Wearable Sensors Incorporating Compensatory Reserve Measurement for Advancing Physiological Monitoring in Critically Injured Trauma Patients
Abstract
1. Introduction
2. Need to Identify New Vital Sign Measurements
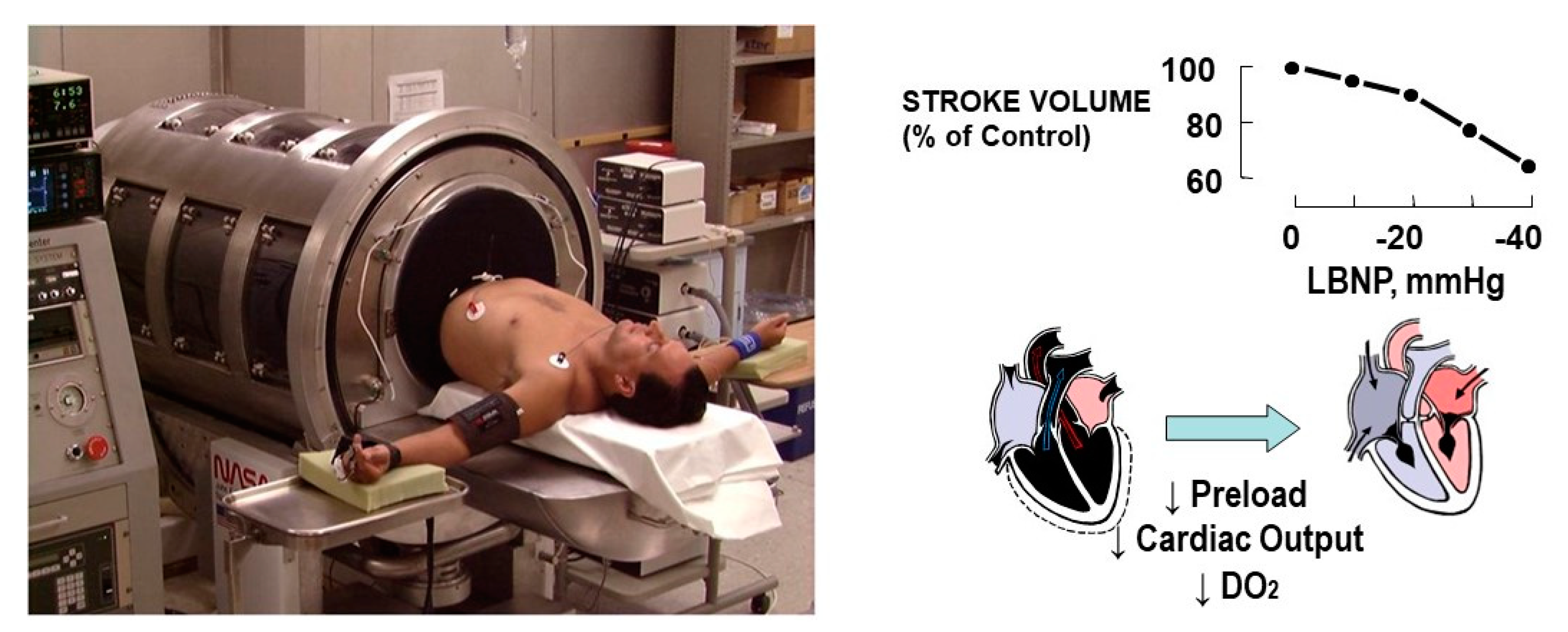
2.1. Compromised DO2—A Primary Clinical Challenge to Effective Medical Monitoring
2.2. Current Vital Sign Monitoring
2.3. Accuracy, Sensitivity, and Specificity
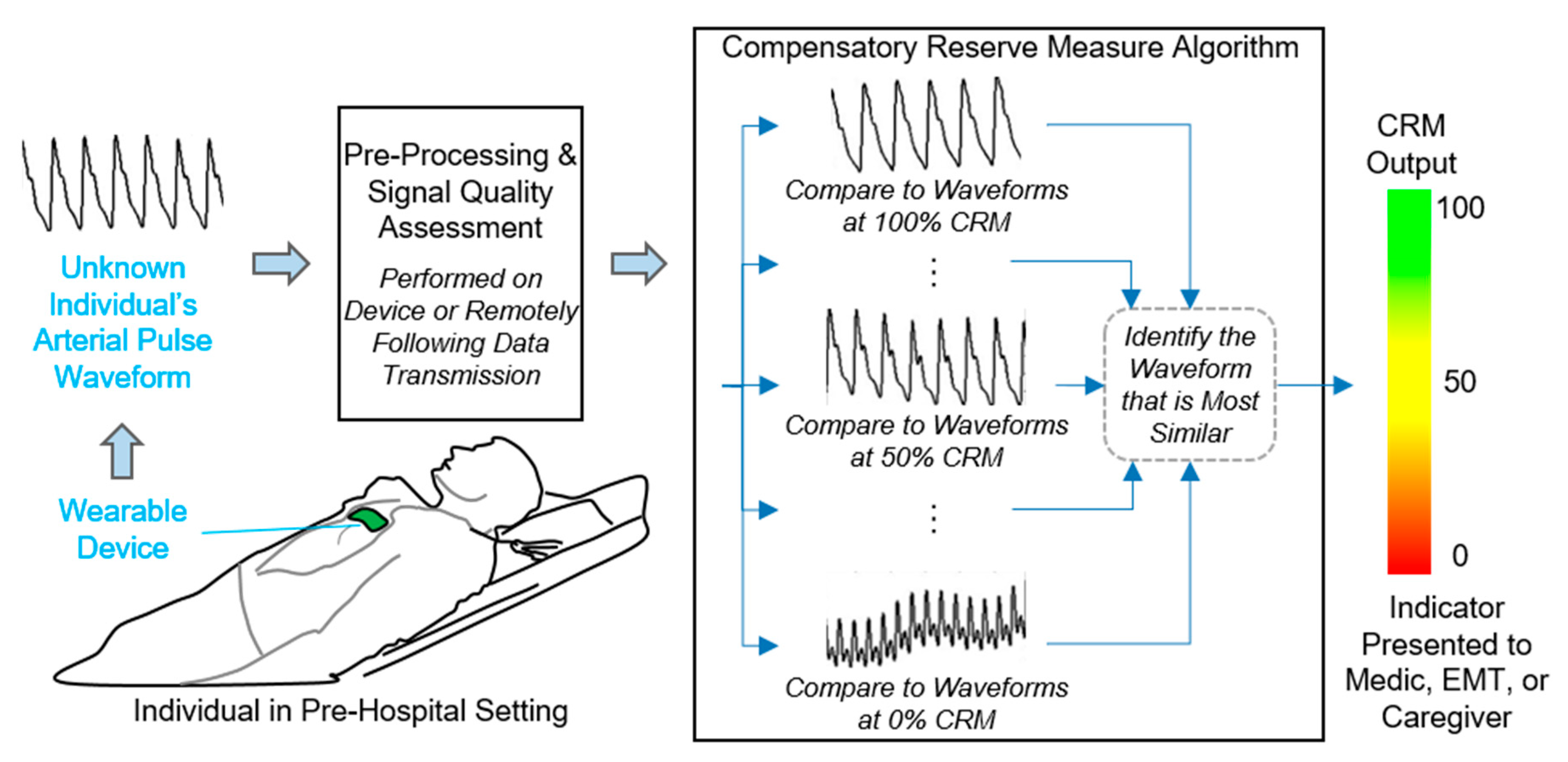
3. New Monitoring Approach: The Compensatory Reserve
3.1. Defining the Compensatory Reserve
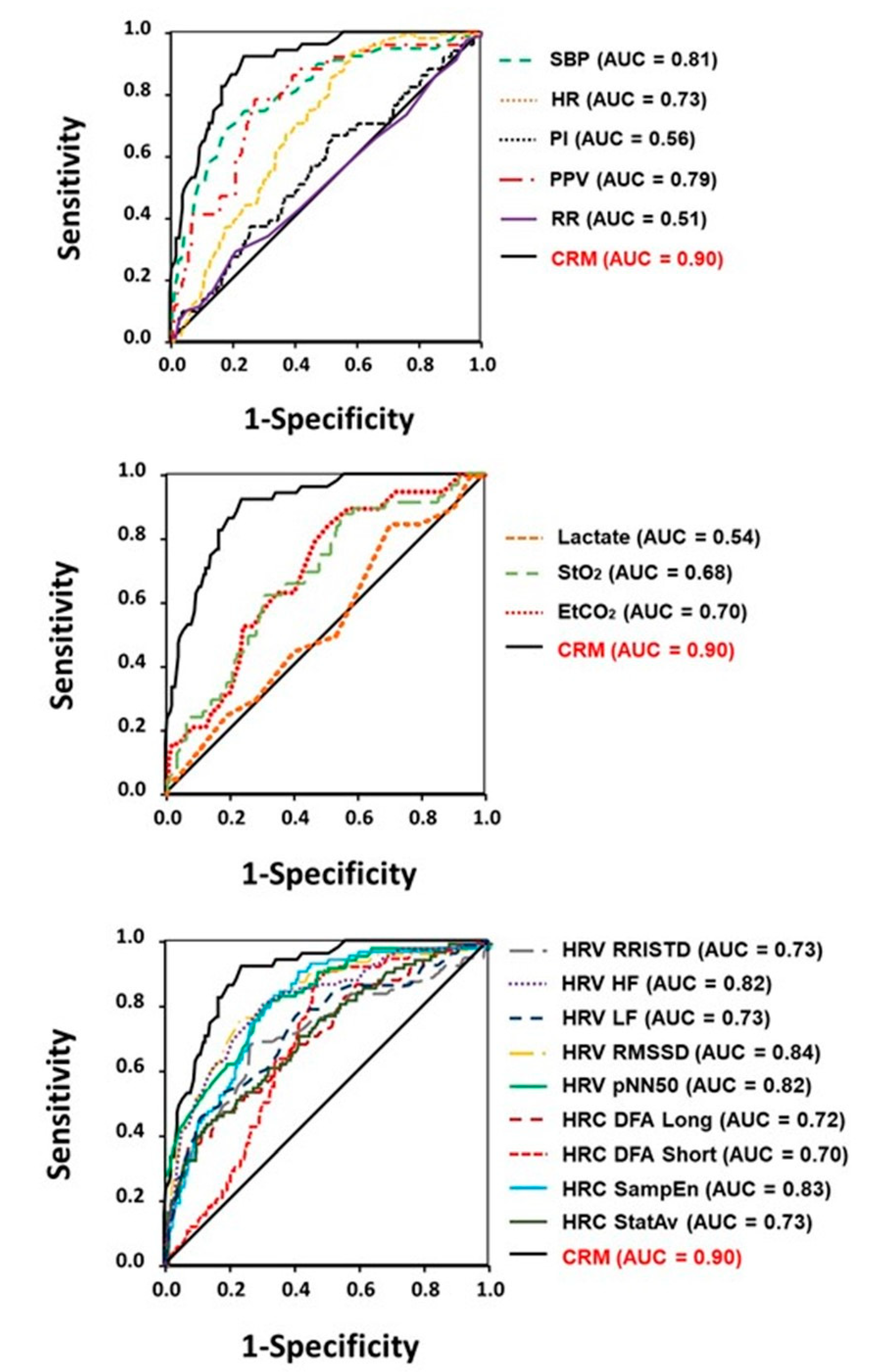
3.2. Performance Comparisons: Compensatory Reserve versus Vital Signs
4. Current Sensor Technology
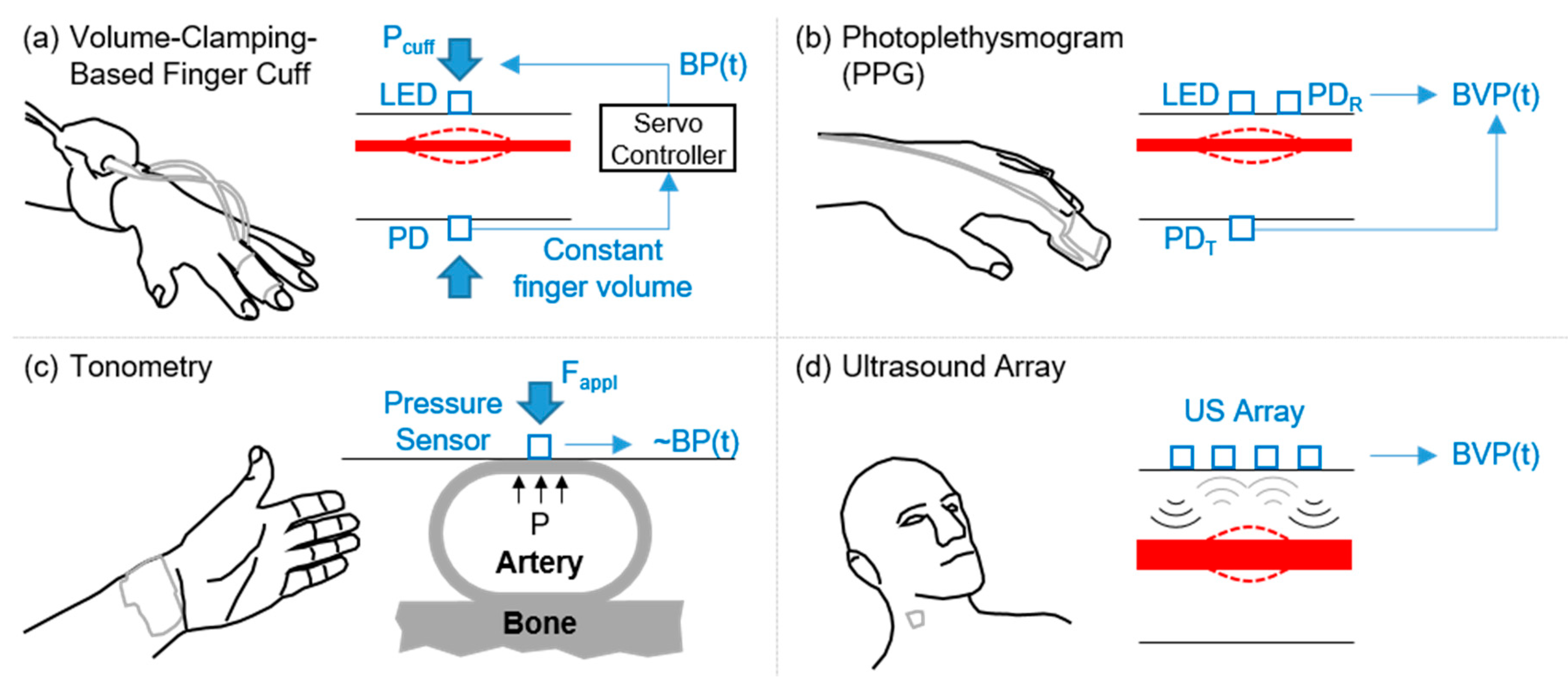
4.1. Arterial Waveform Measurement Modalities Amenable to Wearable Technology: Obtaining Reliable High Signal-to-Noise Features
4.1.1. PPG Signals
4.1.2. Tonometry Signals
4.1.3. Wearable Ultrasound
4.1.4. Cardio-Mechanical Vibrations
4.1.5. Other Emerging Wearable Sensing Devices
4.2. Mitigating the Effects of External Vibrations and Motion Artifacts
4.2.1. Improving the Signal Quality at the Source
4.2.2. Providing Auxiliary Sensors to Detect and Cancel Motion Artifacts
4.2.3. Quantifying Signal Quality for Rejecting Lower Quality Waveforms
4.3. Eliminating the Need for Baseline Measures/Calibration
4.4. Real-Time Measurements and Processing for Display
4.5. Electronic Documentation in the Prehospital Setting
4.6. Military Perspectives and Implications
4.7. Future Directions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Disclaimer
Copyright
References
- Charlari, E.; Intas, G.; Stergiannis, P.; Vezyridis, P.; Fildissis, G. The importance of vital signs in the triage of injured patients. Crit. Care Nurs. Q. 2013, 35, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Booth, J. A short history of blood pressure measurement. Proc. R. Soc. Med. 1977, 70, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Schauer, S.G.; Naylor, J.F.; April, M.D.; Fisher, A.D.; Cunningham, C.W.; Fernandez, J.R.D.; Shreve, B.P.; Bebarta, V.S. Prehospital resuscitation performed on hypotensive trauma patients in afghanistan: The prehospital trauma registry experience. Mil. Med. 2019, 184, e154–e157. [Google Scholar] [CrossRef] [PubMed]
- Naylor, J.F.; Fisher, A.D.; April, M.D.; Schauer, S.G. An analysis of radial pulse strength to recorded blood pressure in the Department of Defense Trauma Registry. Mil. Med. 2020. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Salinas, J.; McManus, J.M.; Miller, C.C.; Cooke, W.H.; Convertino, V.A. Manual vital signs reliably predict need for life-saving intervention in trauma patients. J. Trauma 2005, 59, 821–829. [Google Scholar] [CrossRef]
- McManus, J.; Yershov, A.L.; Ludwig, D.; Holcomb, J.B.; Salinas, J.; Dubick, M.A.; Convertino, V.A.; Hinds, D.; David, W.; Flanagan, T.; et al. Radial pulse character relationships to systolic blood pressure and trauma outcomes. Prehospital Emerg. Care 2005, 9, 423–428. [Google Scholar] [CrossRef]
- Keenan, S.; Riesberg, J.C. Prolonged field care: Beyond the “Golden Hour”. Wilderness Environ. Med. 2017, 28, S135–S139. [Google Scholar] [CrossRef]
- Ball, J.A.; Keenan, S. Prolonged field care working group position paper: Prolonged field care capabilities. J. Spec. Oper. Med. 2015, 15, 76–77. [Google Scholar]
- Bickell, W.H.; Wall, M.J., Jr.; Pepe, P.E.; Martin, R.R.; Ginger, V.F.; Allen, M.K.; Mattox, K.L. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. N. Engl. J. Med. 1994, 331, 1105–1109. [Google Scholar] [CrossRef]
- Griggs, J.E.; Jeyanathan, J.; Joy, M.; Russell, M.Q.; Durge, N.; Bootland, D.; Dunn, S.; Sausmarez, E.D.; Wareham, G.; Weaver, A.; et al. Mortality of civilian patients with suspected traumatic haemorrhage receiving pre-hospital transfusion of packed red blood cells compared to pre-hospital crystalloid. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 100. [Google Scholar] [CrossRef]
- Huang, G.S.; Dunham, C.M. Mortality outcomes in trauma patients undergoing prehospital red blood cell transfusion: A systematic literature review. Int. J. Burns Trauma 2017, 7, 17–26. [Google Scholar] [PubMed]
- Bellamy, R.F. The causes of death in conventional land warfare: Implications for combat casualty care research. Mil. Med. 1984, 149, 55–62. [Google Scholar] [PubMed]
- Eastridge, B.J.; Hardin, M.; Cantrell, J.; Oetjen-Gerdes, L.; Zubko, T.; Mallak, C.; Wade, C.E.; Simmons, J.; Mace, J.; Mabry, R.; et al. Died of wounds on the battlefield: Causation and implications for improving combat casualty care. J. Trauma 2011, 71, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Koons, N.J.; Suresh, M. The physiology of human hemorrhage and compensation. Comp. Physiol. 2020, in press. [Google Scholar]
- April, M.D.; Becker, T.E.; Fisher, A.D.; Naylor, J.F.; Schauer, S.G. Vital sign thresholds predictive of death in the combat setting. Am. J. Emerg. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Eastridge, B.J.; Salinas, J.; McManus, J.G.; Blackburn, L.; Bugler, E.M.; Cooke, W.H.; Convertino, V.A.; Wade, C.E.; Holcomb, J.B. Hypotension begins at 110 mm Hg: Redefining “hypotension” with data. J. Trauma 2007, 63, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Koons, N.J.; Nguyen, B.; Suresh, M.R.; Hinojosa-Laborde, C.; Convertino, V.A. Tracking DO2 with compensatory reserve during whole blood resuscitation in baboons. Shock 2020, 53, 327–334. [Google Scholar] [CrossRef]
- Convertino, V.A.; Koons, N.J. The compensatory reserve: Potential for accurate individualized goal-directed whole blood resuscitation. Transfusion 2020, 60, S150–S157. [Google Scholar] [CrossRef]
- Thiele, R.H.; Nemergut, E.C.; Lynch, C. The physiologic implications of isolated alpha(1) adrenergic stimulation. Anesth. Analg. 2011, 113, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Wampler, M.R.; Johnson, M.; Alarhayem, A.; Le, T.D.; Nicholson, S.; Myers, J.G.; Chung, K.K.; Struck, K.R.; Cuenca, C.; et al. Validating clinical threshold values for a dashboard view of the compensatory reserve measurement for hemorrhage detection. J. Trauma Acute Care Surg. 2020, 89, S169–S174. [Google Scholar] [CrossRef]
- Convertino, V.A.; Grudic, G.; Mulligan, J.; Moulton, S. Estimation of individual-specific progression to impending cardiovascular instability using arterial waveforms. J. Appl. Physiol. 2013, 115, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Wirt, M.D.; Glenn, J.F.; Lein, B.C. The compensatory reserve for early and accurate prediction of hemodynamic compromise: A review of the underlying physiology. Shock 2016, 45, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Schiller, A.M. Measuring the compensatory reserve to identify shock. J. Trauma Acute Care Surg. 2017, 82, S57–S65. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Moulton, S.L.; Grudic, G.Z.; Rickards, C.A.; Hinojosa-Laborde, C.; Ryan, K.L. Use of advanced machine-learning techniques for non-invasive monitoring of hemorrhage. J. Trauma 2011, 71, S25–S32. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Cardin, S.; Batchelder, P.; Grudic, G.Z.; Mulligan, J.; Moulton, S.L.; MacLeod, D. A novel measurement for accurate assessment of clinical status in patients with significant blood loss: The compensatory reserve. Shock 2015, 44, 27–32. [Google Scholar] [CrossRef]
- Orlinsky, M.; Shoemaker, W.; Reis, E.D.; Kerstein, M.D. Current controversies in shock and resuscitation. Surg. Clin. N. Am. 2001, 81, 1217–1262. [Google Scholar] [CrossRef]
- Wo, C.C.; Shoemaker, W.C.; Appel, P.L.; Bishop, M.H.; Kram, H.B.; Hardin, E. Unreliability of blood pressure and heart rate to evaluate cardiac output in emergency resuscitation and critical illness. Crit. Care Med. 1993, 21, 218–223. [Google Scholar] [CrossRef]
- Bruijns, S.R.; Guly, H.R.; Bouamra, O.; Lecky, F.; Lee, W.A. The value of traditional vital signs, shock index, and age-based markers in predicting trauma mortality. J. Trauma Acute Care Surg. 2013, 74, 1432–1437. [Google Scholar] [CrossRef]
- Parks, J.K.; Elliott, A.C.; Gentilello, L.M.; Shafi, S. Systemic hypotension is a late marker of shock after trauma: A validation study of advanced trauma life support principles in a large national sample. Am. J. Surg. 2006, 192, 727–731. [Google Scholar] [CrossRef]
- Moulton, S.L.; Mulligan, J.; Grudic, G.Z.; Convertino, V.A. Running on empty? The compensatory reserve index. J. Trauma Acute Care Surg. 2013, 75, 1053–1059. [Google Scholar] [CrossRef]
- Hinojosa-Laborde, C.; Howard, J.T.; Mulligan, J.; Grudic, G.Z.; Convertino, V.A. Comparison of comensatory reserve during lower-body negative pressure and hemorrhage in nonhuman primates. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 310, R1154–R1159. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Laborde, C.; Shade, R.E.; Muniz, G.W.; Bauer, C.; Goei, K.A.; Pidcoke, H.F.; Chung, K.K.; Cap, A.P.; Convertino, V.A. Validation of lower body negative pressure as an experimental model of hemorrhage. J. Appl. Physiol. 2014, 116, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Convertino, V.A.; Rickards, C.A.; Ryan, K.L. Autonomic mechanisms associated with heart rate and vasoconstrictor reserves. Clin. Auton. Res. 2012, 22, 123–130. [Google Scholar] [CrossRef]
- Engelke, K.A.; Doerr, D.F.; Crandall, C.G.; Convertino, V.A. Application of acute maximal exercise to protect orthostatic tolerance after simulated microgravity. Am. J. Physiol. 1996, 271, R837–R847. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A. G-Factor as a tool in basic research: Mechanisms of orthostatic tolerance. J. Gravit. Physiol. 1999, 6, 73–76. [Google Scholar]
- Carter, R., III; Hinojosa-Laborde, C.; Convertino, V.A. Variability in integration of mechanisms associated with high tolerance to progressive reductions in central blood volume: The compensatory reserve. Physiol. Rep. 2016, 4, e12705. [Google Scholar] [CrossRef]
- Howard, J.T.; Janak, J.C.; Hinojosa-Laborde, C.; Convertino, V.A. Specificity of compensatory reserve and tissue oxygenation as early predictors of tolerance to progressive reductions in central blood volume. Shock 2016, 46, 68–73. [Google Scholar] [CrossRef]
- Janak, J.C.; Howard, J.T.; Goei, K.A.; Weber, R.; Muniz, G.W.; Hinojosa-Laborde, C.; Convertino, V.A. Predictors of the onset of hemodynamic decompensation during progressive central hypovolemia: Comparison of the peripheral perfusion index, pulse pressure variability, and compensatory reserve index. Shock 2015, 44, 548–553. [Google Scholar] [CrossRef]
- Chew, M.S.; Aneman, A. Haemodynamic monitoring using arterial waveform analysis. Curr. Opin. Crit. Care 2013, 19, 234–241. [Google Scholar] [CrossRef]
- Convertino, V.A. Blood pressure measurement for accurate assessment of patient status in emergency medical settings. Aviat. Space Environ. Med. 2012, 83, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Cooke, W.H.; Holcomb, J.B. Arterial pulse pressure and its association with reduced stroke volume during progressive central hypovolemia. J. Trauma 2006, 61, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.J.; Vistisen, S.T.; Jian, Z.; Hatib, F.; Scheeren, T.W.L. Ability of an arterial waveform analysis-derived hypotension prediction index to predict future hypotensive events in surgical patients. Anesth. Analg. 2020, 130, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Hametner, B.; Wassertheurer, S. Pulse Waveform Analysis: Is it ready for prime time? Curr. Hypertens. Rep. 2017, 19, 73. [Google Scholar] [CrossRef]
- Hatib, F.; Jian, Z.; Buddi, S.; Lee, C.; Settels, J.; Sibert, K.; Rinehart, J.; Cannesson, M. Machine-learning algorithm to predict hypotension based on high-fidelity arterial pressure waveform analysis. Anesthesiology 2018, 129, 663–674. [Google Scholar] [CrossRef]
- Kendale, S.; Kulkarni, P.; Rosenberg, A.D.; Wang, J. Supervised machine-learning predictive analytics for prediction of postinduction hypotension. Anesthesiology 2018, 129, 675–688. [Google Scholar] [CrossRef]
- Kim, S.K.; Shin, W.J.; Kim, J.W.; Park, J.Y.; Hwang, G.S. Prediction of hyperdynamic circulation by arterial diastolic reflected waveform analysis in patients undergoing liver transplantation. Blood Press. Monit. 2016, 21, 9–15. [Google Scholar] [CrossRef]
- Thiele, R.H.; Durieux, M.E. Arterial waveform analysis for the anesthesiologist: Past, present, and future concepts. Anesth. Analg. 2011, 113, 766–776. [Google Scholar] [CrossRef]
- Wasicek, P.J.; Teeter, W.A.; Yang, S.; Hu, P.; Gamble, W.B.; Galvagno, S.M.; Hoehn, M.R.; Scalea, T.M.; Morrison, J.J. Arterial waveform morphomics during hemorrhagic shock. Eur. J. Trauma Emerg. Surg. Off. Publ. Eur. Trauma Soc. 2019. [Google Scholar] [CrossRef]
- Van der Ster, B.; Westerhof, B.; Stock, W.; Van Lieshout, J. Detecting central hypovolemia in simulated hypovolemic shock by automated feature extraction with principal component analysis. Physiol. Rep. 2018, 6, e13895. [Google Scholar] [CrossRef]
- Holder, A.L.; Clermont, G. Using what you get: Dynamic physiologic signatures of critical illness. Crit. Care Clin. 2015, 31, 133–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Esper, S.A.; Pinsky, M.R. Arterial waveform analysis. Best Pract. Res. Clin. Anaesthesiol. 2014, 28, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Reisner, A.; Shaltis, P.A.; McCombie, D.; Asada, H.H. Utility of the photoplethysmogram in circulatory monitoring. Anesthesiology 2008, 108, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Schiller, A.M.; Howard, J.T.; Lye, K.R.; Magby, C.G.; Convertino, V.A. Comparisons of traditional metabolic markers and compensatory reserve as early predictors of tolerance to central hypovolemia in humans. Shock 2018, 50, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Convertino, V.A.; Sawka, M.N. Wearable compensatory reserve measurement for hypovolemia sensing. J. Appl. Physiol. 2018, 124, 442–451. [Google Scholar] [CrossRef]
- Schiller, A.M.; Howard, J.T.; Convertino, V.A. The physiology of blood loss and shock: New insights from a human laboratory model of hemorrhage. Exp. Biol. Med. 2017, 242, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Schlotman, T.E.; Akers, K.S.; Nessen, S.C.; Convertino, V.A. Differentiating compensatory mechanisms associated with low tolerance to central hypovolemia in women. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H609–H616. [Google Scholar] [CrossRef]
- Wenner, M.M.; Hinds, K.; Howard, J.; Nawn, C.D.; Carter, R., III; Hinojosa-Laborde, C.; Stachenfeld, N.S.; Convertino, V. Differences in compensatory response to progressive reductions in central blood volume of African American and Caucasian women. J. Trauma Acute Care Surg. 2018, 85, S77–S83. [Google Scholar] [CrossRef]
- Wenner, M.M.; Hinds, K.A.; Howard, J.T.; Nawn, C.D.; Stachenfeld, N.S.; Convertino, V.A. Measurement of compensatory reserve predicts racial differences in tolerance to simulated hemorrhage in women. J. Trauma Acute Care Surg. 2018, 85, S77–S83. [Google Scholar] [CrossRef]
- Johnson, M.; Alarhayem, A.; Convertino, V.; Carter, R., 3rd; Chung, K.; Stewart, R.; Myers, J.; Dent, D.; Liao, L.; Cestero, R.; et al. Compensatory reserve index: Performance of a novel monitoring technology to identify the bleeding trauma patient. Shock 2018, 49, 295–300. [Google Scholar] [CrossRef]
- Stewart, C.L.; Mulligan, J.; Grudic, G.Z.; Convertino, V.A.; Moulton, S.L. Detection of low-volume blood loss: The compensatory reserve index versus traditional vital signs. J. Trauma Acute Care Surg. 2014, 77, 892–897. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Perkins, N.J.; Liu, A.; Bondell, H. Optimal cut-point and its corresponding Youden Index to discriminate individuals using pooled blood samples. Epidemiology 2005, 16, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Schlotman, T.E.; Suresh, M.; Koons, N.J.; Howard, J.T.; Convertino, V.A. Comparisons of measures of compensatory reserve and heart rate variability as early indicators of hemodynamic decompensation in progressive hypovolemia. J. Trauma Acute Care Surg. 2020, 89, S161–S168. [Google Scholar] [PubMed]
- Nadler, R.; Convertino, V.A.; Gendler, S.; Lending, G.; Lipsky, A.M.; Cardin, S.; Lowenthal, A.; Glassberg, E. The value of non-invasive mesurement of the compensatory reserve index in monitoring and triage of patients experiencing minimal blood loss. Shock 2014, 42, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Moulton, S.L.; Mulligan, J.; Santoro, M.A.; Bui, K.; Grudic, G.Z.; MacLeod, D. Validation of a noninvasive monitor to continuously trend individual responses to hypovolemia. J. Trauma Acute Care Surg. 2017, 83, S104–S111. [Google Scholar] [CrossRef] [PubMed]
- Koons, N.J.; Owens, G.A.; Parsons, D.L.; Schauer, S.G.; Buller, J.L.; Convertino, V.A. Compensatory reserve: A novel monitoring capability for early detection of hemorrhage by combat medics. J. Trauma Acute Care Surg. 2020, 89, S146–S152. [Google Scholar]
- Muniz, G.W.; Wampler, D.A.; Manifold, C.A.; Grudic, G.Z.; Mulligan, J.; Moulton, S.; Gerhardt, R.T.; Convertino, V.A. Promoting early diagnosis of hemodynamic instability during simulated hemorrhage with the use of a real-time decision-assist algorithm. J. Trauma Acute Care Surg. 2013, 75, S184–S189. [Google Scholar] [CrossRef]
- Benov, A.; Yaslowitz, O.; Hakim, T.; Amir-Keret, R.; Nadler, R.; Brand, A.; Glassberg, E.; Yitzhak, A.; Convertino, V.A.; Paran, H. The effect of blood transfusion on compensatory reserve: A prospective clinical trial. J. Trauma Acute Care Surg. 2017, 83, S71–S76. [Google Scholar] [CrossRef]
- Benov, A.; Brand, A.; Rozenblat, T.; Antebi, B.; Ben-Ari, A.; Amir-Keret, R.; Nadler, R.; Chen, J.; Chung, K.K.; Convertino, V.A.; et al. Evaluation of sepsis using compensatory reserve measurement: A prospective clinical trial. J. Trauma Acute Care Surg. 2020, 89, S153–S160. [Google Scholar] [CrossRef]
- Stewart, C.L.; Nawn, C.D.; Mulligan, J.; Grudic, G.; Moulton, S.L.; Convertino, V.A. The Compensatory Reserve for Early and Accurate Prediction of Hemodynamic Compromise: Case Studies for Clinical Utility in Acute Care and Physical Performance. J. Spec. Oper. Med. 2016, 16, 6–13. [Google Scholar]
- Stewart, C.L.; Mulligan, J.; Grudic, G.Z.; Talley, M.E.; Jurkovich, G.J.; Moulton, S.L. The Compensatory reserve index following injury: Results of a prospective clinical trial. Shock 2016, 46, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Mulligan, J.; Grudic, G.Z.; Pyle, L.; Moulton, S.L. A noninvasive computational method for fluid resuscitation monitoring in pediatric burns: A preliminary report. J. Burn Care Res. 2015, 36, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Imholz, B.P.M.; Wieling, W.; van Montfrans, G.A.; Wesseling, K.H. Fifteen years experience with finger arterial pressure monitoring. Cardiovasc. Res. 1998, 38, 605–616. [Google Scholar] [CrossRef]
- Parati, G.; Casadei, R.; Groppelli, A.; Di Rienzo, M.; Mancia, G. Comparison of finger and intra-arterial blood pressure monitoring at rest and during laboratory testing. Hypertension 1989, 13, 647–655. [Google Scholar] [CrossRef]
- Lee, J.; Nam, K.C. Tonometric Vascular Function Assessment; INTECH Open Access Publisher: London, UK, 2009. [Google Scholar]
- Wang, Z.; Xu, Y. Design and optimization of an ultra-sensitive piezoresistive accelerometer for continuous respiratory sound monitoring. Sens. Lett. 2007, 5, 450–458. [Google Scholar] [CrossRef]
- Shelley, K.H. Photoplethysmography: Beyond the calculation of arterial oxygen saturation and heart rate. Anesth. Analg. 2007, 105, S31–S36. [Google Scholar] [CrossRef]
- Li, K.; Warren, S. A wireless reflectance pulse oximeter with digital baseline control for unfiltered photoplethysmograms. IEEE Trans. Biomed. Circuits Syst. 2012, 6, 269–278. [Google Scholar] [CrossRef]
- Maeda, Y.; Sekine, M.; Tamura, T. Relationship between measurement site and motion artifacts in wearable reflected photoplethysmography. J. Med. Syst. 2011, 35, 969–976. [Google Scholar] [CrossRef]
- Asada, H.H.; Shaltis, P.; Reisner, A.; Rhee, S.; Hutchinson, R.C. Mobile monitoring with wearable photoplethysmographic biosensors. IEEE Eng. Med. Biol. Mag. 2003, 22, 28–40. [Google Scholar] [CrossRef]
- Chung, H.U.; Rwei, A.Y.; Hourlier-Fargette, A.; Xu, S.; Lee, K.; Dunne, E.C.; Xie, Z.; Liu, C.; Carlini, A.; Kim, D.H.; et al. Skin-interfaced biosensors for advanced wireless physiological monitoring in neonatal and pediatric intensive-care units. Nat. Med. 2020, 26, 418–429. [Google Scholar] [CrossRef]
- Jeong, H.; Wang, L.; Ha, T.; Mitbander, R.; Yang, X.; Dai, Z.; Qiao, S.; Shen, L.; Sun, N.; Lu, N. Modular and reconfigurable wireless E-tattoos for personalized sensing. Adv. Mater. Technol. 2019, 4. [Google Scholar] [CrossRef]
- Biswas, S.; Shao, Y.; Hachisu, T.; Nguyen-Dang, T.; Visell, Y. Integrated soft optoelectronics for wearable health monitoring. Adv. Mater. Technol. 2020, 5, 2000347. [Google Scholar] [CrossRef]
- Han, D.; Khan, Y.; Ting, J.; Zhu, J.; Combe, C.; Wadsworth, A.; McCulloch, I.; Arias, A.C. Pulse oximetry using organic optoelectronics under ambient light. Adv. Mater. Technol. 2020, 5, 1901122. [Google Scholar] [CrossRef]
- Drzewiecki, G.M.; Melbin, J.; Noordergraaf, A. Deformational Forces in Arterial Tonometry; IEEE: New York, NY, USA, 1984; Volume 28. [Google Scholar]
- Eckerle, J.S. Tonometry, arterial. In Encyclopedia of Medical Devices and Instrumentation; Webster, J.G., Ed.; John Wiley & Sons: New York, NY, USA, 1988. [Google Scholar]
- Schwartz, G.; Tee, B.C.-K.; Mei, J.; Appleton, A.L.; Kim, D.H.; Wang, H.; Bao, Z. Flexible polymer transistors with high pressure sensitivity for application in electronic skin and health monitoring. Nat. Commun. 2013, 4, 1859. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, K.; Dumont, G.A.; Houlton, G.; Blaber, A.P. Precordial vibrations provide noninvasive detection of early-stage hemorrhage. Shock 2014, 41, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zia, J.; Kimball, J.; Rolfes, C.; Hahn, J.-O.; Inan, O.T. Enabling the assessment of trauma-induced hemorrhage via smart wearable systems. Sci. Adv. 2020, 6, eabb1708. [Google Scholar] [CrossRef] [PubMed]
- Inan, O.T.; Migeotte, P.F.; Kwang-Suk, P.; Etemadi, M.; Tavakolian, K.; Casanella, R.; Zanetti, J.; Tank, J.; Funtova, I.; Prisk, G.K.; et al. Ballistocardiography and seismocardiography: A review of recent advances. IEEE J. Biomed. Health Inform. 2015, 19, 1414–1427. [Google Scholar] [CrossRef]
- Etemadi, M.; Inan, O.T. Wearable ballistocardiogram and seismocardiogram systems for health and performance. J. Appl. Physiol. 2017, 124, 452–461. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Rizzo, G.; Işılay, Z.M.; Lombardi, P. SeisMote: A Multi-Sensor Wireless platform for cardiovascular monitoring in laboratory, daily life, and telemedicine. Sensors 2020, 20, 680. [Google Scholar] [CrossRef]
- Inan, O.T.; Baran Pouyan, M.; Javaid, A.Q.; Dowling, S.; Etemadi, M.; Dorier, A.; Heller, J.A.; Bicen, A.O.; Roy, S.; De Marco, T.; et al. Novel Wearable Seismocardiography and Machine Learning Algorithms Can Assess Clinical Status of Heart Failure Patients. Circ. Heart Fail. 2018, 11, e004313. [Google Scholar] [CrossRef] [PubMed]
- Shandhi, M.M.H.; Hersek, S.; Fan, J.; Sander, E.; De Marco, T.; Heller, J.A.; Etemadi, M.; Klein, L.; Inan, O.T. Wearable patch based estimation of oxygen uptake and assessment of clinical status during cardiopulmonary exercise testing in patients with heart failure. J. Card. Fail. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Norton, J.J.; Qazi, R.; Zou, Z.; Ammann, K.R.; Liu, H.; Yan, L.; Tran, P.L.; Jang, K.I.; Lee, J.W.; et al. Epidermal mechano-acoustic sensing electronics for cardiovascular diagnostics and human-machine interfaces. Sci. Adv. 2016, 2, e1601185. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.M.; Nguyen, A.; Lawal, Q.O.; Chortos, A.; Rondeau-Gagne, S.; Bao, Z. A sensitive and biodegradable pressure sensor array for cardiovascular monitoring. Adv. Mater. 2015, 27, 6954–6961. [Google Scholar] [CrossRef] [PubMed]
- Imani, S.; Bandodkar, A.J.; Vinu Mohan, A.M.; Kumar, R.; Yu, S.; Wang, J.; Mercier, P.P. A wearable chemical–electrophysiological hybrid biosensing system for real-time health and fitness monitoring. Nat. Commun. 2016, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sel, K.; Ibrahim, B.; Jafari, R. ImpediBands: Body coupled bio-impedance patches for physiological sensing proof of concept. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 757–774. [Google Scholar] [CrossRef]
- Teichmann, D.; Kuhn, A.; Leonhardt, S.; Walter, M. The MAIN Shirt: A textile-integrated magnetic induction sensor array. Sensors 2014, 14, 1039–1056. [Google Scholar] [CrossRef]
- Ganti, V.G.; Carek, A.; Nevius, B.N.; Heller, J.; Etemadi, M.; Inan, O.T. Wearable cuff-less blood pressure estimation at home via pulse transit time. IEEE J. Biomed. Health Inform. 2020, in press. [Google Scholar] [CrossRef]
- Lee, J.; Matsumura, K.; Yamakoshi, K.-i.; Rolfe, P.; Tanaka, S.; Yamakoshi, T. Comparison between Red, Green and Blue Light Reflection Photoplethysmography for Heart Rate Monitoring During Motion. In Proceedings of the Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 1724–1727. [Google Scholar]
- Spigulis, J.; Gailite, L.; Lihachev, A.; Erts, R. Simultaneous recording of skin blood pulsations at different vascular depths by multiwavelength photoplethysmography. Appl. Opt. 2007, 46, 1754–1759. [Google Scholar] [CrossRef]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable photoplethysmographic sensors—past and present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Kaisti, M.; Panula, T.; Leppanen, J.; Punkkinen, R.; Jafari Tadi, M.; Vasankari, T.; Jaakkola, S.; Kiviniemi, T.; Airaksinen, J.; Kostiainen, P.; et al. Clinical assessment of a non-invasive wearable MEMS pressure sensor array for monitoring of arterial pulse waveform, heart rate and detection of atrial fibrillation. NPJ Digit. Med. 2019, 2, 39. [Google Scholar] [CrossRef] [PubMed]
- Digiglio, P.; Li, R.; Wang, W.; Pan, T. Microflotronic arterial tonometry for continuous wearable non-invasive hemodynamic monitoring. Ann. Biomed. Eng. 2014, 42, 2278–2288. [Google Scholar] [CrossRef] [PubMed]
- Hossein, A.; Mirica, D.C.; Rabineau, J.; Rio, J.I.D.; Morra, S.; Gorlier, D.; Nonclercq, A.; van de Borne, P.; Migeotte, P.F. Accurate detection of dobutamine-induced haemodynamic changes by kino-cardiography: A randomised double-blind placebo-controlled validation study. Sci. Rep. 2019, 9, 10479. [Google Scholar] [CrossRef] [PubMed]
- Poh, M.-Z.; Swenson, N.C.; Picard, R.W. Motion-tolerant magnetic earring sensor and wireless earpiece for wearable photoplethysmography. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Kim, M.-J.; Kim, J. Development of Real-Time Motion Artifact Reduction Algorithm for a Wearable Photoplethysmography. In Proceedings of the Engineering in Medicine and Biology Society, Lyon, France, 23–26 August 2007; pp. 1538–1541. [Google Scholar]
- Wood, L.B.; Asada, H.H. Noise Cancellation Model Validation for Reduced Motion Artifact Wearable PPG Sensors Using MEMS Accelerometers. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; pp. 3525–3528. [Google Scholar]
- Javaid, A.Q.; Ashouri, H.; Dorier, A.; Etemadi, M.; Heller, J.A.; Roy, S.; Inan, O.T. Quantifying and reducing Motion artifacts in wearable seismocardiogram measurements during walking to assess left ventricular health. IEEE Trans. Biomed. Eng. 2017, 64, 1277–1286. [Google Scholar] [CrossRef]
- Yang, C.; Tavassolian, N. Motion artifact cancellation of seismocardiographic recording from moving subjects. IEEE Sens. J. 2016, 16, 5702–5708. [Google Scholar] [CrossRef]
- Inan, O.T.; Dookun, P.; Giovangrandi, L.; Kovacs, G.T. Noninvasive measurement of physiological signals on a modified home bathroom scale. IEEE Trans. Biomed. Eng. 2012, 59, 2137–2143. [Google Scholar] [CrossRef]
- Inan, O.T.; Etemadi, M.; Widrow, B.; Kovacs, G.T.A. Adaptive cancellation of floor vibrations in standing ballistocardiogram measurements using a seismic sensor as a noise reference. IEEE Trans. Biomed. Eng. 2010, 57, 722–727. [Google Scholar] [CrossRef]
- Orphanidou, C.; Bonnici, T.; Charlton, P.; Clifton, D.; Vallance, D.; Tarassenko, L. Signal-quality indices for the electrocardiogram and photoplethysmogram: Derivation and applications to wireless monitoring. IEEE J. Biomed. Health Inform. 2014, 19, 832–838. [Google Scholar] [CrossRef]
- Li, Q.; Clifford, G.D. Dynamic time warping and machine learning for signal quality assessment of pulsatile signals. Physiol. Meas. 2012, 33, 1491–1501. [Google Scholar] [CrossRef]
- Zia, J.S.; Kimball, J.; Hersek, S.; Shandhi, M.; Semiz, B.; Inan, O. A unified framework for quality indexing and classification of seismocardiogram signals. IEEE J. Biomed. Health Inform. 2019. [Google Scholar] [CrossRef] [PubMed]
- Keogh, E.; Ratanamahatana, C.A. Exact indexing of dynamic time warping. Knowl. Inf. Syst. 2005, 7, 358–386. [Google Scholar] [CrossRef]
- Zia, J.S.; Kimball, J.; Rozell, C.J.; Inan, O. Harnessing the manifold structure of cardiomechanical signals for physiological monitoring during hemorrhage. IEEE Trans. Biomed. Eng. 2020. [Google Scholar] [CrossRef]
- Karmer, G.C.; Kinsky, M.P.; Prough, D.S.; Salinas, J.; Sondeen, J.L.; Hazel-Scero, M.L.; Mitchell, C.E. Closed-loop control of fluid therapy for treatment of hypovolemia. J. Trauma 2008, 64, S333–S341. [Google Scholar] [CrossRef] [PubMed]
- Libert, N.; Chenegros, G.; Harrois, A.; Baudry, N.; Cordurie, G.; Benosman, R.; Vicaut, E.; Duranteau, J. Performance of closed-loop resuscitation of haemorrhagic shock with fluid alone or in combination with norepinephrine: An experimental study. Ann. Intensive Care 2018, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Bighamian, R.; Kim, C.-S.; Reisner, A.T.; Hahn, J.-O. Closed-loop fluid resuscitation control via blood volume estimation. J. Dyn. Syst. Meas. Control 2016, 138, 111005. [Google Scholar] [CrossRef]
- Schauer, S.G.; Naylor, J.F.; Oliver, J.J.; Maddry, J.K.; April, M.D. An analysis of casualties presenting to military emergency departments in Iraq and Afghanistan. Am. J. Emerg. Med. 2019, 37, 94–99. [Google Scholar] [CrossRef]
- Chen, F.; Chandrakasan, A.P.; Stojanovic, V.M. Design and analysis of a hardware-efficient compressed sensing architecture for data compression in wireless sensors. IEEE J. Solid State Circuits 2012, 47, 744–756. [Google Scholar] [CrossRef]
- Craven, D.; McGinley, B.; Kilmartin, L.; Glavin, M.; Jones, E. Compressed sensing for bioelectric signals: A review. IEEE J. Biomed. Health Inform. 2015, 19, 529–540. [Google Scholar] [CrossRef]
- Butler, F.K.; Holcomb, J.B.; Schreiber, M.A.; Kotwal, R.S.; Jenkins, D.A.; Champion, H.R.; Bowling, F.; Cap, A.P.; Dubose, J.J.; Dorlac, W.C.; et al. Fluid resuscitation for hemorrhagic shock in tactical combat casualty care: TCCC Guidelines Change 14-01-2 June 2014. J. Spec. Oper. Med. 2014, 14, 30–55. [Google Scholar]
- Mulder, M.B.; Eidelson, S.A.; Buzzelli, M.D.; Gross, K.R.; Batchinsky, A.I.; Convertino, V.A.; Schulman, C.I.; Namias, N.; Proctor, K.G. Exercise-induced changes in compensatory reserve and heart rate complexity. Aerosp. Med. Hum. Perform. 2019, 90, 1009–1015. [Google Scholar] [CrossRef] [PubMed]

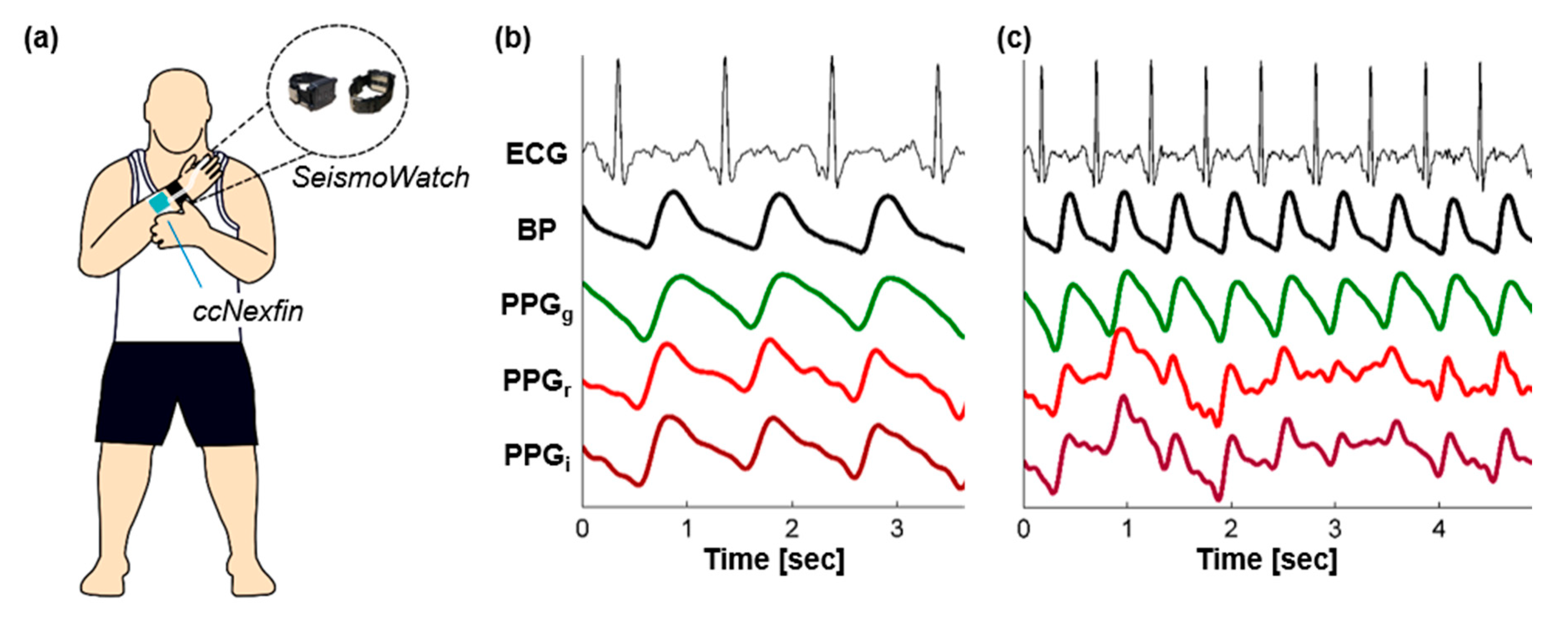
| Vital Sign or Measurement | Change During Progressive Central Hypovolemia |
|---|---|
| Systolic blood pressure | Late |
| Diastolic blood pressure | Late |
| Mean blood pressure | Late |
| Heart rate | Non-specific |
| Shock index (heart rate/systolic pressure) | Late |
| Oxygen saturation | Late |
| Radial pulse character assessment | Late |
| End-tidal CO2 | Late, Non-specific |
| Respiratory rate | Late, Non-specific |
| Glasgow Coma Scale | Late, Non-specific |
| Blood pH, PCO2, Base Excess | Late, Non-specific |
| Blood Lactate | Late, Non-specific |
| Hematocrit, Hemoglobin | Late, Non-specific |
| Vital Sign | Sensitivity | Specificity | Youden’s ‘J’ Index |
|---|---|---|---|
| Systolic Blood Pressure | 0.80 | 0.17 | 0.03 |
| Diastolic Blood Pressure | 0.40 | 0.53 | 0.07 |
| Mean Blood Pressure | 0.60 | 0.33 | 0.07 |
| Heart Rate | 0.80 | 0.02 | 0.18 |
| Stroke Volume | 0.60 | 0.33 | 0.07 |
| Cardiac Output | 0.80 | 0.02 | 0.18 |
| Pulse Pressure Variability | 0.78 | 0.69 | 0.47 |
| Peripheral Capillary Oxygen Saturation (SpO2) | 0.60 | 0.00 | 0.40 |
| Deep Muscle Oxygen Saturation (SmO2) | 0.65 | 0.63 | 0.28 |
| Compensatory Reserve | 0.84–0.87 | 0.78–0.86 | 0.62–0.73 |
| Sensing Modality | Principle of Operation | Typical Location(s) | Advantages | Disadvantages |
|---|---|---|---|---|
| PPG | Optical sensing of blood volume changes in a small volume of tissue | Transmissive: Finger, Earlobe, Toe Reflective: Wrist, Forehead, Forearm | Waveform resembles arterial pressure curves; signal quality is typically high; well-established sensing modality and already used in many clinical settings (i.e., pulse oximetry) | Signal originates mainly from the cutaneous vasculature and thus is affected by hypoperfusion (peripheral vasoconstriction); reflective PPG is more convenient in terms of placement but suffers from motion artifacts and placement-based variability in signal shape; requires substantial current consumption (active sensing) |
| Tonometry | Force/pressure sensing of arterial wall displacement at the surface of the skin | Wrist (Radial Artery) | With applanation of the artery, captures true blood pressure waveform and does not require calibration; low power measurement since it is a passive sensing approach | Applanation is challenging in practice and not reliable; measurement depends highly on placement; coupling to a superficial artery is needed |
| Ultrasound-Based BP | Ultrasound sensing of arterial diameter changes in deeper/larger arteries | Neck (Carotid Artery) | Measurements can be obtained from deeper arteries (e.g., carotid) and thus are less affected by hypoperfusion and/or vasoconstriction; arrayed sensing approach may reduce the variability in signal shape due to sensor positioning | Active measurement which requires substantial power consumption to deliver ultrasound energy to the body and process the resultant signals; may require manual approaches to annotating images |
| Cardiogenic Vibration | Mechanical vibration sensing of blood movement through vasculature | Chest (Sternum) | Passive measurements that can be captured non-intrusively with sensors on the chest; represent more central cardiac activity since the origination is from cardiac vibrations rather than peripheral blood volume pulse; minimal affects due to peripheral vasoconstriction | Not a direct arterial pressure waveform analog; requires coupling to the chest with an adhesive; may be sensitive to positioning of the sensor on the body |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Convertino, V.A.; Schauer, S.G.; Weitzel, E.K.; Cardin, S.; Stackle, M.E.; Talley, M.J.; Sawka, M.N.; Inan, O.T. Wearable Sensors Incorporating Compensatory Reserve Measurement for Advancing Physiological Monitoring in Critically Injured Trauma Patients. Sensors 2020, 20, 6413. https://doi.org/10.3390/s20226413
Convertino VA, Schauer SG, Weitzel EK, Cardin S, Stackle ME, Talley MJ, Sawka MN, Inan OT. Wearable Sensors Incorporating Compensatory Reserve Measurement for Advancing Physiological Monitoring in Critically Injured Trauma Patients. Sensors. 2020; 20(22):6413. https://doi.org/10.3390/s20226413
Chicago/Turabian StyleConvertino, Victor A., Steven G. Schauer, Erik K. Weitzel, Sylvain Cardin, Mark E. Stackle, Michael J. Talley, Michael N. Sawka, and Omer T. Inan. 2020. "Wearable Sensors Incorporating Compensatory Reserve Measurement for Advancing Physiological Monitoring in Critically Injured Trauma Patients" Sensors 20, no. 22: 6413. https://doi.org/10.3390/s20226413
APA StyleConvertino, V. A., Schauer, S. G., Weitzel, E. K., Cardin, S., Stackle, M. E., Talley, M. J., Sawka, M. N., & Inan, O. T. (2020). Wearable Sensors Incorporating Compensatory Reserve Measurement for Advancing Physiological Monitoring in Critically Injured Trauma Patients. Sensors, 20(22), 6413. https://doi.org/10.3390/s20226413







