SnO2/TiO2 Thin Film n-n Heterostructures of Improved Sensitivity to NO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.1.1. SnO2 Thin Films
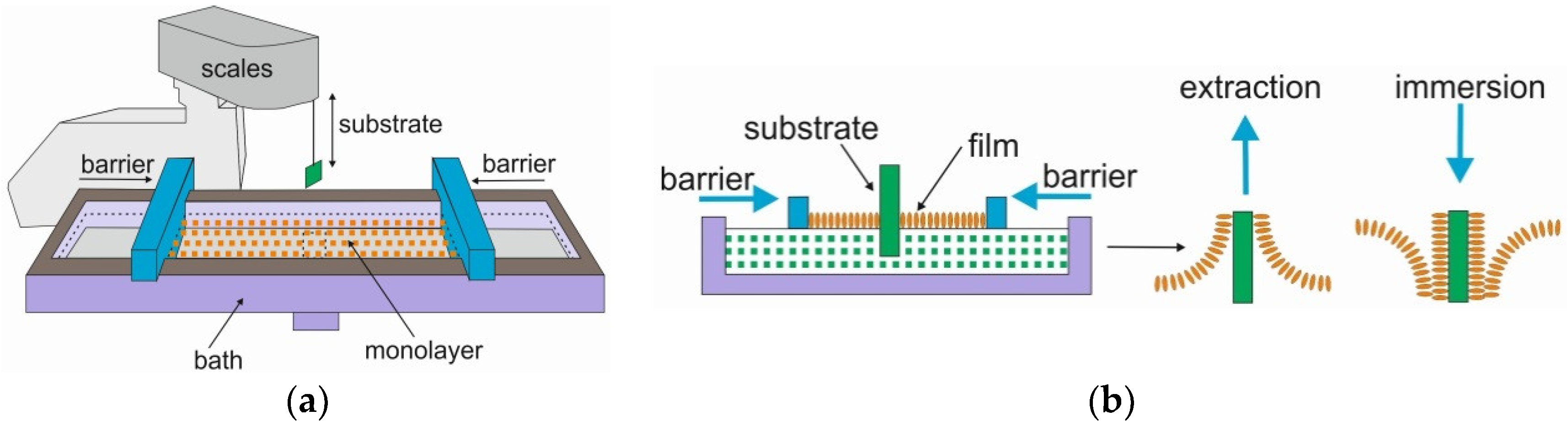
2.1.2. TiO2 Thin Films
2.2. Characterization Methods
3. Results and Discussion
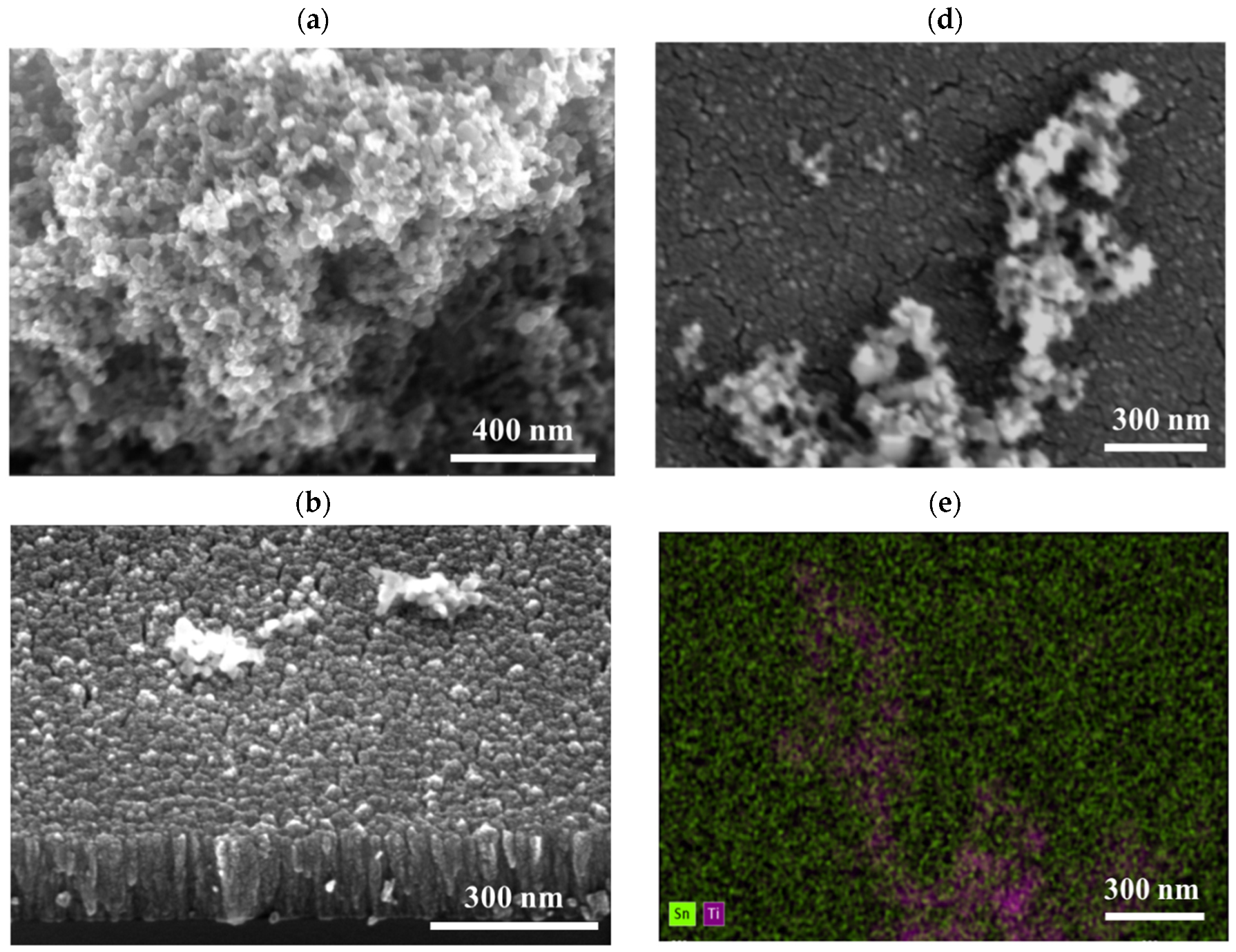
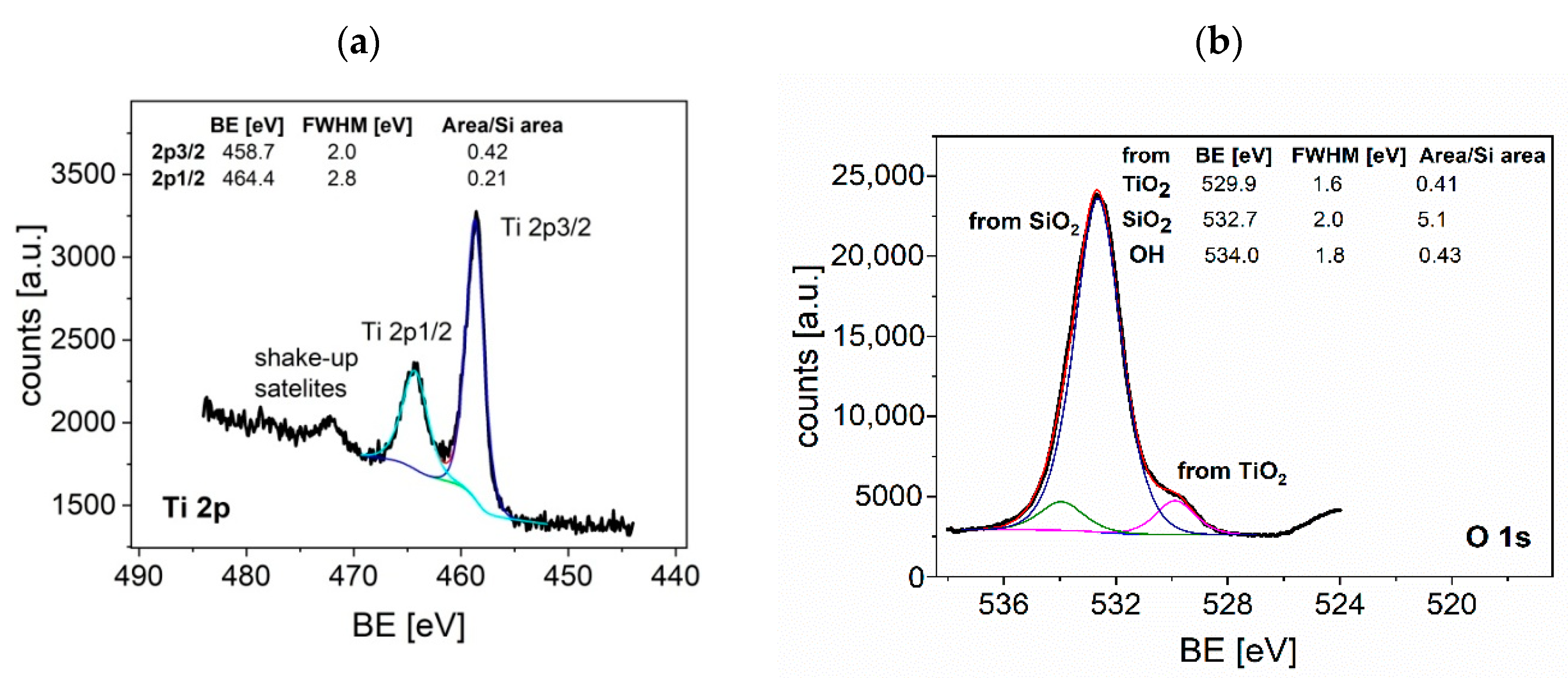
3.1. Film Characterization
3.2. Gas Sensor Measurements
4. Discussion
- physisorption of water in its molecular form that occurs at lower temperatures
- chemisorption of OH− taking place at higher temperatures above 300 °C
5. Conclusions
- The heterostructures composed of TiO2 agglomerated discontinuous layer on the SnO2 thin film with a columnar mode of growth have a higher gas response than pure SnO2 for both reducing (H2) and oxidizing (NO2) gases.
- Amorphous a-SnO2 demonstrate a much higher response to NO2 than their crystalline counterparts c-SnO2, probably because of the size effect.
- SnO2/TiO2 heterostructures are selective and sensitive even to low concentrations of NO2 which can be attributed to the electron injection from the conduction band CB of TiO2 to CB of SnO2.
- The significant increase in NO2 response occurs at an operating temperature below 150 °C where a considerable influence of humidity has been demonstrated; this effect is probably due to the competitive physisorption of water against chemisorption of oxygen and hydroxyl groups.
- SnO2/TiO2 n-n nanoheterostructures in a form of thin films have proven to be highly sensitive and selective to NO2 with a threshold lower than 200 ppb.
Author Contributions
Funding
Conflicts of Interest
References
- U.S. National Library of Medicine, TOXNET—Toxicology Data Network, (n.d.). Available online: https://toxnet.nlm.nih.gov (accessed on 26 November 2018).
- The National Institute for Occupational Safety and Health (NIOSH), Nitrogen Dioxide—International Chemical Safety Cards, (n.d.). Available online: https://www.cdc.gov/niosh/ipcsneng/neng0930.html (accessed on 26 November 2018).
- Ielpo, P.; Mangia, C.; Marra, G.P.; Comite, V.; Rizza, U.; Uricchio, V.F.; Fermo, P. Outdoor spatial distribution and indoor levels of NO2 and SO2 in a high environmental risk site of the South Italy. Sci. Total Environ. 2019, 648, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Casquero-Vera, J.A.; Lyamani, H.; Titos, G.; Borrás, E.; Olmo, F.J.; Alados-Arboledas, L. Impact of primary NO2 emissions at different urban sites exceeding the European NO2 standard limit. Sci. Total Environ. 2019, 646, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Salthammer, T.; Schieweck, A.; Gu, J.; Ameri, S.; Uhde, E. Future trends in ambient air pollution and climate in Germany—Implications for the indoor environment. Build. Environ. 2018, 143, 661–670. [Google Scholar] [CrossRef]
- Mölter, A.; Lindley, S.; de Vocht, F.; Agius, R.; Kerry, G.; Johnson, K.; Ashmore, M.; Terry, A.; Dimitroulopoulou, S.; Simpson, A. Performance of a microenviromental model for estimating personal NO2 exposure in children. Atmos. Environ. 2012, 51, 225–233. [Google Scholar] [CrossRef]
- Beulertz, G.; Groß, A.; Moos, R.; Kubinski, D.J.; Visser, J.H. Determining the total amount of NOx in a gas stream—Advances in the accumulating gas sensor principle. Sens. Actuators B 2012, 175, 157–162. [Google Scholar] [CrossRef]
- Keskinen, H.; Tricoli, A.; Marjamäki, M.; Mäkelä, J.M.; Pratsinis, S.E. Size-selected agglomerates of SnO2 nanoparticles as gas sensors. J. Appl. Phys. 2009, 106, 084316. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Han, S.H.; Gulina, L.B.; Tolstoy, V.P.; Cho, B.K. SnO2 films decorated by Au clusters and their gas sensing properties. Mater. Sci. Forum 2015, 827, 251–256. [Google Scholar] [CrossRef]
- Comini, E.; Faglia, G.; Sberveglieri, G.; Calestani, D.; Zanotti, L.; Zha, M. Tin oxide nanobelts electrical and sensing properties. Sens. Actuators B 2005, 111–112, 2–6. [Google Scholar] [CrossRef]
- Shukla, S.; Patil, S.; Kuiry, S.C.; Rahman, Z.; Du, T.; Ludwig, L.; Parish, C.; Seal, S. Synthesis and characterization of sol–gel derived nanocrystalline tin oxide thin film as hydrogen sensor. Sens. Actuators B 2003, 96, 343–353. [Google Scholar] [CrossRef]
- Alberti, A.; Renna, L.; Sanzaro, S.; Smecca, E.; Mannino, G.; Bongiorno, C.; Galati, C.; Gervasi, L.; Santangelo, A.; La Magna, A. Innovative spongy TiO2 layers for gas detection at low working temperature. Sens. Actuators B 2018, 259, 658–667. [Google Scholar] [CrossRef]
- Huang, L.; Liu, T.; Zhang, H.; Guo, W.; Zeng, W. Hydrothermal synthesis of different TiO2 nanostructures: Structure, growth and gas sensor properties. J. Mater. Sci. Mater. Electron. 2012, 23, 2024–2029. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Qi, Y.; Lun, N.; Liu, N. Large scale synthesis and gas-sensing properties of anatase TiO2 three-dimensional hierarchical nanostructures. Langmuir 2010, 26, 12841–12848. [Google Scholar] [CrossRef] [PubMed]
- Haidry, A.A.; Kind, N.; Saruhan, B. Investigating the influence of Al-doping and background humidity on NO2 sensing characteristics of magnetron-sputtered SnO2 sensors. J. Sens. Sens. Syst. 2015, 4, 271–280. [Google Scholar] [CrossRef]
- Sukunta, J.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S.; Liewhiran, C. WO3 nanotubes−SnO2 nanoparticles heterointerfaces for ultrasensitive and selective NO2 detections. Appl. Surf. Sci. 2018, 458, 319–332. [Google Scholar] [CrossRef]
- Santos, J.; Serrini, P.; O’Beirn, B.; Manes, L. A thin film SnO2 gas sensor selective to ultra-low NO2 concentrations in air. Sens. Actuators B 1997, 43, 154–160. [Google Scholar] [CrossRef]
- Kaur, J.; Kumar, R.; Bhatnagar, M.C. Effect of indium-doped SnO2 nanoparticles on NO2 gas sensing properties. Sens. Actuators B 2007, 126, 478–484. [Google Scholar] [CrossRef]
- Liangyuan, C.; Shouli, B.; Guojun, Z.; Dianqing, L.; Aifan, C.; Liu, C.C. Synthesis of ZnO–SnO2 nanocomposites by microemulsion and sensing properties for NO2. Sens. Actuators B 2008, 134, 360–366. [Google Scholar] [CrossRef]
- Bai, S.; Li, D.; Han, D.; Luo, R.; Chen, A.; Chung, C.L. Preparation, characterization of WO3–SnO2 nanocomposites and their sensing properties for NO2. Sens. Actuators B 2010, 150, 749–755. [Google Scholar] [CrossRef]
- Chen, A.; Huang, X.; Tong, Z.; Bai, S.; Luo, R.; Liu, C.C. Preparation, characterization and gas-sensing properties of SnO2-In2O3 nanocomposite oxides. Sens. Actuators B 2006, 115, 316–321. [Google Scholar] [CrossRef]
- Sharma, A.; Tomar, M.; Gupta, V. Enhanced response characteristics of SnO2 thin film based NO2 gas sensor integrated with nanoscaled metal oxide clusters. Sens. Actuators B 2013, 181, 735–742. [Google Scholar] [CrossRef]
- Leo, G.; Rella, R.; Siciliano, P.; Capone, S.; Alonso, J.C.; Pankov, V.; Ortiz, A. Sprayed SnO2 thin films for NO2 sensors. Sens. Actuators B 1999, 58, 370–374. [Google Scholar] [CrossRef]
- Sauvan, M.; Pijolat, C. Selectivity improvement of SnO2 films by superficial metallic films. Sens. Actuators B 1999, 58, 295–301. [Google Scholar] [CrossRef]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Kwon, Y.J.; Kim, S.S.; Kim, T.W.; Kim, H.W. Selective NO2 sensor based on Bi2O3 branched SnO2 nanowires. Sens. Actuators B 2018, 274, 356–369. [Google Scholar] [CrossRef]
- Srivastava, V.; Jain, K. At room temperature graphene/SnO2 is better than MWCNT/SnO2 as NO2 gas sensor. Mater. Lett. 2016, 169, 28–32. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Wang, Z.; Song, Z.; Zhou, X.; Han, N.; Chen, Y. Sputtered SnO2:NiO thin films on self-assembled Au nanoparticle arrays for MEMS compatible NO2 gas sensors. Sens. Actuators B 2019, 278, 28–38. [Google Scholar] [CrossRef]
- Kamble, D.L.; Harale, N.S.; Patil, V.L.; Patil, P.S.; Kadam, L.D. Characterization and NO2 gas sensing properties of spray pyrolyzed SnO2 thin films. J. Anal. Appl. Pyrolysis 2017, 127, 38–46. [Google Scholar] [CrossRef]
- Gu, D.; Li, X.; Zhao, Y.; Wang, J. Enhanced NO2 sensing of SnO2/SnS2 heterojunction based sensor. Sens. Actuators B 2017, 244, 67–76. [Google Scholar] [CrossRef]
- Hyodo, T.; Urata, K.; Kamada, K.; Ueda, T.; Shimizu, Y. Semiconductor-type SnO2-based NO2 sensors operated at room temperature under UV-light irradiation. Sens. Actuators B 2017, 253, 630–640. [Google Scholar] [CrossRef]
- Yu, H.; Yang, T.; Wang, Z.; Li, Z.; Zhao, Q.; Zhang, M. p-N heterostructural sensor with SnO-SnO2 for fast NO2 sensing response properties at room temperature. Sens. Actuators B Chem. 2018, 258, 517–526. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, Z.; Li, Q.; Zhang, X.; Sun, W.; Sun, J.; Liu, B.; Ha, B. The enhanced NO2 sensing properties of SnO2 nanoparticles/reduced graphene oxide composite. J. Colloid Interface Sci. 2019, 537, 228–237. [Google Scholar] [CrossRef]
- Li, Z.; Yi, J. Synthesis and enhanced NO2-sensing properties of ZnO-decorated SnO2 microspheres. Mater. Lett. 2019, 236, 570–573. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Z.; Zhang, Z. Visible light assisted room-temperature NO2 gas sensor based on hollow SnO2@SnS2 nanostructures. Sens. Actuators B 2020, 324, 128754. [Google Scholar] [CrossRef]
- Sharma, B.; Sharma, A.; Joshi, M.; Myung, J. Sputtered SnO2/ZnO heterostructures for improved NO2 gas sensing properties. Chemosensors 2020, 8, 67. [Google Scholar] [CrossRef]
- Maziarz, W. TiO2/SnO2 and TiO2/CuO thin film nanoheterostructures as gas sensors. Appl. Surf. Sci. 2019, 480, 361–370. [Google Scholar] [CrossRef]
- Kusior, A.; Radecka, M.; Zych, Ł.; Zakrzewska, K.; Reszka, A.; Kowalski, B.J. Sensitization of TiO2/SnO2 nanocomposites for gas detection. Sens. Actuators B 2013, 189, 251–259. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.; Wang, Z. Sensitivity improvement of TiO2-doped SnO2 to volatile organic compounds. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 43, 633–638. [Google Scholar] [CrossRef]
- Park, S.; Kim, S.; Kheel, H.; Park, S.E.; Lee, C. Synthesis and hydrogen gas sensing properties of TiO2-decorated CuO nanorods. Bull. Korean Chem. Soc. 2015, 36, 2458–2463. [Google Scholar] [CrossRef]
- Shao, F.; Hoffmann, M.W.G.; Prades, J.D.; Zamani, R.; Arbiol, J.; Morante, J.R.; Varechkina, E.; Rumyantseva, M.; Gaskov, A.; Giebelhaus, I.; et al. Heterostructured p-CuO (nanoparticle)/n-SnO2 (nanowire) devices for selective H2S detection. Sens. Actuators B 2013, 181, 130–135. [Google Scholar] [CrossRef]
- Kaur, M.; Dadhich, B.K.; Singh, R.; Kailasaganapathi, S.; Bagwaiya, T.; Bhattacharya, S.; Debnath, A.K.; Muthe, K.P.; Gadkari, S.C. RF sputtered SnO2: NiO thin films as sub-ppm H2S sensor operable at room temperature. Sens. Actuators B 2017, 242, 389–403. [Google Scholar] [CrossRef]
- Poloju, M.; Jayababu, N.; Ramana Reddy, M.V. Improved gas sensing performance of Al doped ZnO/CuO nanocomposite based ammonia gas sensor. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2018, 227, 61–67. [Google Scholar] [CrossRef]
- Larin, A.; Womble, P.C.; Dobrokhotov, V. Hybrid SnO2/TiO2 nanocomposites for selective detection of ultra-low hydrogen sulfide concentrations in complex backgrounds. Sensors 2016, 16, 1373. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Lyson-Sypien, B.; Kusior, A.; Rekas, M.; Zukrowski, J.; Gajewska, M.; Michalow-Mauke, K.; Graule, T.; Radecka, M.; Zakrzewska, K. Nanocrystalline TiO2/SnO2 heterostructures for gas sensing. Beilstein J. Nanotechnol. 2017, 8, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewska, K.; Radecka, M. TiO2-SnO2 system for gas sensing-photodegradation of organic contaminants. Thin Solid Films 2007, 515, 8332–8338. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Radecka, M. TiO2-based nanomaterials for gas sensing—influence of anatase and rutile contributions. Nanoscale Res. Lett. 2017, 12, 89. [Google Scholar] [CrossRef]
- McNamee, C.E.; Yamamoto, S.; Butt, H.J.; Higashitani, K. A straightforward way to form close-packed TiO2 particle monolayers at an air/water interface. Langmuir 2011, 27, 887–894. [Google Scholar] [CrossRef]
- Ganguly, P.; Paranjape, D.V.; Sastry, M. Novel structure of Langmuir-Blodgett films of chloroplatinic acid using n-octadecylamine: Evidence for interdigitation of hydrocarbon chains. J. Am. Chem. Soc. 1993, 115, 793–794. [Google Scholar] [CrossRef]
- Choudhury, S.; Bagkar, N.; Dey, G.K.; Subramanian, H.; Yakhmi, J.V. Crystallization of prussian blue analogues at the air-water interface using an octadecylamine monolayer as a template. Langmuir 2002, 18, 7409–7414. [Google Scholar] [CrossRef]
- Amm, D.T.; Johnson, D.J.; Laursen, T.; Gupta, S.K. Fabrication of ultrathin metal oxide films using Langmuir-Blodgett deposition. Appl. Phys. Lett. 1992, 61, 522–524. [Google Scholar] [CrossRef]
- Paranjape, D.V.; Sastry, M.; Ganguly, P. Deposition of thin films of TiO2 from Langmuir-Blodgett film precursors. Appl. Phys. Lett. 1993, 63, 18–20. [Google Scholar] [CrossRef]
- Schurr, M.; Hassmann, J.; Kügler, R.; Tomaschko, C.; Voit, H. Ultrathin layers of rare earth oxides from Langmuir-Blodgett films. Thin Solid Films 1997, 307, 260–265. [Google Scholar] [CrossRef]
- Kwon, H.; Yoon, J.S.; Lee, Y.; Kim, D.Y.; Baek, C.K.; Kim, J.K. An array of metal oxides nanoscale hetero p-n junctions toward designable and highly-selective gas sensors. Sens. Actuators B Chem. 2018, 255, 1663–1670. [Google Scholar] [CrossRef]
- Nowak, P.; Maziarz, W.; Rydosz, A.; Kowalski, K.; Zakrzewska, K. SnO2/TiO2 thin film n-n heterostructures for H2 and NO2 gas sensor. In Proceedings of the 17th International Meeting on Chemical Sensors—IMCS 2018, Vienna, Austria, 15–19 July 2018; pp. 549–550. [Google Scholar] [CrossRef]
- Zakrzewska, K.; Maziarz, W.; Schneider, K.; Mazur, M.; Wojcieszak, D.; Kaczmarek, D. Cu2O/CuO thin film p-p nano-heterostructures for gas sensing. In Proceedings of the 17th International Meeting on Chemical Sensors—IMCS 2018, Vienna, Austria, 15–19 July 2018; pp. 789–790. [Google Scholar] [CrossRef]
- Rydosz, A.; Maziarz, W.; Brudnik, A.; Czapla, A.; Zakrzewska, K. CuO and CuO/TiO2-y thin-film gas sensors of H2 and NO2. In Proceedings of the XV International Scientific Conference on Optoelectronic and Electronic Sensors (COE), Warsaw, Poland, 17–20 June 2018; pp. 2016–2019. [Google Scholar]
- Xu, H.; Ju, J.; Li, W.; Zhang, J.; Wang, J.; Cao, B. Superior triethylamine-sensing properties based on TiO2/SnO2 n-n heterojunction nanosheets directly grown on ceramic tubes. Sens. Actuators B 2016, 228, 634–642. [Google Scholar] [CrossRef]
- Maziarz, W.; Kusior, A.; Trenczek-Zajac, A. Nanostructured TiO2-based gas sensors with enhanced sensitivity to reducing gases. Beilstein J. Nanotechnol. 2016, 7, 1718–1726. [Google Scholar] [CrossRef]
- Choudhary, K.; Manjuladevi, V.; Gupta, R.K.; Bhattacharyya, P.; Hazra, A.; Kumar, S. Ultrathin films of TiO2 nanoparticles at interfaces. Langmuir 2015, 31, 1385–1392. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M.P. (Eds.) Practical Surface Analysis, 2nd ed.; Wiley: Chichester, UK; New York, NY, USA, 1990. [Google Scholar]
- Manifacier, J.C.; Gasiot, J.; Fillard, J.P. A simple method for the determination of the optical constants n, k and the thickness of a weakly absorbing thin film. J. Phys. E 1976, 9, 1002. [Google Scholar] [CrossRef]
- Goldsmith, S.; Çetinörgü, E.; Boxman, R.L. Modeling the optical properties of tin oxide thin films. Thin Solid Films 2009, 517, 5146–5150. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Understanding the fundamental principles of metal oxide based gas sensors; The example of CO sensing with SnO2 sensors in the presence of humidity. J. Phys. Condens. Matter. 2003, 15, R813–R839. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Bielański, A.; Haber, J. Oxygen in catalysis on transition metal oxides. Catal. Rev. 1979, 19, 1–41. [Google Scholar] [CrossRef]
- Giancane, G.; Valli, L. State of art in porphyrin Langmuir-Blodgett films as chemical sensors. Adv. Colloid Interface Sci. 2012, 171–172, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Capan, İ.; Tarımcı, Ç.; Capan, R. Fabrication of Langmuir–Blodgett thin films of porphyrins and investigation on their gas sensing properties. Sens. Actuators B 2010, 144, 126–130. [Google Scholar] [CrossRef]
- Balcerzak, A.; Aleksiejuk, M.; Zhavnerko, G.; Agabekov, V. Sensing properties of two-component Langmuir-Blodgett layer and its porous derivative in SAW sensor for vapors of methanol and ethanol. Thin Solid Films 2010, 518, 3402–3406. [Google Scholar] [CrossRef]
- Penza, M.; Milella, E.; Musio, F.; Alba, M.B.; Cassano, G.; Quirini, A. AC and DC measurements on Langmuir-Blodgett polypyrrole films for selective NH3 gas detection. Mater. Sci. Eng. C. 1998, 5, 255–258. [Google Scholar] [CrossRef]
- Oswald, M.; Hessel, V.; Riedel, R. Formation of ultra-thin ceramic TiO2 films by the Langmuir-Blodgett technique—A two-dimensional sol-gel process at the air-water interface. Thin Solid Films 1999, 339, 284–289. [Google Scholar] [CrossRef]
- Bhullar, G.K.; Kaur, R.; Raina, K.K. Hybrid polyaniline-TiO2 nanocomposite Langmuir-Blodgett thin films: Self-assembly and their characterization. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Choudhury, S.; Betty, C.A.; Girija, K.G.; Kulshreshtha, S.K. Room temperature gas sensitivity of ultrathin SnO2 films prepared from Langmuir-Blodgett film precursors. Appl. Phys. Lett. 2006, 89, 071914. [Google Scholar] [CrossRef]
- Ganguly, P.; Paranjape, D.V.; Sastry, M. Studies on the deposition of titanyl oxalate ions using long-chain hydrocarbon amines. Langmuir 1993, 9, 577–579. [Google Scholar] [CrossRef]
- Takahashi, M.; Kobayashi, K.; Tajima, K. Structural characterization and photocatalytic activity of ultrathin TiO2 films fabricated by Langmuir–Blodgett technique with octadecylamine. Thin Solid Films 2011, 519, 8077–8084. [Google Scholar] [CrossRef]
- Choudhury, S.; Betty, C.A. A heterostructured SnO2–TiO2 thin film prepared by Langmuir–Blodgett technique. Mater. Chem. Phys. 2013, 141, 440–444. [Google Scholar] [CrossRef]
- Betty, C.A.; Choudhury, S.; Girija, K.G. Discerning specific gas sensing at room temperature by ultrathin SnO2 films using impedance approach. Sens. Actuators B 2012, 173, 781–788. [Google Scholar] [CrossRef]
- Betty, C.A.; Choudhury, S.; Girija, K.G. Reliability studies of highly sensitive and specific multi-gas sensor based on nanocrystalline SnO2 film. Sens. Actuators B 2014, 193, 484–491. [Google Scholar] [CrossRef]
- Sahm, T.; Gurlo, A.; Bârsan, N.; Weimar, U.; Mädler, L. Fundamental studies on SnO2 by means of simultaneous work function change and conduction measurements. Thin Solid Films 2005, 490, 43–47. [Google Scholar] [CrossRef]
- Hahn, S.; Bârsan, N.; Weimar, U.; Ejakov, S.; Visser, J.; Soltis, R. CO sensing with SnO2 thick film sensors: Role of oxygen and water vapour. Thin Solid Films 2003, 436, 17–24. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Blinov, I.; Brinzari, V.; Stetter, J.R. Effect of air humidity on gas response of SnO2 thin film ozone sensors. Sens. Actuators B 2007, 122, 519–526. [Google Scholar] [CrossRef]














| NO2-Sensing MOS | Synthesis Method | Operating Temperature | RNO2/Rair | Concentration [ppm] | Reference, Year |
|---|---|---|---|---|---|
| SnO2 | rf-sputtering | 200 °C | 18 | 0.1 | [18], 1997 |
| SnO2 | sol-gel | 150 °C | 72 | 500 | [19], 2007 |
| ZnO–SnO2 | reversed microemulsion | 250 °C | 34.5 | 500 | [20], 2008 |
| WO2–SnO2 | sol precipitation | 200 °C | 186 | 200 | [21], 2010 |
| In2O3–SnO2 | co-precipitation | 200 °C | 7.5 | 1000 | [22], 2006 |
| TiO2/SnO2 | e-beam evaporation | 90 °C | 825 | 10 | [23], 2013 |
| SnO2 | chemical spray deposition | 350 °C | 60 | 500 | [24], 1999 |
| SnO2 | vapor phase deposition | 300 °C | 9 | 0.2 | [11], 2005 |
| SnO2 | chemical vapor deposition | 450 °C | 0.93 | 10 | [25], 1999 |
| SnO2+Bi2O3 | vapor-liquid-solid method | 250 °C | 56.9 | 2 | [26], 2018 |
| SnO2 + graphene SnO2+MWCNT | sol–gel method | RT | ~9.5 ~4.5 | 20 | [27], 2016 |
| Au/SnO2:NiO | sputtering | 200 °C | ∼185 | 5 | [28], 2019 |
| SnO2 | spray pyrolysis | 150 °C | 556 | 40 | [29], 2017 |
| SnO2/SnS2 | high temperature oxidation | 80 °C | 5 | 8 | [30], 2017 |
| Pd/SnO2 Pt/SnO2 | co-precipitation | 30 °C + 7mW uv | 3400 1500 | 5 | [31], 2017 |
| SnO-SnO2 | hydrothermal method | RT | 2.5 4.5 15 | 0.2 1 100 | [32], 2018 |
| SnO2-WO3 | thermal decomposition | 150 °C | 12800 | 5 | [17], 2018 |
| SnO2-graphene | hydrothermal method | 75 °C | 225 | 0.35 | [33], 2019 |
| ZnO+SnO2 | electrospinning | 200 °C | 258 | 100 | [34], 2019 |
| SnO2@SnS2 | hydrothermal method | RT, blue light | 5.2 57.3 | 0.2 5 | [35], 2020 |
| SnO2/ZnO | sputtering | 100 °C | 67 | 100 | [36], 2020 |
| Temperature [°C] | 400 ppb NO2 | 2000 ppb NO2 | ||||
|---|---|---|---|---|---|---|
| tresp [s] | trec [s] | RNO2/R0 | tresp [s] | trec [s] | RNO2/R0 | |
| 123 | 62 | 42 | 696 | 26 | 58 | 847 |
| 183 | 11 | 22 | 97 | 10 | 9 | 350 |
| 253 | 10 | 17 | 62 | 4 | 10 | 136 |
| 320 | 12 | 28 | 37 | 4 | 11 | 101 |
| 385 | 12 | 19 | 20 | 4 | - | - |
| RH % | RNO2/R0 | |||
|---|---|---|---|---|
| 150 °C | 220 °C | 235 °C | 335 °C | |
| 5 | 51 | - | - | 13 |
| 25 | 489 | 135 | 127 | - |
| 50 | 881 | 147 | 128 | - |
| 75 | - | 94 | 60 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, P.; Maziarz, W.; Rydosz, A.; Kowalski, K.; Ziąbka, M.; Zakrzewska, K. SnO2/TiO2 Thin Film n-n Heterostructures of Improved Sensitivity to NO2. Sensors 2020, 20, 6830. https://doi.org/10.3390/s20236830
Nowak P, Maziarz W, Rydosz A, Kowalski K, Ziąbka M, Zakrzewska K. SnO2/TiO2 Thin Film n-n Heterostructures of Improved Sensitivity to NO2. Sensors. 2020; 20(23):6830. https://doi.org/10.3390/s20236830
Chicago/Turabian StyleNowak, Piotr, Wojciech Maziarz, Artur Rydosz, Kazimierz Kowalski, Magdalena Ziąbka, and Katarzyna Zakrzewska. 2020. "SnO2/TiO2 Thin Film n-n Heterostructures of Improved Sensitivity to NO2" Sensors 20, no. 23: 6830. https://doi.org/10.3390/s20236830
APA StyleNowak, P., Maziarz, W., Rydosz, A., Kowalski, K., Ziąbka, M., & Zakrzewska, K. (2020). SnO2/TiO2 Thin Film n-n Heterostructures of Improved Sensitivity to NO2. Sensors, 20(23), 6830. https://doi.org/10.3390/s20236830








