddiquey, I.A. Fabrication of hydrazine sensor based on silica-coated Fe2O3 magnetic nanoparticles prepared by a rapid microwave irradiation method. J. Alloys. Compounds 2017, 698, 921–929. [Google Scholar] [CrossRef]
- Awual, M.R.; Hasan, M.M.; Islam, A.; Rahman, M.M.; Asiri, A.M.; Khaleque, M.A.; Sheikh, M.C. Introducing an an amine functionalized novel conjugate material for toxic nitrite detection and adsorption from wastewater. J. Clean. Prod. 2019, 228, 778–785. [Google Scholar] [CrossRef]
- Rahman, M.M.; Karim, M.R.; Alam, M.M.; Zaman, M.B.; Alharthi, N.; Alharbi, H.; Asiri, A.M. Facile and efficient 3-chlorophenol sensor development based on photolumenescent core-shell CdSe/ZnS quantum dots. Sci. Rep. 2020, 10, 557. [Google Scholar] [CrossRef]






| Modified GCE | * DL | # LDR | Sensitivity | Ref. |
|---|---|---|---|---|
| CdO NP/GCE | 50.0 pM | 0.1 nM~0.1 M | 1.90 μA μM−1 cm−2 | [64] |
| ATO-NPs/Nafion/GCE | 42.0 pM | 0.1 nM~1.0 mM | 0.276 μA μM−1 cm−2 | [65] |
| CMNO NMs/GCE | 21.63 pM | 0.1 nM~0.1 mM | 14.631 μA μM−1 cm−2 | This work |
| Real Samples | Added CA conc. (µM) | Measured CA conc. a by CMNO NMs/GCE (µM) | Average Recovery b (%) | RSD c (%) (n = 3) | ||
|---|---|---|---|---|---|---|
| R1 | R2 | R3 | ||||
| Mouse serum | 0.01 | 0.00992 | 0.00996 | 0.00996 | 99.52 | 0.23 |
| Rabbit serum | 0.01 | 0.00997 | 0.00994 | 0.00995 | 99.56 | 0.15 |
| Human urine | 0.01 | 0.01179 | 0.01182 | 0.01165 | 117.54 | 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.M.; Alam, M.M.; Asiri, A.M.; Opo, F.A.D.M. An Electrochemical Approach for the Selective Detection of Cancer Metabolic Creatine Biomarker with Porous Nano-Formulated CMNO Materials Decorated Glassy Carbon Electrode. Sensors 2020, 20, 7060. https://doi.org/10.3390/s20247060
Rahman MM, Alam MM, Asiri AM, Opo FADM. An Electrochemical Approach for the Selective Detection of Cancer Metabolic Creatine Biomarker with Porous Nano-Formulated CMNO Materials Decorated Glassy Carbon Electrode. Sensors. 2020; 20(24):7060. https://doi.org/10.3390/s20247060
Chicago/Turabian StyleRahman, Mohammed M., Md. M. Alam, Abdullah M. Asiri, and Firoz. A. D. M. Opo. 2020. "An Electrochemical Approach for the Selective Detection of Cancer Metabolic Creatine Biomarker with Porous Nano-Formulated CMNO Materials Decorated Glassy Carbon Electrode" Sensors 20, no. 24: 7060. https://doi.org/10.3390/s20247060
APA StyleRahman, M. M., Alam, M. M., Asiri, A. M., & Opo, F. A. D. M. (2020). An Electrochemical Approach for the Selective Detection of Cancer Metabolic Creatine Biomarker with Porous Nano-Formulated CMNO Materials Decorated Glassy Carbon Electrode. Sensors, 20(24), 7060. https://doi.org/10.3390/s20247060






