Quantification of the Flavor and Taste of Gonads from the Sea Urchin Mesocentrotus nudus Using GC–MS and a Taste-Sensing System
Abstract
1. Introduction
2. Materials and Methods
2.1. Sea Urchin Samples
2.2. Odor-Active Volatile Compound Analysis
2.3. Taste Analysis
3. Results
3.1. Odor-Active Compounds
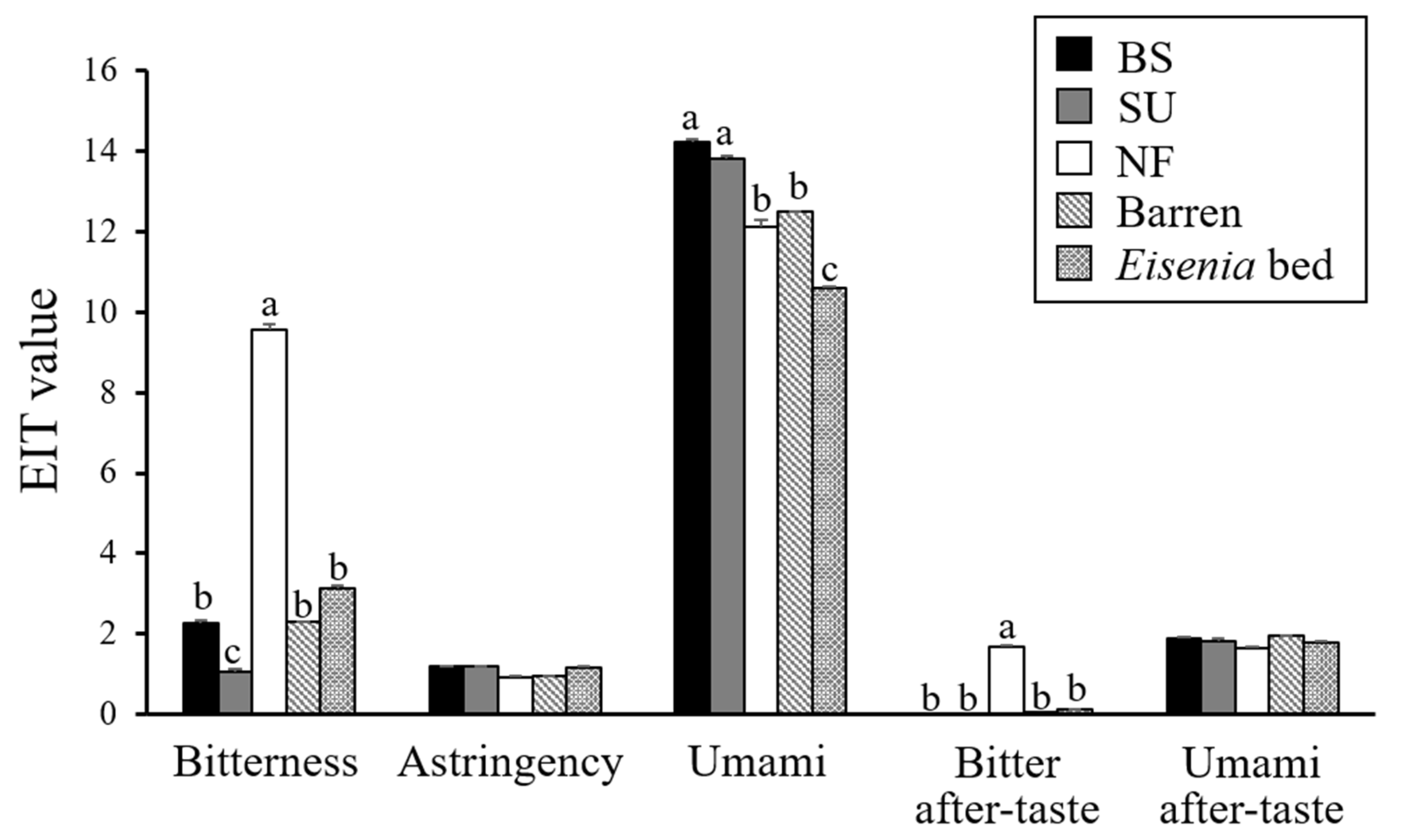
3.2. Taste Analyzed by the Taste-Sensing System
4. Discussion
4.1. Odor-Active Compounds
4.2. Taste Analysis
4.3. Standard of High-Quality Gonad of M. nudus
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walker, C.W.; Böttger, S.; Unuma, T.; Watts, S.A.; Harris, L.G.; Lawrence, A.L.; Eddy, S.D. Enhancing the commercial quality of edible sea urchin gonads—Technologies emphasizing nutritive phagocytes. In Echinoderm Aquaculture; Brown, N.P., Eddy, S.D., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 263–286. [Google Scholar]
- Sun, J.; Chiang, F.S. Use and exploitation of sea urchins. In Echinoderm Aquaculture; Brown, N.P., Eddy, S.D., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 25–45. [Google Scholar]
- Metropolitan Central Wholesale Market. Market Statistical Information. 2019. Available online: http://www.shijou-tokei.metro.tokyo.jp/ (accessed on 29 August 2019). (In Japanese).
- FAO. Food Supply—Livestock and Fish Primary Equivalent. 2019. Available online: http://www.fao.org/faostat/en/#data/CL/visualize (accessed on 17 April 2019).
- FAO. Fishery Commodities and Trade. 2019. Available online: http://www.fao.org/fishery/statistics/global-commodities-production/en (accessed on 29 August 2019).
- McBride, S.C.; Price, R.J.; Tom, P.D.; Lawrence, J.M.; Lawrence, A.L. Comparison of gonad quality factors: Color, hardness and resilience, of Strongylocentrotus franciscanus between sea urchins fed prepared feed or algal diets and sea urchins harvested from the Northern California fishery. Aquaculture 2004, 233, 405–422. [Google Scholar] [CrossRef]
- Woods, C.M.C.; James, P.J.; Moss, G.A.; Wright, J.; Siikavuopio, S. A comparison of the effect of urchin size iand diet on gonad yield and quality in the sea urchin Evechinus chloroticus Valenciennes. Aquacult. Int. 2008, 16, 49–68. [Google Scholar] [CrossRef]
- Takagi, S.; Murata, Y.; Inomata, E.; Endo, H.; Aoki, M.N.; Agatsuma, Y. Improvement of gonad quality of the sea urchin Mesocentrotus nudus fed the kelp Saccharina japonica during offshore cage culture. Aquaculture 2017, 477, 50–61. [Google Scholar] [CrossRef]
- Kelly, M.S.; Symonds, R.C. Carotenoids in sea urchins. In Sea Urchins: Biology and Ecology, 3rd ed.; Lawrence, J.M., Ed.; Academic Press: San Diego, CA, USA, 2013; pp. 171–177. [Google Scholar]
- Takagi, S.; Murata, Y.; Inomata, E.; Aoki, M.N.; Agatsuma, Y. Production of high quality gonads in the sea urchin Mesocentrotus nudus (A. Agassiz, 1864) from a barren by feeding on the kelp Saccharina japonica at the late sporophyte stage. J. Appl. Phycol. 2019, 31, 4037–4048. [Google Scholar] [CrossRef]
- De Quirós, A.R.B.; López-Hernández, J.; González-Castro, M.J.; de la Cruz-García, C.; Simal-Lozano, J. Comparison of volatile components in fresh and canned sea urchin (Paracentrotus lividus, Lamarck) gonads by GC–MS using dynamic headspace sampling and microwave desorption. Eur. Food Res. Technol. 2001, 212, 643–647. [Google Scholar] [CrossRef]
- Niimi, J.; Leus, M.; Silcock, P.; Hamid, N.; Bremer, P. Characterisation of odour active volatile compounds of New Zealand sea urchin (Evechinus chloroticus) roe using gas chromatography–olfactometry–finger span cross modality (GC–O–FSCM) method. Food Chem. 2010, 121, 601–607. [Google Scholar] [CrossRef]
- Phillips, K.; Niimi, J.; Hamid, N.; Silcock, P.; Delahunty, C.; Barker, M.; Sewell, M.; Bremer, P. Sensory and volatile analysis of sea urchin roe from different geographical regions in New Zealand. LWT-Food Sci. Technol. 2010, 43, 202–213. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Habara, M.; Ikezazki, H.; Chen, R.; Naito, Y.; Toko, K. Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. Sensors 2010, 10, 3411–3443. [Google Scholar] [CrossRef]
- Yamada, J.; Tokunaga, N.; Nashimoto, A.; Inamori, M.; Matsuda, H. Inhibitory effect of Katsuo-dashi dried bonito stock on the taste and odor of lactic acid. J. Cookery Sci. Jpn. 2011, 44, 122–127, (In Japanese with English Abstract). [Google Scholar]
- Touhata, K.; Habara, M.; Ikezaki, H.; Ishida, N. Applicability of a taste sensing system to objectively assess taste of seafood. In Proceedings of the JSFS 85th Anniversary Commemorative International Symposium 2017, Tokyo, Japan, 22–24 September 2017; p. 10021. [Google Scholar]
- Uchida, M.; Kurushima, H.; Ishihara, K.; Murata, Y.; Touhata, K.; Ishida, N.; Niwa, K.; Araki, T. Characterization of fermented seaweed sauce prepared from nori (Pyropia yezoensis). J. Biosci. Bioeng. 2017, 123, 332–372. [Google Scholar] [CrossRef]
- Kitaoka, C.; Hosoe, J.; Hakamatsuka, T.; Araya, K.; Habara, M.; Ikezaki, H.; Hamada-Sato, N.; Shinagawa, A.; Yamamoto, J.; Kato-Yoshinaga, Y. Taste component analysis of Pacific oysters cultured in Konagai, Nagasaki and taste evaluation using a taste-sensing system. Jpn. J. Food Chem. Safety 2016, 23, 63–71. [Google Scholar]
- Murayama, F.; Sato, J.; Touhata, K.; Ishida, N. Taste evaluations of ovigerous and soft-shelled female swimming crab Portunus trituberculatus meat extracts. Nippon Suisan Gakkaishi 2018, 84, 425–433, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Unuma, T. Introduction: Sea urchin fisheries in Japan. In Echinoderm Aquaculture; Brown, N.P., Eddy, S.D., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 77–85. [Google Scholar]
- Agatsuma, Y. Ecological studies on the population dynamics of the sea urchin Strongylocentrotus nudus. Sci. Rep. Hokkaido Fish. Exp. Stn. 1997, 51, 1–66, (In Japanese with English Abstract). [Google Scholar]
- Agatsuma, Y.; Sato, M.; Taniguchi, K. Factors causing brown-colored gonads of the sea urchin Strongylocentrotus nudus in northern Honshu, Japan. Aquaculture 2005, 249, 449–458. [Google Scholar] [CrossRef]
- Takagi, S.; Murata, Y.; Inomata, E.; Endo, H.; Aoki, M.N.; Agatsuma, Y. Dietary effect of kelp (Saccharina japonica) on gonad quantity and quality in sea urchins (Mesocentrotus nudus) collected from a barren before the fishing season. J. Shellfish Res. 2018, 37, 659–669. [Google Scholar] [CrossRef]
- 24. Takagi, S.; Sato, Y.; Kokubun, A.; Inomata, E.; Agatsuma, Y. Odor-active compounds from the gonads of Mesocentrotus nudus sea urchins fed Saccharina japonica. PLoS ONE 2020, 15, e0231673. [Google Scholar] [CrossRef]
- Takagi, S.; Murata, Y.; Agatsuma, Y. Feeding the sporophyll of Undaria pinnatifida kelp shortens the culture duration for the production of high-quality gonads of Mesocentrotus nudus sea urchins from a barren. Aquaculture 2020, 528, 735503. [Google Scholar] [CrossRef]
- Sato, Y.; Takagi, S.; Inomata, E.; Agatsuma, Y. Odor-active volatile compounds from the gonads of the sea urchin Mesocentrotus nudus in the wild in Miyagi Prefecture, Tohoku, Japan. Food Nutr. Sci. 2019, 10, 860–875. [Google Scholar]
- Zhu, X.; Gao, Y.; Chen, Z.; Su, Q. Development of a Chromatogrophic fingerprint of tobacco flavor by use of GC and GC-MS. Chromatographia 2009, 69, 735–742. [Google Scholar] [CrossRef]
- McDonnell, E.; Hulin-Bertaud, S.; Sheehan, E.M.; Delahunty, C.M. Development and learning process of a sensory vocabulary for the odor evaluation of selected distilled beverages using descriptive analysis. J. Sens. Stud. 2001, 16, 425–445. [Google Scholar] [CrossRef]
- Phillips, K.; Bremer, P.; Silcock, P.; Hamid, N.; Delahunty, C.; Barker, M.; Kissick, J. Effect of gender, diet and storage time on the physical properties and sensory quality of sea urchin (Evechinus chloroticus) gonads. Aquaculture 2009, 288, 205–215. [Google Scholar] [CrossRef]
- Anjiki, N.; Kawahara, N.; Goda, Y. Studies on the taste profile analysis of Setsucha products by a taste-sensing system. Jpn. J. Food Chem. 2007, 14, 121–127, (In Japanese with English Abstract). [Google Scholar]
- Whitfield, F.B. Microbiology of food taints. Int. J. Food Sci. Technol. 1998, 33, 31–51. [Google Scholar] [CrossRef]
- Le Guen, S.; Prost, C.; Demaimay, M. Characterization of odorant compounds of mussels (Mytilus edulis) according to their origin using gas chromatography–olfactometry and gas chromatography–mass spectrometry. J. Chromatogr. A 2000, 896, 361–371. [Google Scholar] [CrossRef]
- Cruz-Romero, M.; Kerry, J.P.; Kelly, A.L. Changes in the microbiological and physicochemical quality of high-pressure-treated oyster (Crassostrea gigas) during chilled storage. Food Control 2008, 19, 1139–1147. [Google Scholar] [CrossRef]
- Oishi, K.; Kunisaki, N. Free amino acid composition of acceleratedly cultured makombu, Laminaria japonica, at different growing stages. Bull. Jpn. Soc. Sci. Fish. 1970, 36, 1181–1185, (In Japanese with English Abstract). [Google Scholar] [CrossRef][Green Version]
- Fukushi, A. Seasonal variation of components in different parts of cultivated Japanese kelp (Laminaria japonica). Sci. Rep. Hokkaido Fish. Exp. Stn. 1988, 31, 55–61, (In Japanese with English Abstract). [Google Scholar]
- Takagi, S.; Murata, Y.; Inomata, E.; Aoki, M.N.; Agatsuma, Y. Pronounced effects of the basal frond portion of the kelp Saccharina japonica on gonad qualities of the sea urchin Mesocentrotus nudus from a barren. Aquaculture 2020, 516, 734623. [Google Scholar] [CrossRef]
- Komata, Y. Studies on the extractives of “Uni”–IV. Taste of each component in the extractives. Bull. Jpn. Soc. Sci. Fish. 1964, 30, 749–756, (In Japanese with English Abstract). [Google Scholar] [CrossRef]
- Agatsuma, Y. Aquaculture of the sea urchin (Strongylocentrotus nudus) transplanted from coralline flats in Hokkaido, Japan. J. Shellfish Res. 1998, 17, 1541–1547. [Google Scholar]
- Hoshikawa, H.; Takahashi, K.; Sugimoto, T.; Tuji, K.; Nobuta, S. The effects of fish meal feeding on the gonad quality of cultivated sea urchins, Strongylocentrotus nudus (A. AGASSIZ). Sci. Rep. Hokkaido Fish. Exp. Stn. 1998, 52, 17–24, (In Japanese with English Abstract). [Google Scholar]
- Murata, Y.; Sata, N.U. Isolation and structure of pulcherrimine, a novel bitter-tasting amino acid, from the sea urchin (Hemicentrotus pulcherrimus) ovaries. J. Agric. Food Chem. 2000, 48, 5557–5560. [Google Scholar] [CrossRef] [PubMed]
- Osako, K.; Kiriyama, T.; Ruttanapornvareesakul, Y.; Kuwahara, K.; Okamoto, A.; Nagano, N. Free amino acid compositions of the gonad of the wild and cultured sea urchins Anthocidaris crassispina. Aquaculture Sci. 2006, 54, 301–304. [Google Scholar]
- Inomata, E.; Murata, Y.; Matsui, T.; Agatsuma, Y. Gonadal production and quality in the sea urchin Mesocentrotus nudus fed a high-protein concentrated red alga Pyropia yezoensis. Aquaculture 2016, 454, 184–191. [Google Scholar] [CrossRef]
- Akitomi, H.; Tahara, Y.; Yasuura, M.; Kobayashi, Y.; Ikezaki, H.; Toko, K. Quantification of tastes of amino acids using taste sensors. Sens. Actuators B Chem. 2013, 179, 276–281. [Google Scholar] [CrossRef]
- Ninomiya, K. Science of umami taste: Adaptation to gastronomic culture. Flavour 2015, 4, 13. [Google Scholar] [CrossRef]
- Kirimura, J.; Shimizu, A.; Kimizuka, A.; Ninomiya, T.; Katsuya, N. The contribution of peptides and amino acids to the taste of feedstuffs. J. Agric. Food Chem. 1969, 17, 689–695. [Google Scholar] [CrossRef]
- Kurihara, K. Umami the fifth basic taste: History of studies on receptor mechanisms and role as a food flavor. BioMed Res. Int. 2015, 189402. [Google Scholar] [CrossRef]
- Unuma, T.; Murata, Y.; Hasegawa, N.; Sawaguchi, S.; Takahashi, K. Improving the food quality of sea urchins collected from barren grounds by short-term aquaculture under controlled temperature. Bull. Fish. Res. Agen. 2015, 40, 145–153. [Google Scholar]
- Pert, C.; Swearer, S.E.; Dworjanyn, S.A.; Kriegisch, N.; Turchini, G.; Francis, D.; Dempster, T. Barrens of gold: Gonad conditioning of an overabundant sea urchin. Aquacult. Environ. Interact. 2018, 10, 345–361. [Google Scholar] [CrossRef]
- FAO. Global Aquaculture Production. 2020. Available online: http://www.fao.org/fishery/statistics/global-aquaculture-production/en (accessed on 24 May 2020).
- UN. Transforming our World: The 2030 Agenda for Sustainable Development. 2015. Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed on 24 May 2020).

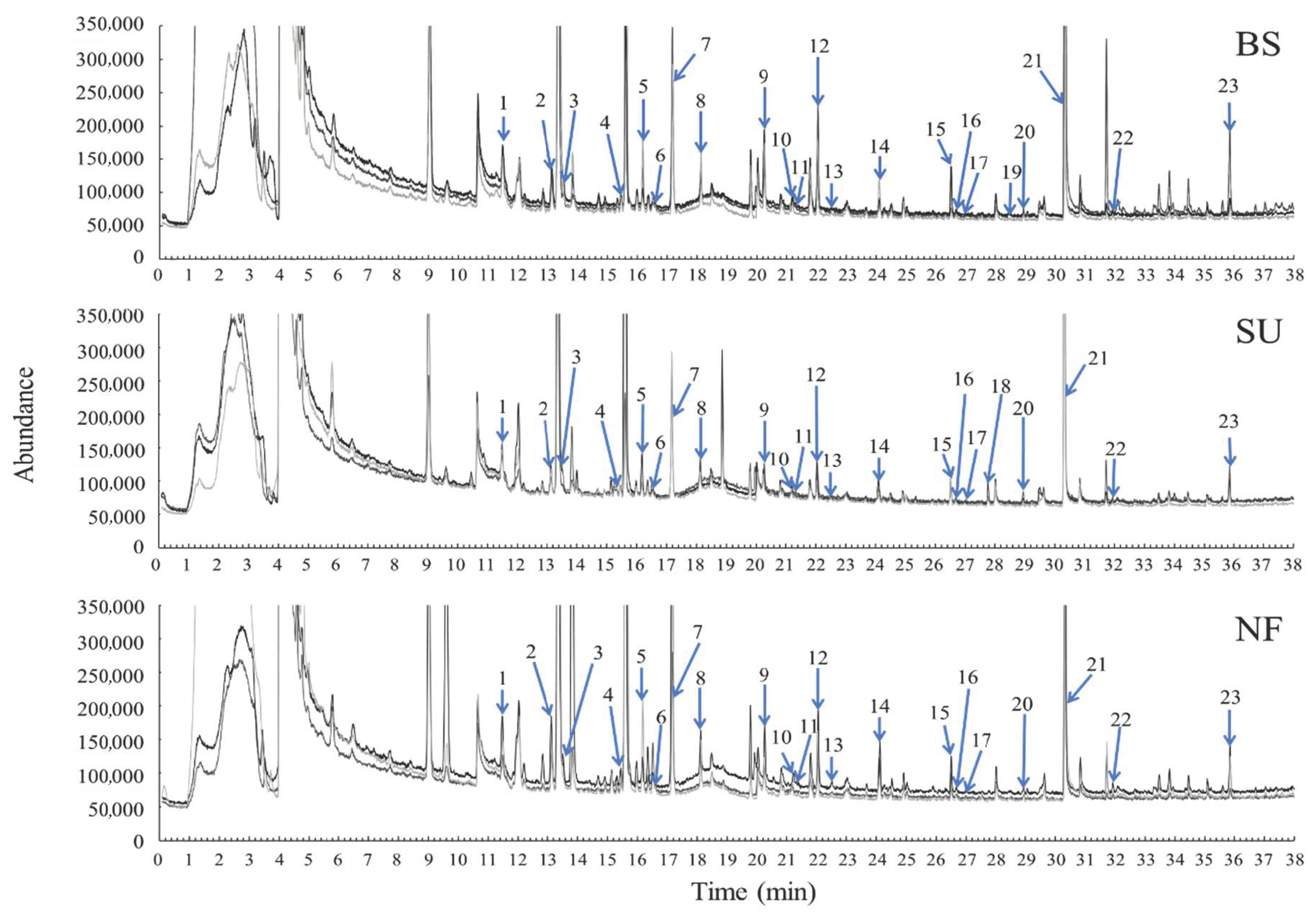
| SNF-RT | GCMS-RT | Compound | NO. | Description | SNF Detection | Peak Area | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BS | SU | NF | BS | SU | NF | p | |||||
| 11.50 | 11.47 | Ethyl acetate | 1 | Fresh, green * | + | 186.07 ± 12.24 | 133.22 ± 27.31 | 154.65 ± 44.16 | 0.514 | ||
| 12.60–12.70 | Unknown | Sea urchin * | + | ||||||||
| 13.20 | 13.13 | 2-Propanol | 2 | Fresh, green, fishy † | + | + | 224.42 ± 27.05 | 168.95 ± 28.73 | 307.15 ± 59.18 | 0.134 | |
| 13.50–13.60 | 13.52 | Benzene | 3 | Sweet, caramel * | + | + | 34.62 ± 9.12 | 42.31 ± 10.80 | 49.83 ± 17.04 | 0.716 | |
| 15.48 | 15.45 | Decane | 4 | Green * | + | 83.07 ± 13.52 | 61.35 ± 5.68 | 69.84 ± 16.20 | 0.511 | ||
| 16.20–16.30 | 16.17 | Chloroform | 5 | Putrid, fishy † | + | + | 238.68 ± 86.48 | 126.32 ± 49.58 | 287.84 ± 121.82 | 0.481 | |
| 16.73–16.75 | 16.60 | α-Pinene | 6 | Sea urchin † | + | + | 9.83 ± 2.01 | 5.35 ± 0.93 | 9.44 ± 1.92 | 0.198 | |
| 17.18 | 17.18 | Toluene | 7 | Green, chemical | + | + | 933.53 ± 80.79 | 757.52 ±33.75 | 1695.73 ± 643.82 | 0.196 | |
| 18.03–18.04 | 18.08 | Butyl acetate | 8 | Fruit, sour, green * | + | + | 8.16 ± 0.77 | 6.88 ± 1.27 | 8.82 ± 3.47 | 0.846 | |
| 18.30 | Unknown | Sea urchin * | + | ||||||||
| 20.38–20.41 | 20.25 | Xylene | 9 | Orange, fresh, sweet * | + | 313.71 ± 51.19 a | 104.56 ±11.22 b | 126.15 ± 18.83 b | 0.004 | ||
| 21.30 | 21.23 | Dodecane | 10 | Fishy † | + | 8.89 ± 0.79 | 7.05 ± 0.85 | 9.89 ± 1.24 | 0.198 | ||
| 21.32 | Ethyl toluene | 11 | 9.61 ± 1.97 | 6.23 ± 1.03 | 6.72 ± 2.22 | 0.418 | |||||
| 21.69 | Unknown | Sea urchin * | + | ||||||||
| 22.05 | 22.06 | Limonene | 12 | Sea urchin † | + | 408.43 ± 78.32 | 153.03 ± 24.23 | 306.63 ± 59.68 | 0.060 | ||
| 22.61 | 22.52 | Propyl benzene | 13 | Putrid, smoke † | + | 8.70 ± 0.33 | 6.42 ± 0.57 | 8.97 ± 1.82 | 0.284 | ||
| 22.78 | Unknown | Fishy † | + | ||||||||
| 24.13 | 24.12 | Styrene | 14 | Putrid, Undaria † | + | 30.38 ± 3.86 b | 18.57 ± 2.62 b | 60.43 ± 11.53 a | 0.005 | ||
| 26.63–26.66 | 26.50 | 6-Methyl-5-hepten-2-one | 15 | Sea urchin † | + | + | 113.72 ± 56.79 | 104.01 ± 19.40 | 117.70 ± 33.72 | 0.969 | |
| 26.67 | Isopropyl benzene | 16 | 9.03 ± 0.60 | 5.95 ± 0.40 | 8.69 ± 1.33 | 0.088 | |||||
| 27.18 | 27.00 | Mentha-1,4,8-triene | 17 | Sea urchin after taste | + | 4.07 ± 0.19 | 3.11 ± 0.35 | 4.12 ± 0.41 | 0.123 | ||
| 27.30 | Unknown | Sea urchin, citrus * | + | ||||||||
| 27.85 | 27.79 | 3-Octanol | 18 | Sea urchin * | + | ND b | 59.66 ± 17.09 a | ND b | <0.001 | ||
| 28.50 | 28.47 | 2-Butoxyethanol | 19 | Green, heavy, seaweed | + | 4.43 ± 1.35 a | ND b | ND b | <0.001 | ||
| 29.00–29.04 | 28.95 | Ethyl octanoate | 20 | Sea urchin, sour and sweet † | + | + | + | 8.28 ± 2.13 | 9.00 ± 3.73 | 8.16 ± 0.47 | 0.973 |
| 30.50 | 30.32 | 2-Ethylhexanol | 21 | Green * | + | 5198.37 ± 3375.74 | 2396.09 ± 403.79 | 4171.60 ± 1338.62 | 0.731 | ||
| 31.87–31.89 | 31.92 | Benzaldehyde | 22 | Sweet, floral, fresh * | + | + | + | 8.60 ± 1.09 | 8.21 ± 0.81 | 13.01 ± 3.38 | 0.276 |
| 32.30 | Unknown | Sea urchin, fresh shellfish * | + | ||||||||
| 33.39–33.50 | Unknown | Heavy, butter † | + | + | |||||||
| 34.81 | Unknown | Sea urchin * | + | ||||||||
| 35.90 | 35.86 | 2-Methyl-6-methylene-2,7-octadienal | 23 | Sweet * | + | 218.91 ± 125.88 | 122.85 ± 27.19 | 169.77 ± 59.04 | 0.885 | ||
| 36.60 | Unknown | Sea urchin, fresh shellfish | + | + | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takagi, S.; Sato, Y.; Murata, Y.; Kokubun, A.; Touhata, K.; Ishida, N.; Agatsuma, Y. Quantification of the Flavor and Taste of Gonads from the Sea Urchin Mesocentrotus nudus Using GC–MS and a Taste-Sensing System. Sensors 2020, 20, 7008. https://doi.org/10.3390/s20247008
Takagi S, Sato Y, Murata Y, Kokubun A, Touhata K, Ishida N, Agatsuma Y. Quantification of the Flavor and Taste of Gonads from the Sea Urchin Mesocentrotus nudus Using GC–MS and a Taste-Sensing System. Sensors. 2020; 20(24):7008. https://doi.org/10.3390/s20247008
Chicago/Turabian StyleTakagi, Satomi, Yoichi Sato, Yuko Murata, Atsuko Kokubun, Ken Touhata, Noriko Ishida, and Yukio Agatsuma. 2020. "Quantification of the Flavor and Taste of Gonads from the Sea Urchin Mesocentrotus nudus Using GC–MS and a Taste-Sensing System" Sensors 20, no. 24: 7008. https://doi.org/10.3390/s20247008
APA StyleTakagi, S., Sato, Y., Murata, Y., Kokubun, A., Touhata, K., Ishida, N., & Agatsuma, Y. (2020). Quantification of the Flavor and Taste of Gonads from the Sea Urchin Mesocentrotus nudus Using GC–MS and a Taste-Sensing System. Sensors, 20(24), 7008. https://doi.org/10.3390/s20247008







