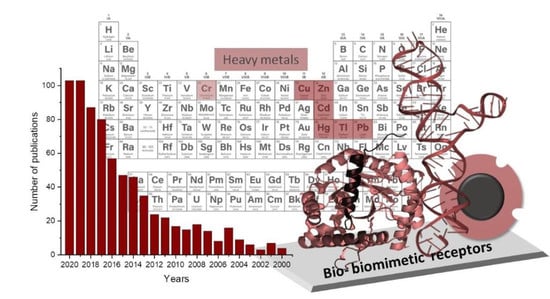
Bio- and Biomimetic Receptors for Electrochemical Sensing of Heavy Metal Ions
Abstract
1. Introduction
2. Bio- and Biomimetic Receptors for HMI
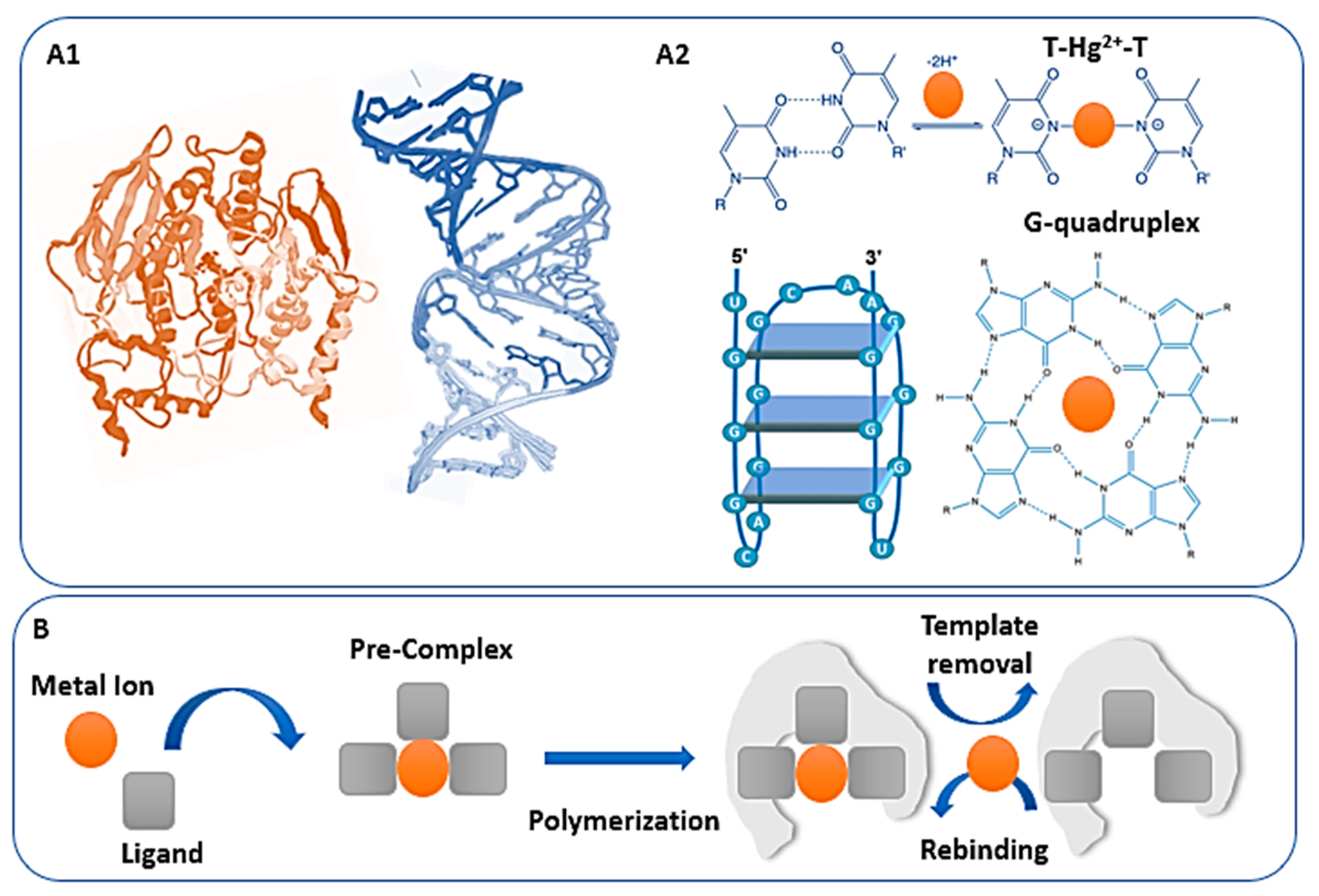
2.1. Peptides, Enzymes and Functional Nucleic Acids
2.2. Imprinted Polymers
3. HMI Sensor Overview
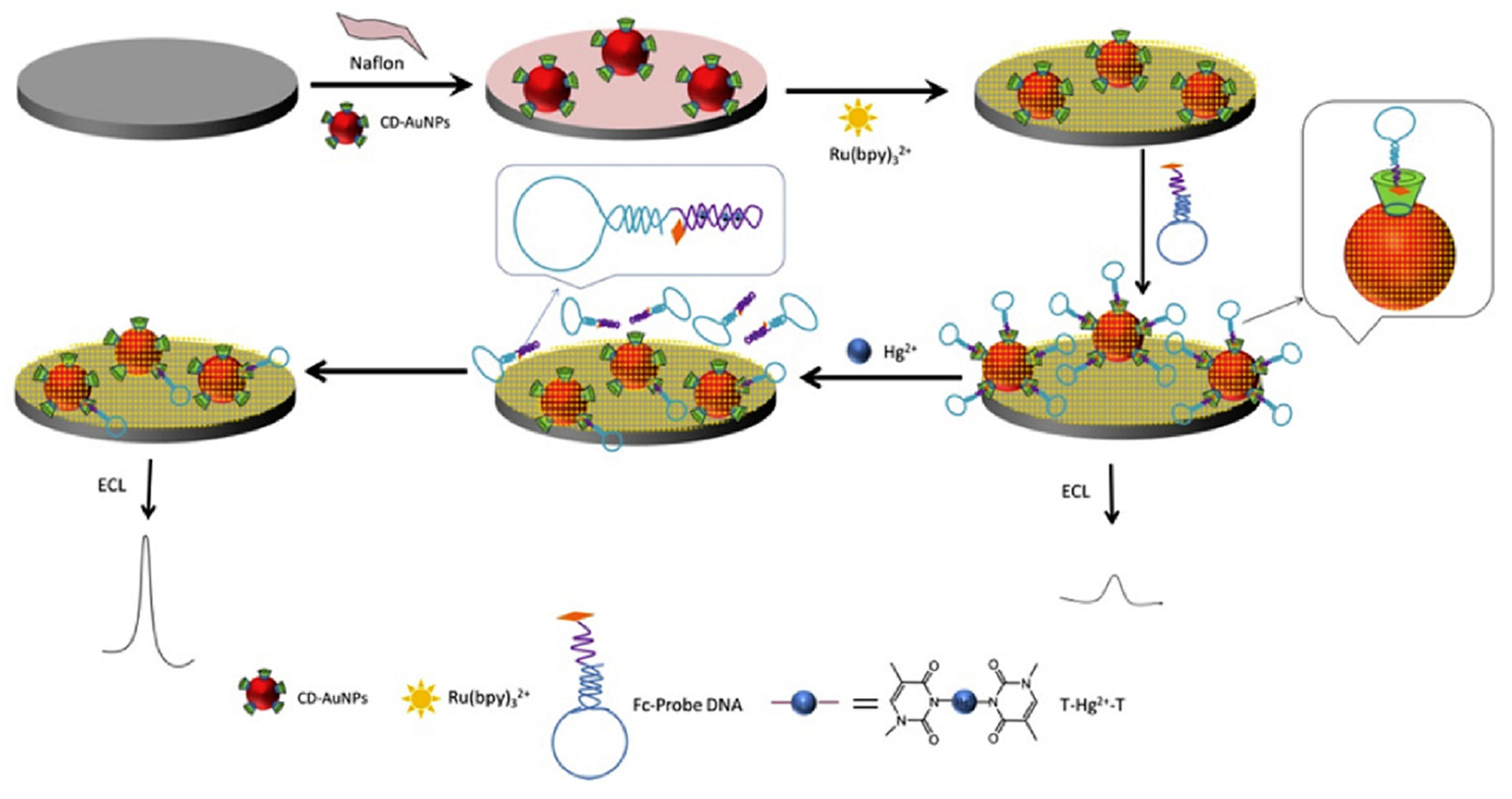
3.1. Mercury
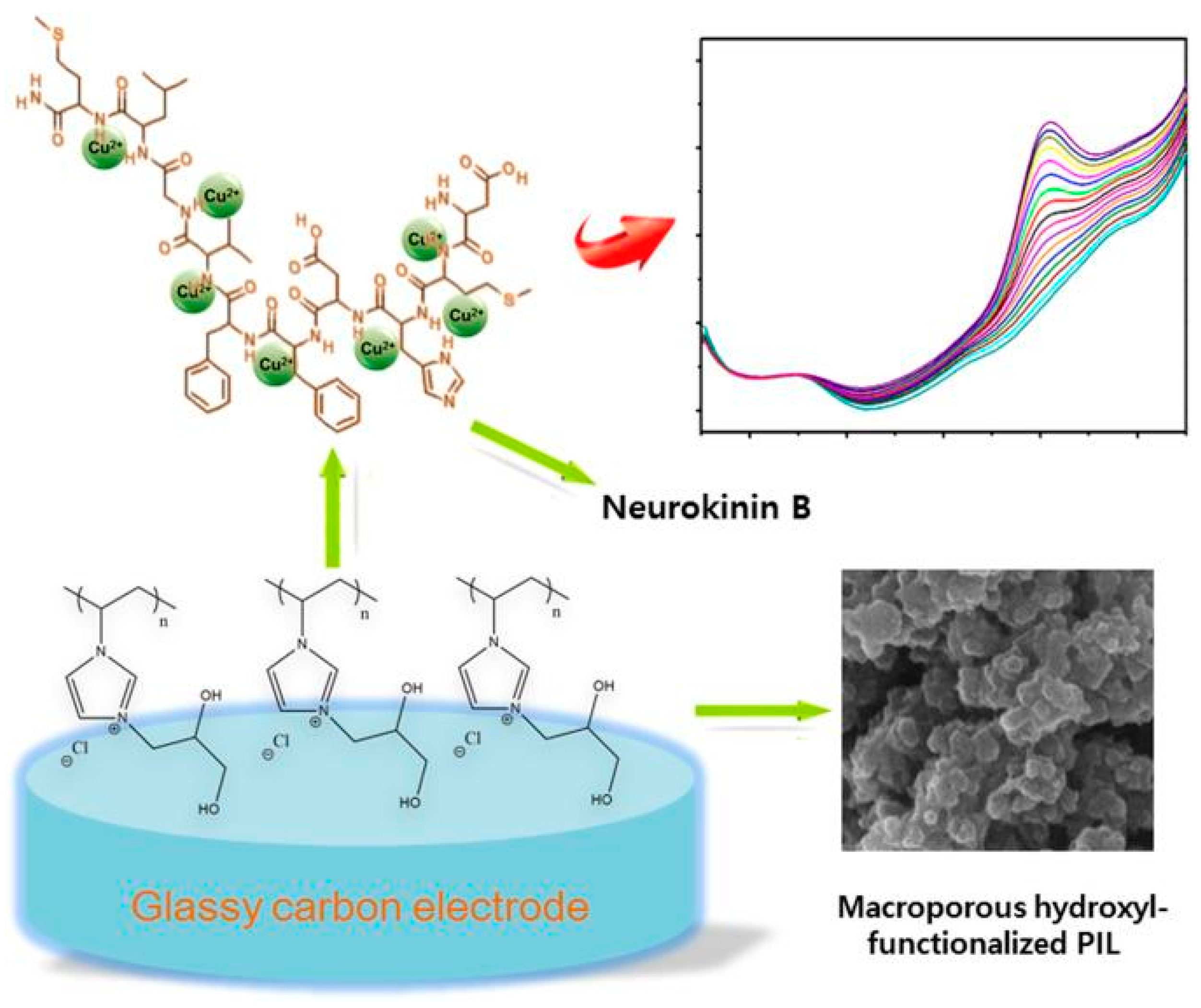
3.2. Copper
3.3. Lead
3.4. Cadmium
3.5. Chromium
3.6. Zinc
3.7. Thallium
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, L.; Zeng, G.; Zhang, C.; Zhang, Y.; Xie, X. Current progress in biosensors for heavy metal ions based on DNAzymes/DNA molecules functionalized nanostructures: A review. Sens. Actuators B Chem. 2016, 223, 280–294. [Google Scholar] [CrossRef]
- Wang, L.; Peng, X.; Fu, H.; Huang, C.; Li, Y.; Liu, Z. Recent advances in the development of electrochemical aptasensors for detection of heavy metals in food. Biosens. Bioelectron. 2020, 147, 111777. [Google Scholar] [CrossRef]
- De Benedetto, G.; Di Masi, S.; Pennetta, A.; Malitesta, C. Response Surface Methodology for the Optimisation of Electrochemical Biosensors for Heavy Metals Detection. Bionsensors 2019, 9, 26. [Google Scholar] [CrossRef]
- Nikolaevna, K.A.; Svalova, T.; Malysheva, N.N.; Okhokhonin, A.V.; Vidrevich, M.B.; Brainina, K. Sensors Based on Bio and Biomimetic Receptors in Medical Diagnostic, Environment, and Food Analysis. Bionsensors 2018, 8, 35. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, P.S.; Machado, A.; Oliveira, H.P.; Bordalo, A.A.; Segundo, M.A. Paper-Based Biosensors for Analysis of Water. In Biosensors for Environmental Monitoring; IntechOpen: Rijeka, Croatia, 2019. [Google Scholar]
- Reynoso, E.C.; Torres, E.; Bettazzi, F.; Palchetti, I. Trends and Perspectives in Immunosensors for Determination of Currently-Used Pesticides: The Case of Glyphosate, Organophosphates, and Neonicotinoids. Bionsensors 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yao, Y.; Ying, Y.; Ping, J. Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection. TrAC Trends Anal. Chem. 2019, 115, 187–202. [Google Scholar] [CrossRef]
- Ferrari, A.G.-M.; Carrington, P.; Rowley-Neale, S.J.; Banks, C.E. Recent advances in portable heavy metal electrochemical sensing platforms. Environ. Sci. Water Res. Technol. 2020, 6, 2676–2690. [Google Scholar] [CrossRef]
- Moro, G.; Bottari, F.; Van Loon, J.; Du Bois, E.; De Wael, K.; Moretto, L.M. Disposable electrodes from waste materials and renewable sources for (bio)electroanalytical applications. Biosens. Bioelectron. 2019, 146, 111758. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Molecular, Clinical and Environmental Toxicology. In Clinical and Environmental Toxicology. Experientia Supplementum; Springer: Berlin/Heidelberg, Germany, 2012; pp. 133–164. ISBN1 978-3-7643-8339-8. ISBN2 978-3-7643-8340-4. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.G. 5—Sources of water pollution. In Natural Water Remediation; Speight, J.G., Ed.; Butterworth-Heinemann: Oxford, UK, 2020; pp. 165–198. ISBN 978-0-12-803810-9. [Google Scholar]
- Council of the European. Union European Drinking Water Directive (DWD) 6230/20 of 7 October 2020 on the Quality of Water Intended for Human Consumption (Recast); EU Parliament: Brussels, Belgium, 2020. [Google Scholar]
- US Environmental Protection Agency. 2018 Edition of the Drinking Water Standards and Health Advisories Tables. 2018. Available online: https://www.epa.gov/sites/production/files/2018-03/documents/dwtable2018.pdf (accessed on 24 November 2020).
- Council Directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption (OJ L 330 05.12.1998 p. 32). Doc. Eur. Community Environ. Law 2010, L, 865–878. [CrossRef]
- Lucentini, L.; Diddi, E.; Di Martino, E.F.; Ferretti, E.; Fuscoletti, V.; Nigro Di Gregorio, F.; Veschetti, E. Piani di Sicurezza dell’Acqua nella Gestione di Emergenze Idropotabili: Il Caso del tallio a Pietrasanta e Valdicastello (Lucca). Rapporti ISTISAN 20/8; Istituto Superiore di Sanità: Rome, Italy, 2020. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th Edition, Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- European Union. O.J. of the E. Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010; EU Parliament: Brussels, Belgium, 2010; pp. 17–119. [Google Scholar]
- Council of the European Union. Commision Regulation (EC) No. 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881&from=IT (accessed on 24 November 2020).
- ICH. Guideline for Elemental Impurities Q3D (R1); European Medicines Agency: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Heena; Malik, A.K. Review on Metal Speciation and Their Applications since 2010; Elsevier: Amsterdam, The Netherlands, 2020; pp. 813–868. [Google Scholar]
- Xing, G.; Sardar, M.R.; Lin, B.; Lin, J.-M. Analysis of trace metals in water samples using NOBIAS chelate resins by HPLC and ICP-MS. Talanta 2019, 204, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Barich, H.; Driesen, K.; Montiel, N.F.; Neven, L.; Mendonça, C.D.; Shanmugam, S.T.; Daems, E.; De Wael, K. Unlocking the full power of electrochemical fingerprinting for on-site sensing applications. Anal. Bioanal. Chem. 2020, 412, 5955–5968. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; De Wael, K.; Moretto, L.M. Challenges in the electrochemical (bio)sensing of nonelectroactive food and environmental contaminants. Curr. Opin. Electrochem. 2019, 16, 57–65. [Google Scholar] [CrossRef]
- Moretto, L.M.; Kalcher, K. Environmental Analysis by Electrochemical Sensors and Biosensors; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1, ISBN 9781493906758. [Google Scholar]
- Wang, R.; Wang, X. Sensing of inorganic ions in microfluidic devices. Sens. Actuators B Chem. 2020, 129171. [Google Scholar] [CrossRef]
- Turdean, G.L. Design and Development of Biosensors for the Detection of Heavy Metal Toxicity. Int. J. Electrochem. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Narakathu, B.; Atashbar, M.Z.; Bejcek, B. Improved detection limits of toxic biochemical species based on impedance measurements in electrochemical biosensors. Biosens. Bioelectron. 2010, 26, 923–928. [Google Scholar] [CrossRef]
- Xie, S.; Tang, Y.; Tang, D. Highly sensitive electrochemical detection of mercuric ions based on sequential nucleic acid amplification and guanine nanowire formation. Anal. Methods 2017, 9, 5478–5483. [Google Scholar] [CrossRef]
- Reddy, G.N.; Prasad, M. Heavy metal-binding proteins/peptides: Occurrence, structure, synthesis and functions. A review. Environ. Exp. Bot. 1990, 30, 251–264. [Google Scholar] [CrossRef]
- Heaton, I.; Platt, M. Peptide Nanocarriers for Detection of Heavy Metal Ions Using Resistive Pulse Sensing. Anal. Chem. 2019, 91, 11291–11296. [Google Scholar] [CrossRef] [PubMed]
- Janyasupab, M.; Liu, C.-C. Enzymatic Electrochemical Biosensors BT. In Encyclopedia of Applied Electrochemistry; Kreysa, G., Ota, K., Savinell, R.F., Eds.; Springer: New York, NY, USA, 2014; pp. 872–882. ISBN 978-1-4419-6996-5. [Google Scholar]
- Ashrafi, A.M.; Sýs, M.; Sedlackova, E.; Farag, A.S.; Adam, V.; Přibyl, J.; Richtera, L. Application of the Enzymatic Electrochemical Biosensors for Monitoring Non-Competitive Inhibition of Enzyme Activity by Heavy Metals. Sensors 2019, 19, 2939. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Z.; Lu, Y. Functional Nucleic Acid Sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef]
- Zhan, S.; Wu, Y.; Wang, L.; Zhan, X.; Zhou, P. A mini-review on functional nucleic acids-based heavy metal ion detection. Biosens. Bioelectron. 2016, 86, 353–368. [Google Scholar] [CrossRef]
- Komarova, N.; Kuznetsov, A. Inside the Black Box: What Makes SELEX Better? Molecules 2019, 24, 3598. [Google Scholar] [CrossRef]
- Sola, M.; Menon, A.P.; Moreno, B.; Meraviglia-Crivelli, D.; Soldevilla, M.M.; Cartón-García, F.; Pastor, F. Aptamers Against Live Targets: Is In Vivo SELEX Finally Coming to the Edge? Mol. Ther. Nucleic Acids 2020, 21, 192–204. [Google Scholar] [CrossRef]
- Zhuo, Z.; Yu, Y.; Wang, M.; Li, J.; Zhang, Z.-K.; Liu, J.; Wu, X.; Lu, A.; Zhang, G.; Zhang, B.-T. Recent Advances in SELEX Technology and Aptamer Applications in Biomedicine. Int. J. Mol. Sci. 2017, 18, 2142. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Liu, G. Reusable resistive aptasensor for Pb (II) based on the Pb(II)-induced despiralization of a DNA duplex and formation of a G-quadruplex. Microchim. Acta 2018, 185, 142. [Google Scholar] [CrossRef]
- Farzinb, L.; Shamsipur, M.; Sheibani, S. A review: Aptamer-based analytical strategies using the nanomaterials for environmental and human monitoring of toxic heavy metals. Talanta 2017, 174, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Amaya, S.; Lin, L.-K.; DiNino, R.E.; Ostos, C.; Stanciu, L.A. Inkjet printed electrochemical aptasensor for detection of Hg2+ in organic solvents. Electrochim. Acta 2019, 316, 33–42. [Google Scholar] [CrossRef]
- Dairaku, T.; Furuita, K.; Sato, H.; Šebera, J.; Yamanaka, D.; Otaki, H.; Kikkawa, S.; Kondo, Y.; Katahira, R.; Bickelhaupt, F.M.; et al. Direct detection of the mercury–nitrogen bond in the thymine–HgII–thymine base-pair with 199Hg NMR spectroscopy. Chem. Commun. 2015, 51, 8488–8491. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, Z.; Zhu, J.; Liu, Y.; Zhou, Y.; He, J. Studies on the thymine–mercury–thymine base pairing in parallel and anti-parallel DNA duplexes. New J. Chem. 2015, 39, 8752–8762. [Google Scholar] [CrossRef]
- Yu, S.H.; Kim, T.H. T-T Mismatch-Based Electrochemical Aptasensor for Ultratrace Level Detection of Hg2+ Using Electrochemically Reduced Graphene Oxide-Modified Electrode. J. Biomed. Nanotechnol. 2019, 15, 1824–1831. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, P.; Xiong, W.; Qi, B.; Shi, R.; Xiang, D.; Zhai, K. Simultaneous detection of mercury (II), lead (II) and silver (I) based on fluorescently labelled aptamer probes and graphene oxide. Environ. Technol. 2020, 1–8. [Google Scholar] [CrossRef]
- Dolati, S.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Recent nucleic acid based biosensors for Pb2+ detection. Sens. Actuators B Chem. 2017, 246, 864–878. [Google Scholar] [CrossRef]
- Cui, H.; Xiong, X.; Gao, B.; Chen, Z.; Luo, Y.; He, F.; Deng, S.; Chen, L. A Novel Impedimetric Biosensor for Detection of Lead (II) with Low-cost Interdigitated Electrodes Made on PCB. Electroanalytical 2016, 28, 2000–2006. [Google Scholar] [CrossRef]
- Han, S.; Wang, R.; Tang, Y.; He, M.; Zhang, X.; Shi, H.; Xiang, Y. Practical, highly sensitive, and regenerable evanescent-wave biosensor for detection of Hg2+ and Pb2+ in water. Biosens. Bioelectron. 2016, 80, 265–272. [Google Scholar] [CrossRef]
- Perera, R.; Ashraf, S.; Mueller, A. The binding of metal ions to molecularly-imprinted polymers. Water Sci. Technol. 2017, 75, 1643–1650. [Google Scholar] [CrossRef]
- Malitesta, C.; Di Masi, S.; Mazzotta, E. From Electrochemical Biosensors to Biomimetic Sensors Based on Molecularly Imprinted Polymers in Environmental Determination of Heavy Metals. Front. Chem. 2017, 5, 47. [Google Scholar] [CrossRef]
- An, Z.; Liu, W.; Liang, Q.; Yan, G.; Qin, L.; Chen, L.; Wang, M.; Yang, Y.; Liu, X. Ion-Imprinted Polymers Modified Sensor for Electrochemical Detection of Cu2+. Nano 2018, 13, 1850140. [Google Scholar] [CrossRef]
- Branger, C.; Meouche, W.; Margaillan, A. Recent advances on ion-imprinted polymers. React. Funct. Polym. 2013, 73, 859–875. [Google Scholar] [CrossRef]
- Florea, A.; Feier, B.; Cristea, C. Chapter Eight—In Situ Analysis Based on Molecularly Imprinted Polymer Electrochemical Sensors. In Mip Synthesis, Characteristics and Analytical Application; Marć, M., Ed.; Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 86, pp. 193–234. [Google Scholar]
- Gui, R.; Guo, H.; Jin, H. Preparation and applications of electrochemical chemosensors based on carbon-nanomaterial-modified molecularly imprinted polymers. Nanoscale Adv. 2019, 1, 3325–3363. [Google Scholar] [CrossRef]
- Crapnell, R.; Hudson, A.; Foster, C.W.; Eersels, K.; Van Grinsven, B.; Cleij, T.J.; Banks, C.E.; Peeters, M. Recent Advances in Electrosynthesized Molecularly Imprinted Polymer Sensing Platforms for Bioanalyte Detection. Sensors 2019, 19, 1204. [Google Scholar] [CrossRef] [PubMed]
- Rico-Yuste, A.; Carrasco, S. Molecularly Imprinted Polymer-Based Hybrid Materials for the Development of Optical Sensors. Polymers 2019, 11, 1173. [Google Scholar] [CrossRef] [PubMed]
- Bala, A.; Górski, Ł. Peptide nucleic acid as a selective recognition element for electrochemical determination of Hg2+. Bioelectrochemistry 2018, 119, 189–195. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, M.; Jiang, Y.; Wang, X.; Guo, Z.; Shi, J.; Zou, X.; Han, E. Simple electrochemical sensing for mercury ions in dairy product using optimal Cu2+-based metal-organic frameworks as signal reporting. J. Hazard. Mater. 2020, 400, 123222. [Google Scholar] [CrossRef]
- Cheng, L.; Wei, B.; He, L.L.; Mao, L.; Zhang, J.; Ceng, J.; Kong, D.; Chen, C.; Cui, H.; Hong, N.; et al. “Off-On” switching electrochemiluminescence biosensor for mercury(II) detection based on molecular recognition technology. Anal. Biochem. 2017, 518, 46–52. [Google Scholar] [CrossRef]
- Bala, A.; Górski, Ł. Determination of mercury cation using electrode modified with phosphorothioate oligonucleotide. Sens. Actuators B Chem. 2016, 230, 731–735. [Google Scholar] [CrossRef]
- Jin, H.; Zhang, M.; Wei, M.; Cheng, J. A voltammetric biosensor for mercury(II) using reduced graphene oxide@gold nanorods and thymine-Hg(II)-thymine interaction. Microchim. Acta 2019, 186, 264. [Google Scholar] [CrossRef] [PubMed]
- Yaddaden, C.; Benamar, M.; Gabouze, N.; Berouaken, M.; Ayat, M. Investigations on mercury ion detection in aqueous solution by triglycine surface activated porous silicon nanowires. Phys. E Low Dimens. Syst. Nanostruct. 2019, 108, 147–152. [Google Scholar] [CrossRef]
- Shah, A.; Nisar, A.; Khan, K.; Nisar, J.; Niaz, A.; Ashiq, M.N.; Akhter, M.S. Amino acid functionalized glassy carbon electrode for the simultaneous detection of thallium and mercuric ions. Electrochim. Acta 2019, 321, 134658. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Q.; Chai, Y.; Yuan, Y.; Yuan, R. Enzyme-assisted cycling amplification and DNA-templated in-situ deposition of silver nanoparticles for the sensitive electrochemical detection of Hg2+. Biosens. Bioelectron. 2016, 86, 630–635. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Wang, J.; Xiong, B.; Hou, X. Electrochemical impedance biosensor array based on DNAzyme-functionalized single-walled carbon nanotubes using Gaussian process regression for Cu(II) and Hg(II) determination. Microchim. Acta 2020, 187, 207–209. [Google Scholar] [CrossRef]
- Zhang, X.; Jianga, Y.; Zhua, M.; Xua, Y.; Guoa, Z.; Shi, J.; Hana, E.; Zou, X.; Wangb, D. Electrochemical DNA sensor for inorganic mercury(II) ion at attomolar level in dairy product using Cu(II)-anchored metal-organic framework as mimetic catalyst. Chem. Eng. J. 2020, 383, 123182. [Google Scholar] [CrossRef]
- Mohammadi, A.; Heydari-Bafrooei, E.; Foroughi, M.M.; Mohammadi, M. Heterostructured Au/MoS2-MWCNT nanoflowers: A highly efficient support for the electrochemical aptasensing of solvated mercuric ion. Microchem. J. 2020, 158, 105154. [Google Scholar] [CrossRef]
- Xie, H.; Niu, Y.; Deng, Y.; Cheng, H.; Ruan, C.; Li, G.; Sun, W. Electrochemical aptamer sensor for highly sensitive detection of mercury ion with Au/Pt@carbon nanofiber-modified electrode. J. Chin. Chem. Soc. 2020. [Google Scholar] [CrossRef]
- Ma, F.; Chen, Y.; Zhu, Y.; Liu, J. Electrogenerated chemiluminescence biosensor for detection of mercury (II) ion via target-triggered manipulation of DNA three-way junctions. Talanta 2019, 194, 114–118. [Google Scholar] [CrossRef]
- Hasanjani, H.R.A.; Zarei, K. An electrochemical sensor for attomolar determination of mercury(II) using DNA/poly-L-methionine-gold nanoparticles/pencil graphite electrode. Biosens. Bioelectron. 2019, 128, 1–8. [Google Scholar] [CrossRef]
- Tang, W.; Wang, Z.; Yu, J.; Zhang, F.; He, P.-G. Internal Calibration Potentiometric Aptasensors for Simultaneous Detection of Hg2+, Cd2+, and As3+ Based on a Screen-Printed Carbon Electrodes Array. Analyst Chem. 2018, 90, 8337–8344. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ràfols, C.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C.; Esteban, M. Glutathione modified screen-printed carbon nanofiber electrode for the voltammetric determination of metal ions in natural samples. Talanta 2016, 155, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, C.; Yin, T.; Ou, S.; Sun, X.; Wen, X.; Zhang, L.; Tang, D.; Yin, X.-X. Functionalized poly (ionic liquid) as the support to construct a ratiometric electrochemical biosensor for the selective determination of copper ions in AD rats. Biosens. Bioelectron. 2017, 87, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Wang, P.; Zhu, X.; Peng, Q.; Zhou, Y.; Yin, T.; Liang, Y.; Yin, X. Combined determination of copper ions and β-amyloid peptide by a single ratiometric electrochemical biosensor. Analyst 2018, 143, 323–331. [Google Scholar] [CrossRef]
- Tadi, K.K.; Alshanski, I.; Mervinetsky, E.; Marx, G.; Petrou, P.; Dimitrios, K.M.; Gilon, C.; Hurevich, M.; Yitzchaik, S. Oxytocin-Monolayer-Based Impedimetric Biosensor for Zinc and Copper Ions. ACS Omega 2017, 2, 8770–8778. [Google Scholar] [CrossRef]
- Mervinetsky, E.; Alshanski, I.; Buchwald, J.; Dianat, A.; Lončarić, I.; Lazić, P.; Crljen, Ž.; Gutierrez, R.; Cuniberti, G.; Hurevich, M.; et al. Direct Assembly and Metal-Ion Binding Properties of Oxytocin Monolayer on Gold Surfaces. Langmuir 2019, 35, 11114–11122. [Google Scholar] [CrossRef]
- Tian, R.; Chen, X.; Liu, D.; Yao, C. A Sensitive Biosensor for Determination of Cu2+ by One-step Electrodeposition. Electroanalytical 2016, 28, 1617–1624. [Google Scholar] [CrossRef]
- Wei, P.; Zhu, Z.; Song, R.; Li, Z.; Chen, C. An ion-imprinted sensor based on chitosan-graphene oxide composite polymer modified glassy carbon electrode for environmental sensing application. Electrochim. Acta 2019, 317, 93–101. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Wei, G.; Zhang, Y.; Zeng, Y. Highly sensitive and doubly orientated selective molecularly imprinted electrochemical sensor for Cu2+. Biosens. Bioelectron. 2015, 69, 316–320. [Google Scholar] [CrossRef]
- Qiu, B.; Qiu, J.; Cui, M.; Wei, X.; Zhao, M.; Qiu, B.; Chen, G. An immobilization free DNAzyme based electrochemical biosensor for lead determination. Analyst 2016, 141, 1121–1126. [Google Scholar] [CrossRef]
- Wang, L.; Wen, Y.; Li, L.; Yang, X.; Jia, N.; Li, W.; Meng, J.; Duan, M.; Sun, X.; Liu, G. Sensitive and label-free electrochemical lead ion biosensor based on a DNAzyme triggered G-quadruplex/hemin conformation. Biosens. Bioelectron. 2018, 115, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, M.; Mathew, B. Ion imprinting approach for the fabrication of an electrochemical sensor and sorbent for lead ions in real samples using modified multiwalled carbon nanotubes. J. Mater. Sci. 2018, 53, 3557–3572. [Google Scholar] [CrossRef]
- Alizadeh, T.; Hamidi, N.; Ganjali, M.R.; Rafiei, F. An extraordinarily sensitive voltammetric sensor with picomolar detection limit for Pb 2+ determination based on carbon paste electrode impregnated with nano-sized imprinted polymer and multi-walled carbon nanotubes. J. Environ. Chem. Eng. 2017, 5, 4327–4336. [Google Scholar] [CrossRef]
- Ghanei-Motlagh, M.; Taher, M.A. An Electrochemical Sensor Based on Novel Ion Imprinted Polymeric Nanoparticles for Selective Detection of Lead Ions. Anal. Bioanal. Chem. Res. 2017, 4, 295–306. [Google Scholar]
- Dahaghin, Z.; Kilmartin, P.A.; Mousavi, H.Z. Novel ion imprinted polymer electrochemical sensor for the selective detection of lead (II). Food Chem. 2020, 303, 125374. [Google Scholar] [CrossRef]
- Dali, M.; Zinoubi, K.; Chrouda, A.; Abderrahmane, S.; Cherrad, S.; Jaffrezic-Renault, N. A biosensor based on fungal soil biomass for electrochemical detection of lead (II) and cadmium (II) by differential pulse anodic stripping voltammetry. J. Electroanal. Chem. 2018, 813, 9–19. [Google Scholar] [CrossRef]
- Florescu, M.; David, M.; Badea, M. Development And Evaluation of Sol-Gel-Based Biosensors for Cadmium Ions Detection. Environ. Eng. Manag. J. 2018, 17, 317–326. [Google Scholar] [CrossRef]
- Fourou, H.; Zazoua, A.; Braiek, M.; Jaffrezic-Renault, N. An enzyme biosensor based on beta-galactosidase inhibition for electrochemical detection of cadmium (II) and chromium (VI). Int. J. Environ. Anal. Chem. 2016, 96, 872–885. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Veerapandian, M.; Krishnan, U.M.; Rayappan, J.B.B. Amperometric determination of As(III) and Cd(II) using a platinum electrode modified with acetylcholinesterase, ruthenium(II)-tris(bipyridine) and graphene oxide. Microchim. Acta 2018, 185, 297. [Google Scholar] [CrossRef]
- Zhad, H.R.L.Z.; Torres, Y.M.R.; Lai, R.Y. A reagentless and reusable electrochemical aptamer-based sensor for rapid detection of Cd (II). J. Electroanal. Chem. 2017, 803, 89–94. [Google Scholar] [CrossRef]
- Li, Y.; Ran, G.; Lu, G.; Ni, X.; Liu, D.; Sun, J.; Xie, C.; Yao, D.; Bai, W. Highly Sensitive Label-Free Electrochemical Aptasensor Based on Screen-Printed Electrode for Detection of Cadmium (II) Ions. J. Electrochem. Soc. 2019, 166, B449–B455. [Google Scholar] [CrossRef]
- Fakude, C.T.; Arotiba, O.; Mabuba, N. Electrochemical aptasensing of cadmium (II) on a carbon black-gold nano-platform. J. Electroanal. Chem. 2020, 858, 113796. [Google Scholar] [CrossRef]
- Liu, Y.; Lai, Y.; Yang, G.; Tang, C.; Deng, Y.; Li, S.; Wang, Z. Cd-Aptamer Electrochemical Biosensor Based on AuNPs/CS Modified Glass Carbon Electrode. J. Biomed. Nanotechnol. 2017, 13, 1253–1259. [Google Scholar] [CrossRef]
- Wang, X.; Gao, W.; Yan, W.; Li, P.; Zou, H.; Wei, Z.; Guan, W.; Ma, Y.; Zou, H.; Wenyu, G.; et al. A Novel Aptasensor Based on Graphene/Graphite Carbon Nitride Nanocomposites for Cadmium Detection with High Selectivity and Sensitivity. ACS Appl. Nano Mater. 2018, 1, 2341–2346. [Google Scholar] [CrossRef]
- Aravind, A.; Mathew, B. Tailoring of nanostructured material as an electrochemical sensor and sorbent for toxic Cd(II) ions from various real samples. J. Anal. Sci. Technol. 2018, 9, 22. [Google Scholar] [CrossRef]
- Hu, S. An Electrochemical Sensor Based on ion Imprinted PPy/rGO Composite for Cd(II) Determination in Water. Int. J. Electrochem. Sci. 2019, 14, 11714–11730. [Google Scholar] [CrossRef]
- Xie, T.; Gao, Y.-M.; Zheng, Q.; Rt, H.-; Wang, X.-H.; Li, Y.; Luo, N.; Liu, R. A Double-microbial Fuel Cell Heavy Metals Toxicity Sensor. DEStech Trans. Environ. Energy Earth Sci. 2017, 5–10. [Google Scholar] [CrossRef]
- Behrmann, I.C.L.; Grattieri, M.; Minteer, S.D.; Ramirez, S.A.; Vullo, D.L. Online self-powered Cr(VI) monitoring with autochthonous Pseudomonas and a bio-inspired redox polymer. Anal. Bioanal. Chem. 2020, 412, 6449–6457. [Google Scholar] [CrossRef]
- Garavaglia, L.; Cerdeira, S.B.; Vullo, D.L. Chromium (VI) biotransformation by β- and γ-Proteobacteria from natural polluted environments: A combined biological and chemical treatment for industrial wastes. J. Hazard. Mater. 2010, 175, 104–110. [Google Scholar] [CrossRef]
- Prabhakaran, D.C.; Riotte, J.; Sivry, Y.; Subramanian, S. Electroanalytical Detection of Cr(VI) and Cr(III) Ions Using a Novel Microbial Sensor. Electroanalytical 2017, 29, 1222–1231. [Google Scholar] [CrossRef]
- Wu, L.-C.; Tsai, T.-H.; Liu, M.-H.; Kuo, J.-L.; Chang, Y.-C.; Chung, Y.-C. A Green Microbial Fuel Cell-Based Biosensor for In Situ Chromium (VI) Measurement in Electroplating Wastewater. Sensors 2017, 17, 2461. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, Y.; Zhang, L.; Ge, S.; Yan, M.; Yu, J. Steric paper based ratio-type electrochemical biosensor with hollow-channel for sensitive detection of Zn2+. Sci. Bull. 2017, 62, 1114–1121. [Google Scholar] [CrossRef]
- Labro, J.; Craig, T.; Wood, S.A.; Packer, M. Demonstration of the use of a photosynthetic microbial fuel cell as an environmental biosensor. Int. J. Nanotechnol. 2017, 14, 213. [Google Scholar] [CrossRef]
- Nasiri-Majd, M.; Taher, M.A.; Fazelirad, H. Synthesis and application of nano-sized ionic imprinted polymer for the selective voltammetric determination of thallium. Talanta 2015, 144, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Marnane, I.; Jeroen, K.; Carlijn, H.; Visschedijk, A.; Tycho, S.; Vermeulen, J.; Grandjean, P.; Hagström, P.; Per, K.; Fold, N.; et al. EU Publications Mercury in Europe’s Environment a Priority for European and Global Action—1977-8449EEA Report No 11/2018; EEA: Copenhagen, Denmark, 2018. [Google Scholar]
- Selin, N.E. Global Biogeochemical Cycling of Mercury: A Review. Annu. Rev. Environ. Resour. 2009, 34, 43–63. [Google Scholar] [CrossRef]
- Streets, D.G.; Horowitz, H.M.; Jacob, D.J.; Lu, Z.; Levin, L.; Ter Schure, A.F.H.; Sunderland, E.M. Total Mercury Released to the Environment by Human Activities. Environ. Sci. Technol. 2017, 51, 5969–5977. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-D.; Zheng, W. Human Exposure and Health Effects of Inorganic and Elemental Mercury. J. Prev. Med. Public Health 2012, 45, 344–352. [Google Scholar] [CrossRef]
- Bernhoft, R.A. Mercury Toxicity and Treatment: A Review of the Literature. J. Environ. Public Health 2011, 2012, 460508. [Google Scholar] [CrossRef]
- Hong, Y.-S.; Kim, Y.-M.; Lee, K.-E. Methylmercury Exposure and Health Effects. J. Prev. Med. Public Health 2012, 45, 353–363. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Carocci, A.; Lauria, G.; Catalano, A. Mercury Exposure and Heart Diseases. Int. J. Environ. Res. Public Health 2017, 14, 74. [Google Scholar] [CrossRef]
- Bose-O’Reilly, S.; Mccarty, K.M.; Steckling, N.; Lettmeier, B. Mercury Exposure and Children’s Health. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 186–215. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chu, Z.; Jin, W. Electrochemical mercury biosensors based on advanced nanomaterials. J. Mater. Chem. B 2019, 7, 3620–3632. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Pournara, A.; Manos, M.; Spanopoulos, I.; Kanatzidis, M.; Tziotzi, T.; Petkov, V.; Margariti, A.; Oikonomopoulos, P.; et al. 3D-printed lab-in-a-syringe voltammetric cell based on a working electrode modified with a highly efficient Ca-MOF sorbent for the determination of Hg(II). Sens. Actuators B Chem. 2020, 321, 128508. [Google Scholar] [CrossRef]
- Desai, V.; Kaler, S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008, 88, 855S–858S. [Google Scholar] [CrossRef] [PubMed]
- Witt, B.; Schaumlöffel, D.; Schwerdtle, T. Subcellular Localization of Copper—Cellular Bioimaging with Focus on Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 2341. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Barceloux, D.G. Copper. J. Toxicol. Clin. Toxicol. 1999, 37, 217–230. [Google Scholar] [CrossRef]
- Li, F.; Ma, W.; Liu, J.; Wu, X.; Wang, Y.; He, J. Luminol, horseradish peroxidase, and glucose oxidase ternary functionalized graphene oxide for ultrasensitive glucose sensing. Anal. Bioanal. Chem. 2018, 410, 543–552. [Google Scholar] [CrossRef]
- Grant, L.D. Lead and Compounds. Environ. Toxic. 2009, 757–809. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef]
- Godt, J.C.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; A Groneberg, D. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Huff, J.; Lunn, R.M.; Waalkes, M.P.; Tomatis, L.; Infante, P.F. Cadmium-induced Cancers in Animals and in Humans. Int. J. Occup. Environ. Health 2007, 13, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Han, H.; Liu, P.; Xiong, J.; Tian, F.; Li, X. Microbial Fuels Cell-Based Biosensor for Toxicity Detection: A Review. Sensors 2017, 17, 2230. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Bai, L.; Zhai, J.; Wang, Y.; Dong, S. Toxicity detection in water containing heavy metal ions with a self-powered microbial fuel cell-based biosensor. Talanta 2017, 168, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Okamura, H. Study on Incineration Method of Leather Scraps and Recovery of Chromium from Incinerated Residues; Elsevier: Amsterdam, The Netherlands, 1994; pp. 281–284. [Google Scholar]
- Frassinetti, S.; Bronzetti, G.L.; Caltavuturo, L.; Cini, M.; Della Croce, C. The Role of Zinc in Life: A Review. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 597–610. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The composition of the continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- Riley, J.; Siddiqui, S. The determination of thallium in sediments and natural waters. Anal. Chim. Acta 1986, 181, 117–123. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, S.; Cheng, H.; Zhang, W.; Ma, J.; Zhang, C.; Guo, Z. Thallium pollution and potential ecological risk in the vicinity of coal mines in Henan Province, China. Chem. Speciat. Bioavailab. 2018, 30, 107–111. [Google Scholar] [CrossRef]
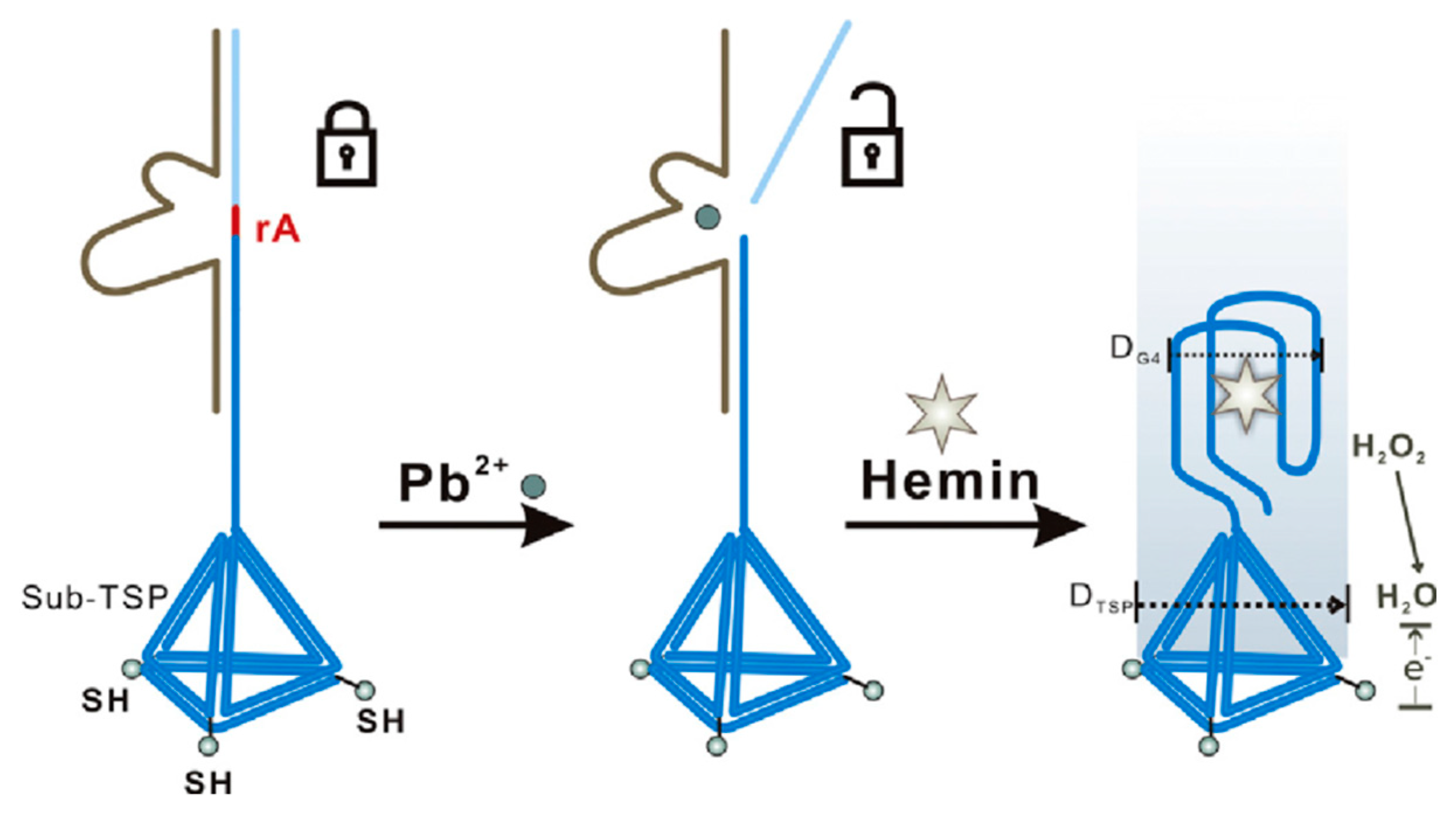
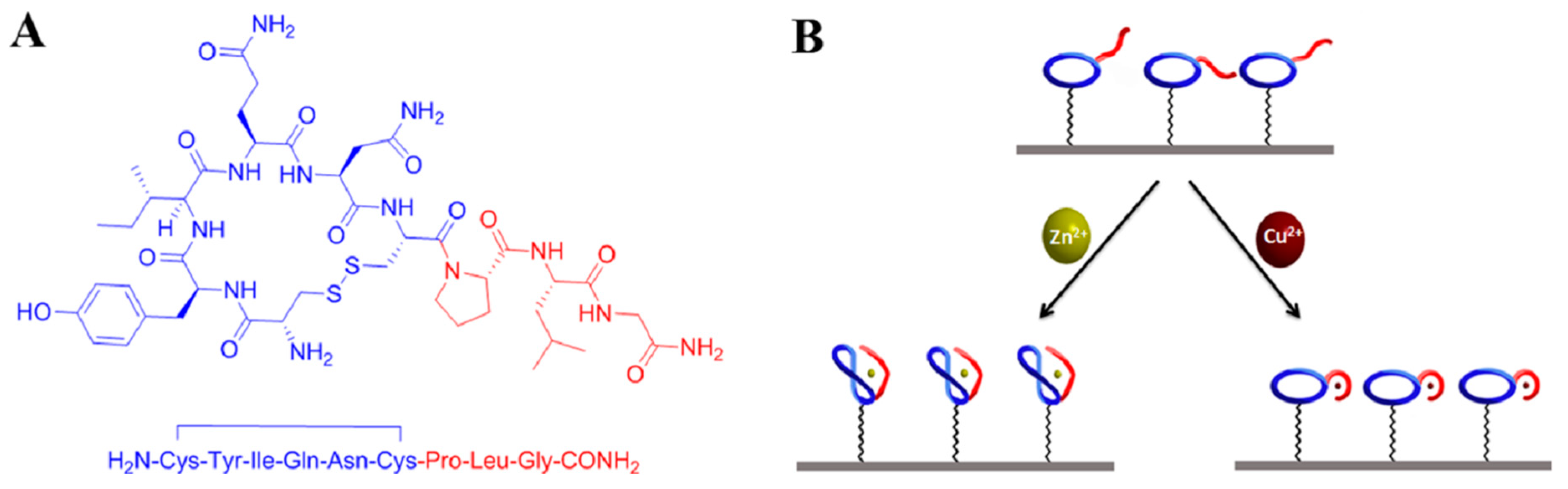
| Matrix | HMI | Concentration Limit | Maximum Level | Units | Notes | ||
|---|---|---|---|---|---|---|---|
| EPA [17] | EU Directis [18] | Updated EU directive 2020 [16] | |||||
| Drinking water | Cd | 5 | 5 | 5 | - | μg L−1 | |
| Cr | 100 | 50 | 25 | - | Total inorganic Cr. The updated value shall be met, at the latest, by 15 years after the day of entry into force of the new EU directive [16] | ||
| Pb | 15 | 10 | 5 | - | The updated value shall be met, at the latest, by 15 years after the day of entry into force of the new EU directive [16] | ||
| Hg | 2 | 1 | 1 | - | |||
| Cu | 1300 | 2000 | 2000 | - | |||
| Tl | 2 | - | - | 0.002 [19] | mg L−1 | ||
| Zn | - | - | - | 3 [20] | |||
| Waste water [21] | Cd | 0.05 | mg L−1 | Emission limit values for discharges of wastewaters from the cleaning of waste gases [21] | |||
| Cr | 0.5 | ||||||
| Cu | 0.5 | ||||||
| Pb | 0.2 | ||||||
| Hg | 0.03 | ||||||
| Tl | 0.05 | ||||||
| Zn | 1.5 | ||||||
| Food [22] Fats and oils, milk and derivates, meat and fish products, cereals, legumes, vegetables | Pb | - | - | - | 0.020 to 1.5 | mg kg−1 | |
| Meat, mollusks, cereals, vegetables and fruit | Cd | - | - | - | 0.050 to 1.0 | ||
| Fish products | Hg | - | - | - | 0.50 | ||
| Drugs [23] Products Substances Excipients | Cd | - | - | - | 0.5/0.2/0.3 | μg g−1 | Oral/Parenteral/Inhalation |
| Pb | - | - | - | 0.5/0.5/0.5 | |||
| Hg | - | - | - | 3/0.3/0.1 | |||
| Tl | - | - | - | 0.8/0.8/0.8 | |||
| Cu | - | - | - | 300/150/1 | |||
| Cr | - | - | - | 1100/110/0.3 | |||
| Metal Ion | Recognition Layer | Linear Range | Limit of Detection | Matrix | Reference |
|---|---|---|---|---|---|
| Hg2+ | Polythymine peptide nucleic acid | 5–500 nM | 4.5 nM | tap water | [60] |
| CuMOFs Thymine DNA strands (T-rich) | 10 fM–100 nM | 4.8 fM | pure fresh milk | [61] | |
| Thymine ssDNA (T-rich) | 0.02–800 ng/mL | 0.1 nM | lily | [62] | |
| Phosphorothioate oligonucleotide (PTO) | 10−11–10−7 M | 2.34 × 10−11 M | reference material | [63] | |
| RGO@AuNR-TH-SA Thymine | 1–200 nM | 0.24 nM | tap water wastewater | [64] | |
| Silicon nanowires Triglycine (Glyl-Gly-Gly) | 10−3–10−8 M | 10−6 M | - | [65] | |
| Gly modified GCE | 2 nM–0.2 mM | 0.23 nM | drinking water, spring water, river water, industrial wastewater | [66] | |
| ssDNA for signal output and nicking endonuclease assisted cycling amplification | 0.01–100 nM | 3 pM | river water | [67] | |
| Hgzyme/SWNTs/FET DNAzyme | 10–10,000 nM | 3.43 nM | pait, soil | [68] | |
| sDNA/MOF-Au | 0.10 aM–100 nM | 0.001 aM | fresh milk, yogurt and infant milk powder | [69] | |
| (APT/Au/MoS2-MWCNT) | 0.1 nM–1 μM | 0.05 nM | tap water | [70] | |
| Aptamer/Au/Pt@CNF/CILE | 1.0 × 10−15–1.0 × 10−6 M | 0.33 fM | domestic and mineral water | [71] | |
| Thiolated DNA strand | 0.01–0.1 mg L−1 | 0.01 mg L−1 0.005 mg L−1 | water dmso (20%) | [44] | |
| DNA three-way junction structure (DNA-TWJ) | 0.1–10 pM | 0.04 pM | water pipes | [72] | |
| DNA/PMET-AuNPs/PGE | 0.1 aM–0.1 nM | 0.004 aM | sea water fish | [73] | |
| Aptamer | 2.5 pM–2.5 μM | 2.0 pM | tap water, lake water, river water | [74] | |
| GlyGlyGlycine-modified PSiNWs | 10−3 –10−9 M | 10−7 M | - | [65] | |
| Cu2+ | Glutathione modified SPE with carbon nanofiber electrode (GSH-SPCNFE) | 10.1–150.1 mg L−1 | 3.0 mg L−1 | wastewater certified reference material (ERMs-CA71) | [75] |
| DHF-PIL-ABTS/NKB/Glu | 0.9–36.1 μM | LOD 0.24 μM LOQ 0.6 μM | cerebrospinal fluid hippocampus | [76] | |
| Neurokinin B (NKB) ABTS-PDDA/CNTs-NKB | 0.1–10 μM | 0.04 μM | plasma hippocampus | [77] | |
| Oxytocin (OT) | - | 500 fM | healthy and MS sera patients | [78] | |
| Oxytocin (OT) | 10−13–10−9 M | - | - | [79] | |
| Cuzyme/SWNTs/FET | 0,01–10,000 nM | 0.0064 nM | pait, soil | [68] | |
| 3DOM CS-PB-SWCNTs | 10−18–10−5 M | 10−19 M | river water | [80] | |
| CS/GO/Cu (II) | 0.5–100 μ M | 0.15 μM | tap water, river water | [81] | |
| MIECS (MIP/Cu-Gly) | 0.5–30 nM | 42.4 pM | running water, citric fruit juice, rainwater, beer, standard food | [82] | |
| Pb2+ | Glutathione modified SPE with carbon nanofiber electrode (GSH-SPCNFE) | 10.8–150.1 mg L−1 | 3.2 mg L−1 | wastewater certified reference material (ERMs-CA71) | [75] |
| DNAzymes and ITO based immobilization | 0.05–1 μM | 0.018 μM | river water, tap water | [83] | |
| DNA nanostructure DNAzyme and G-quadruplex/hemin | 0.01–1000 nM | 0.008 nM | tap water, pool water | [84] | |
| MWCNT-IIP | 1–5 mg L−1 | 2 × 10−2 µM | mining effluent, lake water, food, cosmetics | [85] | |
| Itaconic acid-Pb2+ complex and ethylene glycol dimethacrylate (IIP/MWCNT-CP) | 1.0 × 10−11–5 × 10−10 M 1.0 × 10−9–8 × 10−8 M | 3.8 × 10−12 M | seawater, river water | [86] | |
| IIP-MWCNTs-CPE | 3–55 μg L−1 | 0.5 μg L−1 | river water, aqueduct water, copper factory, wastewater, coal processing wastewater | [87] | |
| GCE modified with magnetic IIP nanoparticles (IIP-GCE) | 0.1–5 ng mL−1 5–80 ng mL−1 | LOD 0.05 ng mL−1 LOQ 0.16 ng mL−1 | tap water, river water, rainwater, fruit juice | [88] | |
| GCE functionalized with carbon nanotubes (SWCNTs-COOH) + filamentous fungi | validated | 0.01 μM | unknown | [89] | |
| Cd2+ | Acetylcholinesterase (AChE) | 2.50–25.00 mg L−1 | 0.19 mg L−1 | river water | [90] |
| Beta galactosidase enzyme (β-gal) on bare gold electrode | EIS 2.36 × 10−3–2.36 × 107 mg L−1 SWV 2.36 × 10−3–2.94 × 107 mg L−1 | EIS 6.95 mg L−1 SWV 7.61 × 10−3 mg L−1 | river water | [91] | |
| Pt/Ru(II)-tris(bipy)-GO/AChE electrode | 0.02–0.7 μM | 0.07 μM | river water, wastewater | [92] | |
| 5′HS-(CH2)6-GGACTGTTGTGGTATTATTTTTGGTTGTGCAGTATG-MB 3′ | 250 nM–1μM | 92 nM | tap water, synthetic saliva | [93] | |
| Aptamer issAP08-Cd | 0.1–1000.0 ng mL−1 | 0.05 ng mL−1 | fish, river water | [94] | |
| Aptamer | 2.5 pM–2.5 μM | 0.62 pM | tap water, lake water, river water | [74] | |
| 36-base thiolated ssDNA aptamer on the SPCE-CB-AuNPs | 1–50 μg L−1 | 0.14 μg L−1 | tap water, industrial effluent | [95] | |
| aptamer on GCE—chitosan (CS) | 0.001–100 nM (1.124 × 10⁻13–1.124 × 10⁻⁸ g mL−1) | 0.04995 pM (5.614 × 10⁻1⁵ g mL−1) | tap water | [96] | |
| carboxyl-terminated aptamers with an appropriately regulated rGO/g-C3N4 nanocomposite | 1 nM–1 μM 1 μM–1 mM | 0.337 nM | tap water lake water industrial waste | [97] | |
| MWCNT-IIP | - | 0.03 μM | lake water, pigments, cosmetics, fertilizers | [98] | |
| (PPy/rGO) composite for trace level determination of Cd(II) | 1–100 μg L−1 | 0.26 μg L−1 | lake water, river water | [99] | |
| Double-chamber MFC | 0.4–10 mg L−1 | --- | wastewater | [100] | |
| Cr6+ | Beta galactosidase enzyme (β-gal) on bare gold electrode | EIS 2.94 × 10−2–2.94 × 104 mg L−1 SWV 2.94 × 10−2–2.94 × 104 mg L−1 | EIS 9.17 × 10−2 mg L−1 SWV 9,17 × 10−2 mg L−1 | river water | [91] |
| self-powered microbial electrochemical sensor—(Pseudomonas P. veronii 2E) | 4–18.5 mg L−1 | 2.4 mg L−1 | [101] | ||
| b and g-Proteobacteria | - | 5 mg L−1 | [102] | ||
| Double-chamber MFC | 0.3–10 mg L−1 | - | wastewater | [100] | |
| Carbon paste electrode modified with Citrobacter freundii (Cf–CPE) | - | CV 1 × 10−4 M Cr(VI) 5 × 10−4 M Cr(III) DPV1 × 10−9 M Cr(VI) 1 × 10−7 M Cr(III) | water | [103] | |
| Cr(VI)-MFC biosensor with E. aestuarii YC211 | - | - | artificial wastewater electroplating waste-water | [104] | |
| Zn2+ | Oxytocin (OT) | - | 100 fM | healthy and ms sera patients (zinc to copper ions) | [78] |
| Oxytocin (OT) | 10−13–10−3 M | - | - | [79] | |
| Steric paper-based ratio-type | 0.1–7000 nM | 0.03 nM | water | [105] | |
| Double-chamber MFC | 15–80 mg L−1 | - | wastewater | [100] | |
| Microbial fuel cells (MFCs) microalgae and cyanobacteria | 2.5–1000 μM | - | ecotoxicology assays | [106] | |
| Tl+ | Gly modified GCE | 2 nM–0.2 mM | 0.175 nM | drinking water, spring water, river water, industrial wastewater | [66] |
| Tl-IP–MWCNT–CPE | 3.0–240 ng mL−1 | 0.76 ng mL−1 | tap water, well water, wastewater | [107] | |
| Microbial fuel cells (MFCs) microalgae and cyanobacteria | 0.1–3000 μM | - | ecotoxicology assays | [106] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stortini, A.M.; Baldo, M.A.; Moro, G.; Polo, F.; Moretto, L.M. Bio- and Biomimetic Receptors for Electrochemical Sensing of Heavy Metal Ions. Sensors 2020, 20, 6800. https://doi.org/10.3390/s20236800
Stortini AM, Baldo MA, Moro G, Polo F, Moretto LM. Bio- and Biomimetic Receptors for Electrochemical Sensing of Heavy Metal Ions. Sensors. 2020; 20(23):6800. https://doi.org/10.3390/s20236800
Chicago/Turabian StyleStortini, Angela Maria, Maria Antonietta Baldo, Giulia Moro, Federico Polo, and Ligia Maria Moretto. 2020. "Bio- and Biomimetic Receptors for Electrochemical Sensing of Heavy Metal Ions" Sensors 20, no. 23: 6800. https://doi.org/10.3390/s20236800
APA StyleStortini, A. M., Baldo, M. A., Moro, G., Polo, F., & Moretto, L. M. (2020). Bio- and Biomimetic Receptors for Electrochemical Sensing of Heavy Metal Ions. Sensors, 20(23), 6800. https://doi.org/10.3390/s20236800