HyCHEED System for Maintaining Stable Temperature Control during Preclinical Irreversible Electroporation Experiments at Clinically Relevant Temperature and Pulse Settings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
2.1.1. Hydraulic Unit
2.1.2. Electric Unit
2.1.3. Control Unit
2.2. Target-Holder Architecture
2.3. Temperature Measurements
2.4. Cell Line and Cell Culture
2.5. Validation of HyCHEED
2.5.1. Choice and Evaluation of Pulse Settings and Target Temperatures
- a significant temperature difference compared to 37 °C, allowing us to demonstrate the capability of HyCHEED to operate at different temperatures;
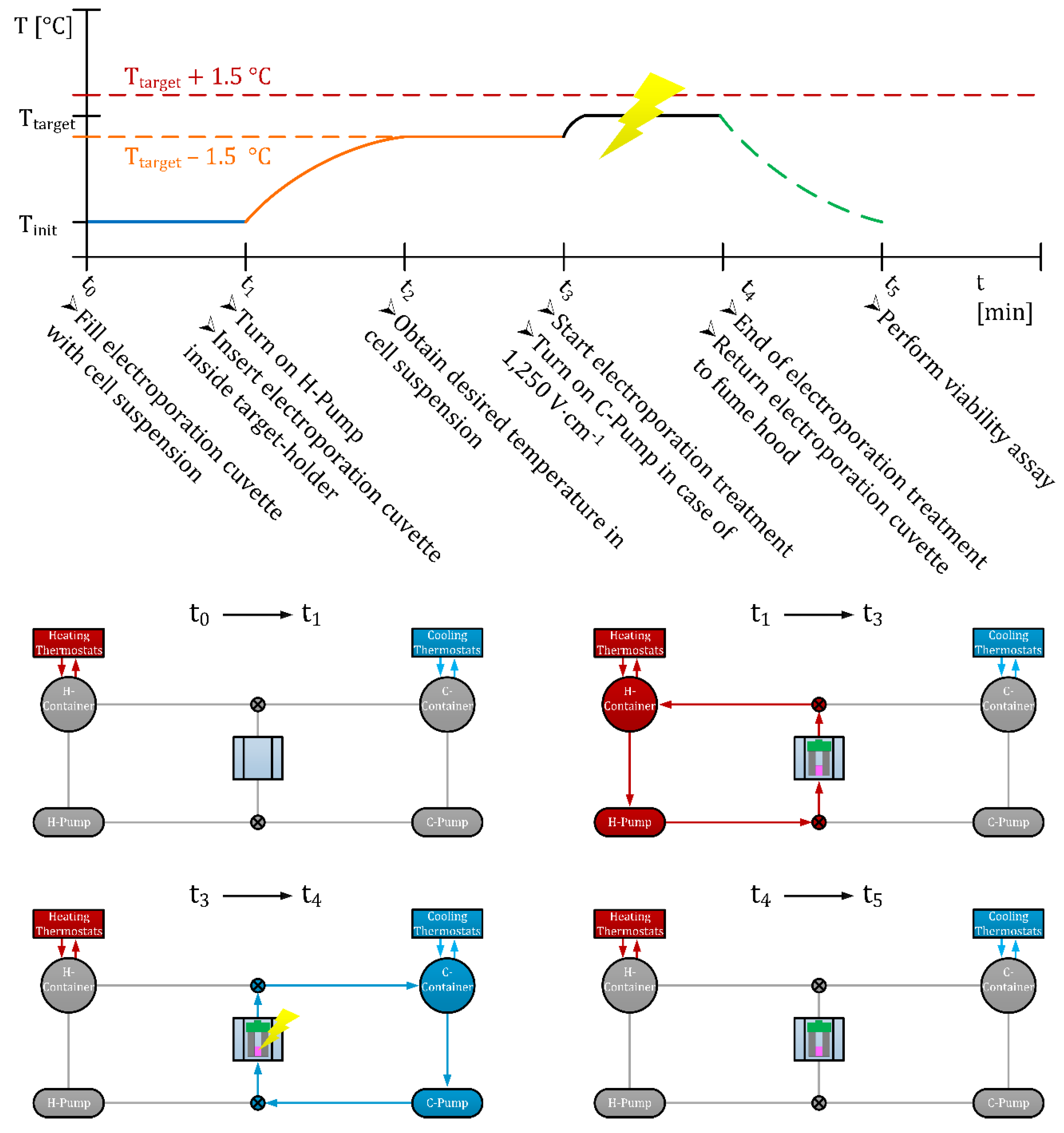
2.5.2. Operation Protocol of HyCHEED
2.6. Evaluation of In Vitro Experiments Using HyCHEED
2.6.1. Establishing the Electrical Specific Conductivity of the Target Volume
2.6.2. Cell Viability Assays/Reagents
2.6.3. Statistical Analyses
3. Results
3.1. Validation of HyCHEED
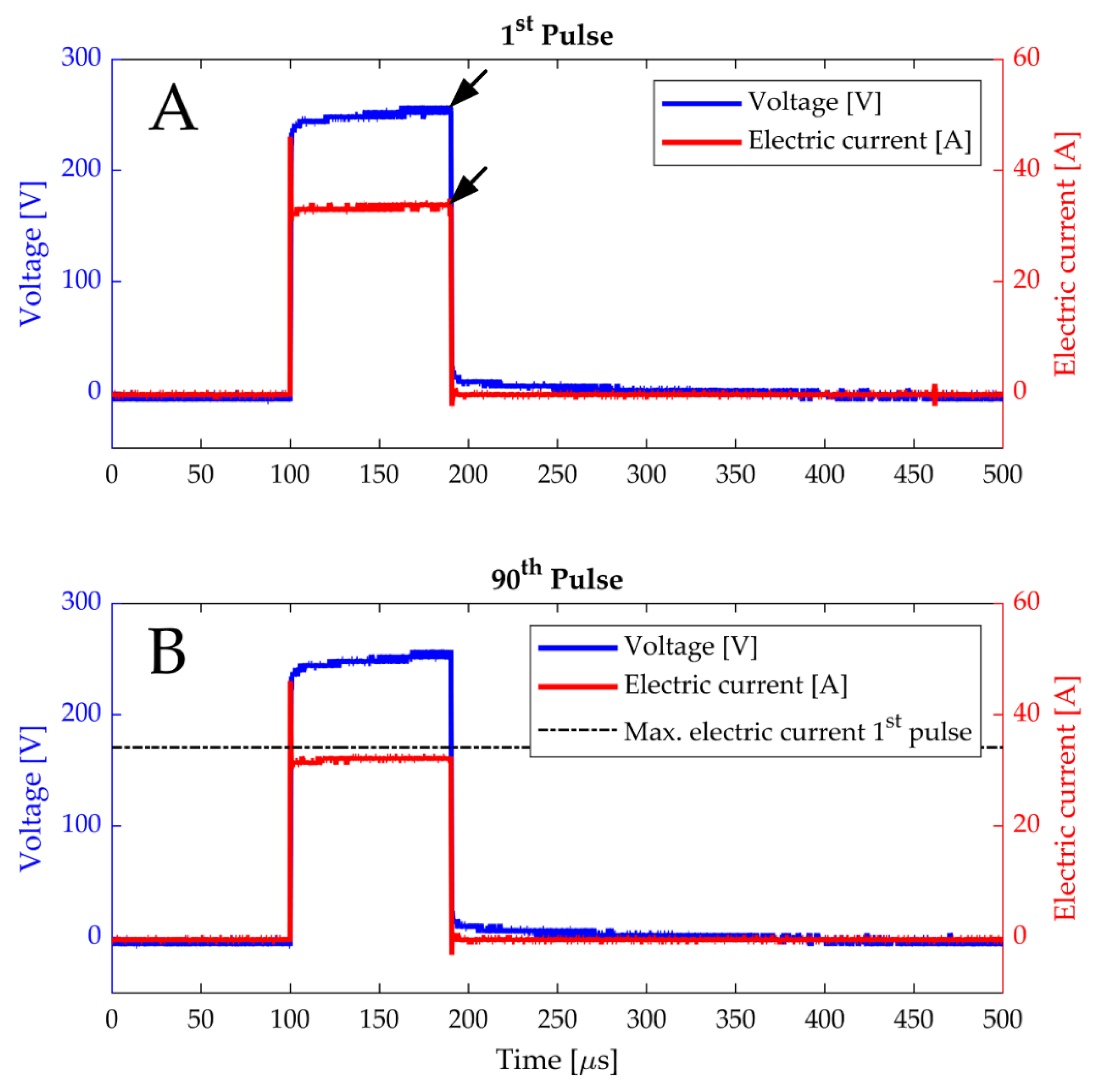
3.1.1. Read-Outs of Electrical Parameters
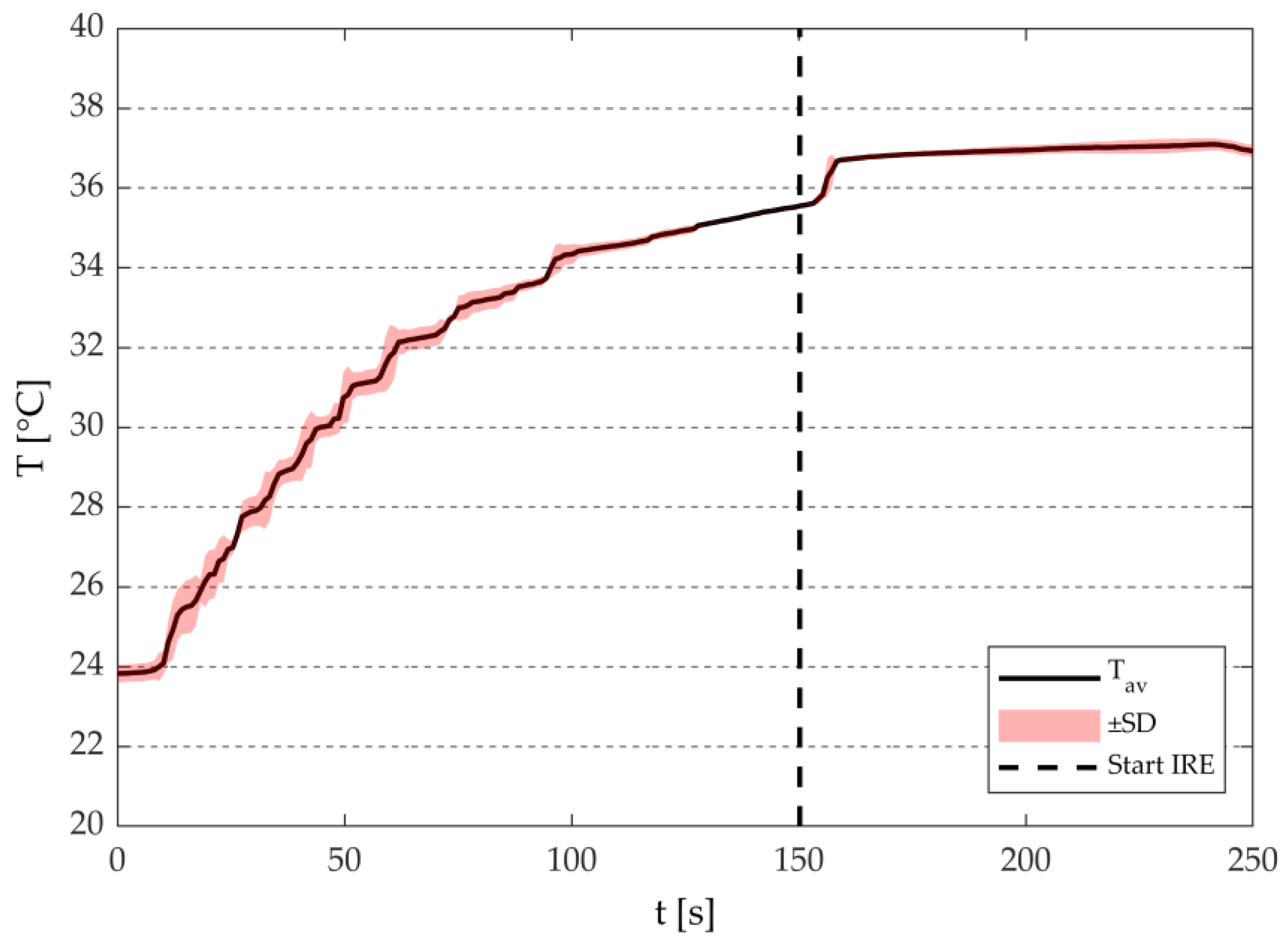
3.1.2. Temperature Measurements
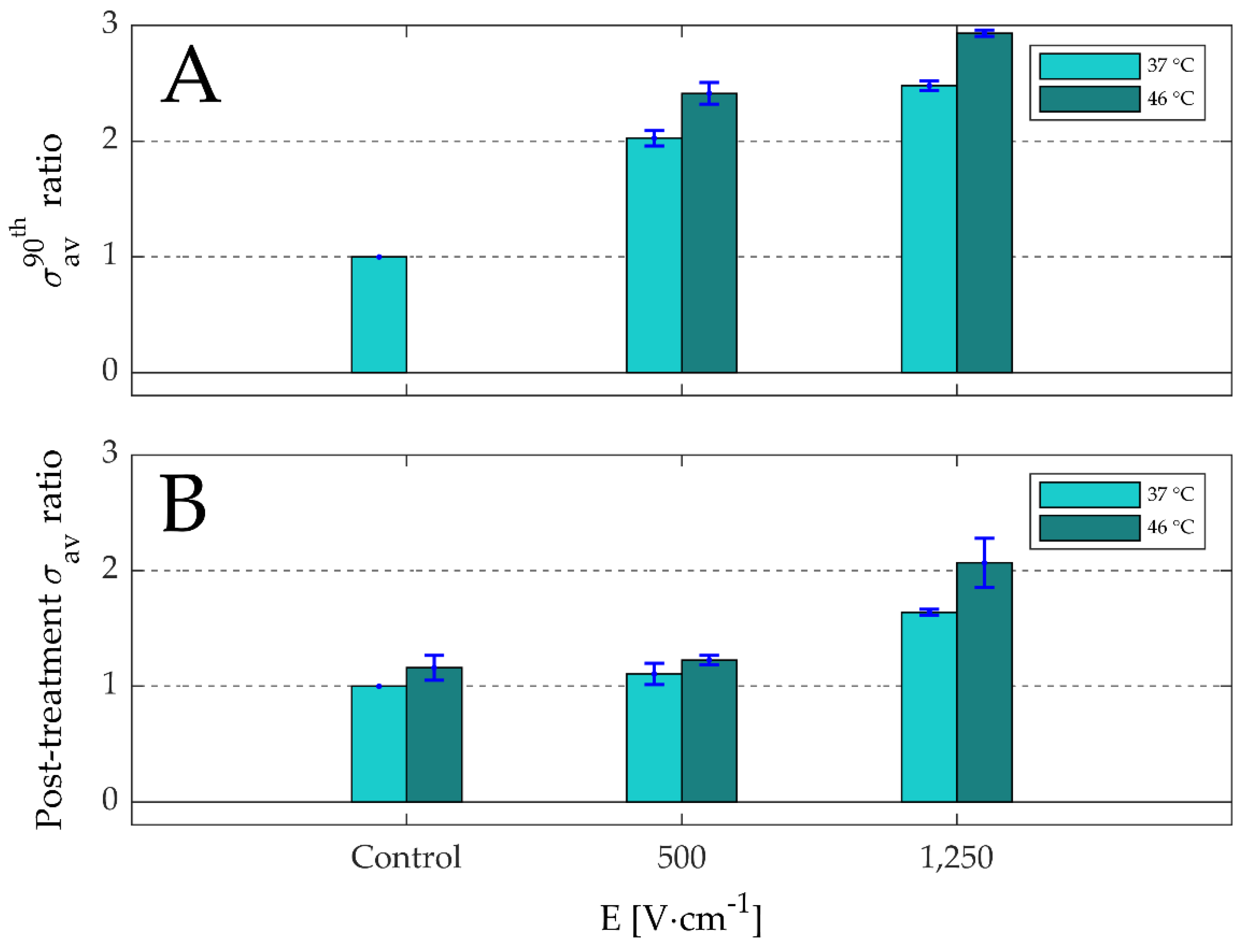
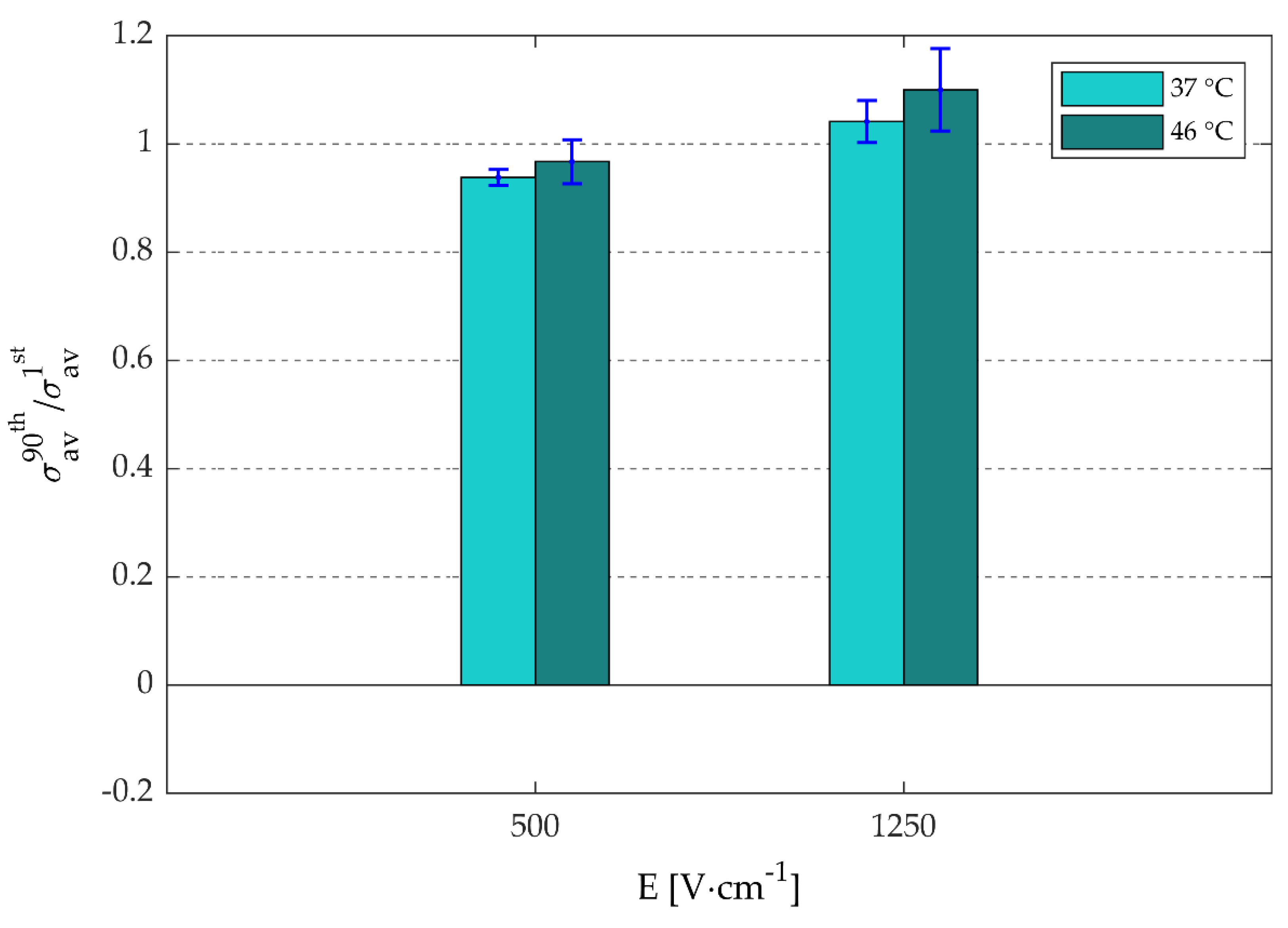
3.2. Effects of Thermally Controlled Irreversible Permeabilization on the Electrical Specific Conductivity and Cell Viability
3.2.1. Electrical Specific Conductivity
- Temperature only:
- In Figure 7B, the σav ratio for the control at 46 °C increased by 1.2-fold with respect to the control at 37 °C.
- Electric-field strength only; during the pulse in the presence of the electric field (focus on Figure 7A):
- In Figure 7A, the σav ratios for 1250 V·cm−1 at 37 °C and 46 °C increased by 2.5 and 2.9-folds, while the σav ratios for 500 V·cm−1 at 37 °C and 46 °C increased by 2.0 and 2.4-folds.
- Electric-field strength only; post-treatment in the absence of the electric field (focus on Figure 7B):
- In Figure 7B, the σav ratios for 1250 V·cm−1 at 37 °C and 46 °C increased by 1.2 and 2.1-folds, while the σav ratios for 500 V·cm−1 at 37 °C and 46 °C increased by 1.1 and 1.6-folds.
- In Figure 7B, the σav ratio for the control at 46 °C increased by 1.2-fold, while in the σav ratios for 500 V·cm−1 and 1250 V·cm−1 at 46 °C increased by 1.6 and 2.1-fold.
- Presence of electric field during the treatment:
- Combined effects of electric-field strength and temperature; during the 90th pulse in the presence of the electric field (focus on Figure 7A):
- In Figure 7A, the σav ratios for 500 V·cm−1 and 1250 V·cm−1 during the 90th pulse at 46 °C increased by 2.4 and 2.9-folds, while the σav ratios for the same pulse parameters at 37 °C increased by 2.0 and 2.5-folds.
- Combined effects of electric-field strength and temperature; post-treatment in the absence of the electric field (focus on Figure 7B):
- In Figure 7B, the σav ratios for 500 V·cm−1 and 1250 V·cm−1 post-treatment at 46 °C increased by 1.2 and 2.1-folds, while the σav ratios for the same pulse parameters at 37 °C increased by 1.1 and 1.6-folds.
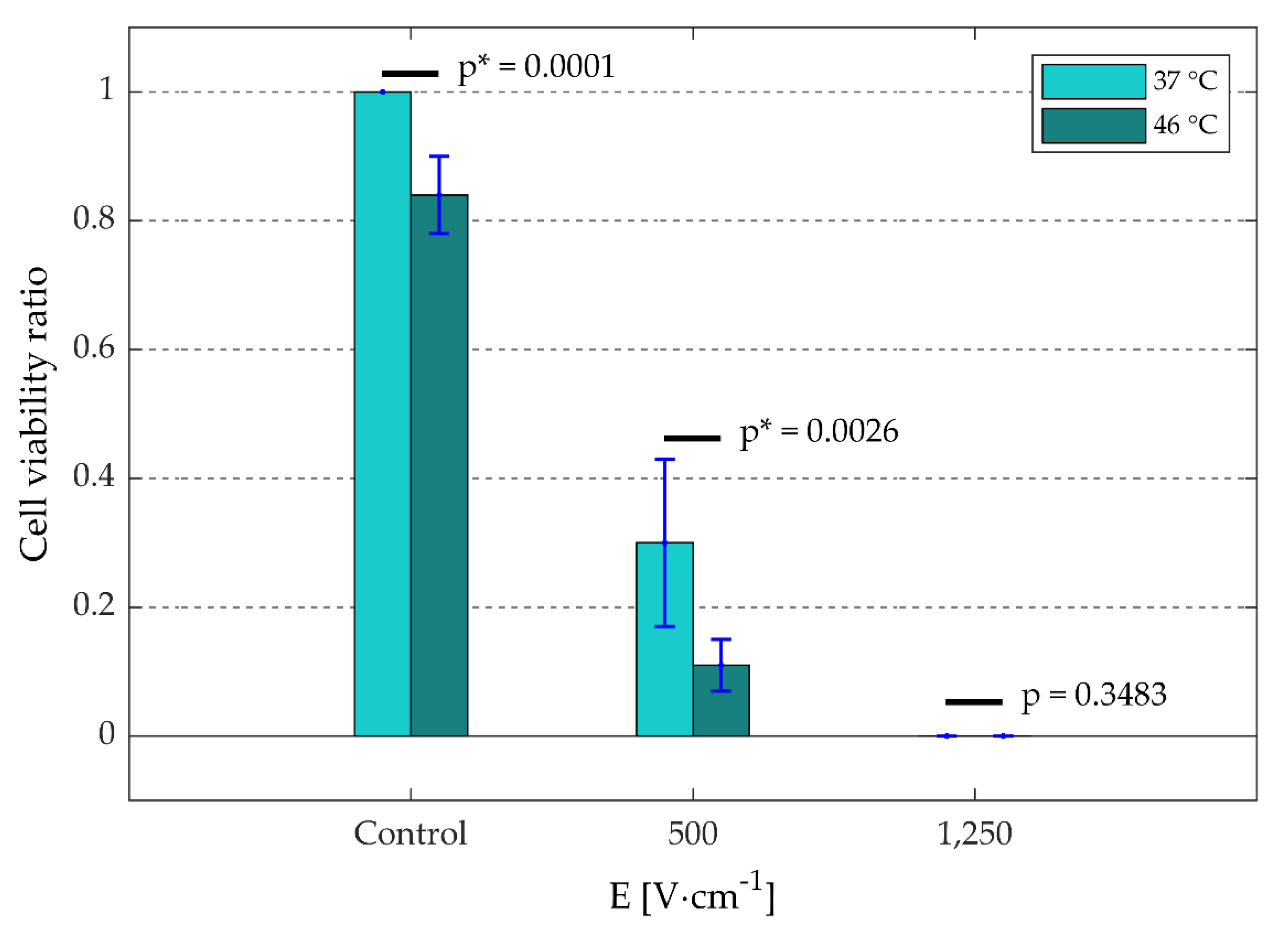
3.2.2. Cell Viability
4. Discussion
4.1. Main Research Objective: HyCHEED Was Validated
4.2. Secondary Research Objective: Irreversible Electroporation Characteristics Are Influenced by Electric-Field Strength and Temperature
4.3. HyCHEED Is Suitable for Investigation of Electroporation Treatment Modalities
4.4. HyCHEED Is Suitable for Investigation of Radio-Frequency Treatments
4.5. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations and Definitions
| Abbreviations and symbols of quantities | Unit | Definition |
| C-container | Container with demineralized water intended to cool off a target volume. | |
| Demi-water | Demineralized water. | |
| IRE | Irreversible electroporation. | |
| IPE | Irreversible permeabilization effect (we chose IPE instead of IRE effect to distinguish between only the permeabilization effect, and the permeabilization and the thermal effects jointly produced by IRE). | |
| H-container | Container with demineralized water intended to heat a target volume. | |
| HF-IRE | High-frequency irreversible electroporation. | |
| HyCHEED | Hydraulically controllable heat exchange electroporation device. | |
| NA | Not applicable. | |
| Post-treatment σav | [S·m−1] | Average electrical specific conductivity of a target volume that is measured after the treatment. |
| T-holder | Target holder. | |
| TV | Target volume. | |
| E | [V·m−1] or [V·cm−1] | Electric-field vector. |
| J | [A·m−2] | Electric-current density. |
| J · E | [W·m−3] | The Joule Heating term; the heat generation rate per unit volume. |
| cp | [J·kg−1·°C−1] | Specific heat capacity of a medium. |
| d | [m] | Distance between electrode plates of an electroporation cuvette. |
| fP | [Hz] | Pulse frequency. |
| HV | [W·m−3·°C−1] | Volumetric heat transfer coefficient between the target volume and its direct ambiance. |
| t | [s] or [min] | Time. |
| t0, t1, …, t5 | [min] | Time points. |
| tinit | [s] | Initial time at which irreversible electroporation treatment starts. |
| tP | [s] | Pulse duration. |
| σ | [S·m−1] | Electrical specific conductivity of a target volume. |
| σav | [S·m−1] | Average electrical specific conductivity of a target volume. |
| A | [m2] | Surface of a target volume that is contacting the electrode plate of an electroporation cuvette. |
| E = |E | | [V·m−1] or [V·cm−1] | Magnitude of an electric-field vector. |
| EIRE(th) | [V·m−1] | Electric-field threshold of irreversible electroporation; minimum electric-field value that ablates target cells/tissue during the irreversible electroporation. |
| I | [A] | Measured electric current. |
| SD | Standard deviation. | |
| T | [°C] | Temperature. |
| Ttarget | [°C] | Target temperature. |
| TA | [°C] | Temperature of the direct ambient of the target volume. |
| Tav | [°C] | Average temperature evolution over time. |
| Φ | [V] | Measured voltage. |
Appendix A—Mathematical Description of Stable Temperature Control in Treated Volume
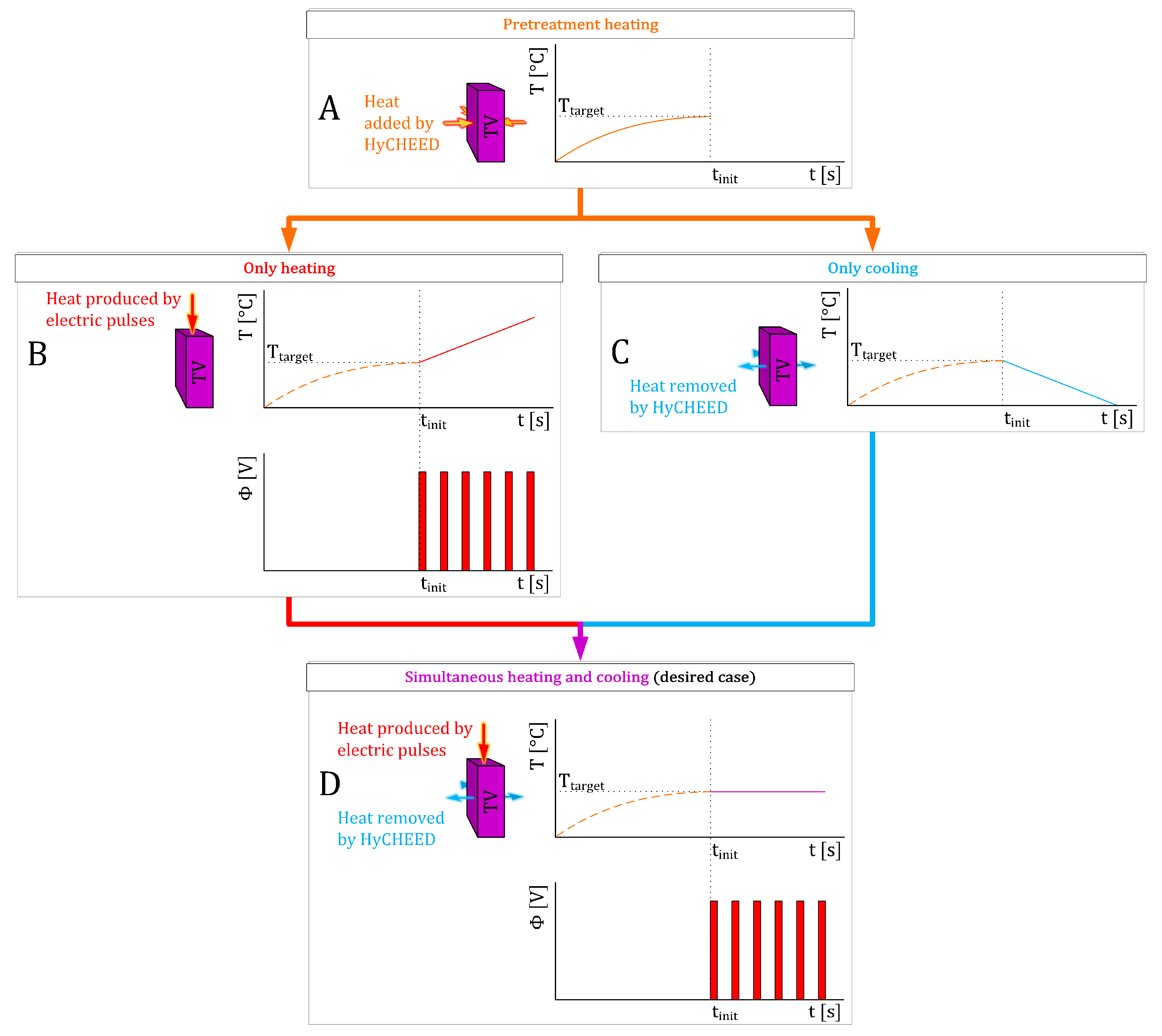
- Pretreatment (E = 0 V·m−1), HyCHEED has to heat the TV up to Ttarget. This implies that at t = 0 s the temperature of the TV is lower than TA, and in time the temperature must reach Ttarget. Therefore, TA = Ttarget. See Figure 1A.
- During the treatment (E > 0 V·m−1), a stable temperature is maintained when the temperature rise caused purely by the electric pulses (Figure 1B) is combined with the temperature decrease caused solely by the cooling effect of term HV · (T − TA) (Figure 1C) to obtain a steady temperature as in Figure 1D. To satisfy this condition, TA must be adjusted at t = tinit such that TA < Ttarget. Using the combination of Equations (A1) and (A3), we can calculate TA by reducing Equation (A1) into
References
- Davalos, R.V.; Mir, L.M.; Rubinsky, B. Tissue ablation with irreversible electroporation. Ann. Biomed. Eng. 2005, 33, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; de Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grüll, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef] [PubMed]
- Neal, R.E.; Garcia, P.A.; Robertson, J.L.; Davalos, R.V. Experimental Characterization and Numerical Modeling of Tissue Electrical Conductivity during Pulsed Electric Fields for Irreversible Electroporation Treatment Planning. IEEE Trans. Biomed. Eng. 2012, 59, 1076–1085. [Google Scholar] [CrossRef]
- Mercadal, B.; Beitel-White, N.; Aycock, K.N.; Castellvi, Q.; Davalos, R.V.; Ivorra, A. Dynamics of Cell Death After Conventional IRE and H-FIRE Treatments. Ann. Biomed. Eng. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.A.; van Veldhuisen, E.; Agnass, P.; Crezee, J.; Dijk, F.; Verheij, J.; van Gunk, T.M.; Meijerink, M.R.; Vroomen, L.G.; van Lienden, K.P.; et al. Time-Dependent Impact of Irreversible Electroporation on Pancreas, Liver, Blood Vessels and Nerves: A Systematic Review of Experimental Studies. PLoS ONE 2016, 11, e0166987. [Google Scholar] [CrossRef] [PubMed]
- Pillai, K.; Akhter, J.; Chua, T.C.; Shehata, M.; Alzahrani, N.; Al-Alem, I.; Morris, D.L. Heat Sink Effect on Tumor Ablation Characteristics as Observed in Monopolar Radiofrequency, Bipolar Radiofrequency, and Microwave, Using Ex Vivo Calf Liver Model. Medicine 2015, 94. [Google Scholar] [CrossRef]
- Faroja, M.; Ahmed, M.; Appelbaum, L.; Ben-David, E.; Moussa, M.; Sosna, J.; Nissenbaum, I.; Goldberg, S.N. Irreversible Electroporation Ablation: Is All the Damage Nonthermal? Radiology 2013, 266, 462–470. [Google Scholar] [CrossRef]
- Dunki-Jacobs, E.M.; Philips, P.; Martin, R.C.G. Evaluation of thermal injury to liver, pancreas and kidney during irreversible electroporation in an in vivo experimental model. Br. J. Surg. 2014, 101, 1113–1121. [Google Scholar] [CrossRef]
- Wagstaff, P.G.K.; de Bruin, D.M.; van den Bos, W.; Ingels, A.; van Gemert, M.J.C.; Zondervan, P.J.; Verdaasdonk, R.M.; van Lienden, K.P.; van Leeuwen, T.G.; de la Rosette, J.; et al. Irreversible electroporation of the porcine kidney: Temperature development and distribution. Urol. Oncol. Semin. Orig. Investig. 2015, 33. [Google Scholar] [CrossRef]
- Agnass, P.; van Veldhuisen, E.; Vogel, J.A.; Kok, H.P.; de Keijzer, M.J.; Schooneveldt, G.; de Haan, L.R.; Klaessens, J.H.; Scheffer, H.J.; Meijerink, M.R.; et al. Thermodynamic profiling during irreversible electroporation in porcine liver and pancreas: A case study series. J. Clin. Transl. Res. 2020, 5, 109–132. [Google Scholar] [CrossRef]
- Bakker, A.; van der Zee, J.; van Tienhoven, G.; Kok, H.P.; Rasch, C.R.N.; Crezee, H. Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: A systematic review. Int. J. Hyperth. 2019, 36, 1024–1039. [Google Scholar] [CrossRef] [PubMed]
- Kroesen, M.; Mulder, H.T.; van Holthe, J.M.L.; Aangeenbrug, A.A.; Mens, J.W.M.; van Doorn, H.C.; Paulides, M.M.; Oomen-de Hoop, E.; Vernhout, R.M.; Lutgens, L.C.; et al. Confirmation of thermal dose as a predictor of local control in cervical carcinoma patients treated with state-of-the-art radiation therapy and hyperthermia. Radiother. Oncol. 2019, 140, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Helderman, R.F.C.P.A.; Loke, D.R.; Kok, H.P.; Oei, A.L.; Tanis, P.J.; Franken, N.A.P.K.; Crezee, J. Variation in Clinical Application of Hyperthermic Intraperitoneal Chemotherapy: A Review. Cancers 2019, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.; Kok, H.P.; Oei, S.B.; Horsman, M.R.; Stalpers, L.J.A.; Franken, N.A.P.; Crezee, J. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol./Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Bull, J.M.C. A review of immune therapy in cancer and a question: Can thermal therapy increase tumor response? Int. J. Hyperth. 2018, 34, 840–852. [Google Scholar] [CrossRef]
- Gerweck, L.E. Hyperthermia in Cancer Therapy: The Biological Basis and Unresolved Questions. Cancer Res. 1985, 45, 3408–3414. [Google Scholar]
- Horsman, M.R. Tissue physiology and the response to heat. Int. J. Hyperth. 2006, 22, 197–203. [Google Scholar] [CrossRef]
- Agnass, P.; van Veldhuisen, E.; van Gemert, M.J.C.; van der Geld, C.W.M.; van Lienden, K.P.; van Gulik, T.M.; Meijerink, M.R.; Besselink, M.G.; Kok, H.P.; Crezee, J. Mathematical modeling of the thermal effects of irreversible electroporation for in vitro, in vivo, and clinical use: A systematic review. Int. J. Hyperth. 2020, 37, 486–505. [Google Scholar] [CrossRef]
- Dewhirst, M.W.; Viglianti, B.L.; Lora-Michiels, M.; Hanson, M.; Hoopes, P.J. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. Hyperth. 2003, 19, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.T.; Hines, J.R.; Gordon, D. Intracellular hyperthermia: A biophysical approach to cancer treatment via intracellular temperature and biophysical alterations. Med. Hypotheses 1979, 5, 83–102. [Google Scholar] [CrossRef]
- Ivkov, R. Magnetic nanoparticle hyperthermia: A new frontier in biology and medicine? Int. J. Hyperth. 2013, 29, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Raaijmakers, E.A.L.; Mestrom, R.M.C.; Sumser, K.; Salim, G.; Rhoon, G.C.; Essers, J.; Paulides, M.M. An MR-compatible antenna and application in a murine superficial hyperthermia applicator. Int. J. Hyperth. 2018, 34, 697–703. [Google Scholar] [CrossRef]
- Crezee, J.; Tienhoven, G.v.; Kolff, M.W.; Sijbrands, J.; Stam, G.v.; Oldenborg, S.; Geysen, E.D.; Hulshof, M.C.C.M.; Kok, H.P. Development of a 70 MHz unit for hyperthermia treatment of deep seated breast tumors. In Proceedings of the 2016 46th European Microwave Conference (EuMC), London, UK, 4–6 October 2016; pp. 906–909. [Google Scholar]
- Zhu, L.F.; Altman, M.B.; Laszlo, A.; Straube, W.; Zoberi, I.; Hallahan, D.E.; Chen, H. Ultrasound Hyperthermia Technology for Radiosensitization. Ultrasound Med. Biol. 2019, 45, 1025–1043. [Google Scholar] [CrossRef] [PubMed]
- Edelblute, C.M.; Hornef, J.; Burcus, N.I.; Norman, T.; Beebe, S.J.; Schoenbach, K.; Heller, R.; Jiang, C.Q.; Guo, S.Q. Controllable Moderate Heating Enhances the Therapeutic Efficacy of Irreversible Electroporation for Pancreatic Cancer. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.J.; Ding, L.J.; Moser, M.A.J.; Zhang, E.M.; Zhang, W.J. Tumor Ablation Enhancement by Combining Radiofrequency Ablation and Irreversible Electroporation: An In Vitro 3D Tumor Study. Ann. Biomed. Eng. 2019, 47, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.A.; Rombouts, S.J.; de Rooij, T.; van Delden, O.M.; Dijkgraaf, M.G.; van Gulik, T.M.; van Hooft, J.E.; van Laarhoven, H.W.; Martin, R.C.; Schoorlemmer, A.; et al. Induction Chemotherapy Followed by Resection or Irreversible Electroporation in Locally Advanced Pancreatic Cancer (IMPALA): A Prospective Cohort Study. Ann. Surg. Oncol. 2017, 24, 2734–2743. [Google Scholar] [CrossRef]
- Timmer, F.E.F.; Geboers, B.; Ruarus, A.H.; Schouten, E.A.C.; Nieuwenhuizen, S.; Puijk, R.S.; de Vries, J.J.J.; Meijerink, M.R.; Scheffer, H.J. Irreversible Electroporation for Locally Advanced Pancreatic Cancer. Tech. Vasc. Interv. Radiol. 2020, 23, 443–449. [Google Scholar] [CrossRef]
- Ruarus, A.H.; Vroomen, L.; Geboers, B.; van Veldhuisen, E.; Puijk, R.S.; Nieuwenhuizen, S.; Besselink, M.G.; Zonderhuis, B.M.; Kazemier, G.; de Gruijl, T.D.; et al. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology 2020, 294, 212–220. [Google Scholar] [CrossRef]
- Garcia, P.A.; Arena, C.B.; Davalos, R.V. Towards a predictive model of electroporation-based therapies using pre-pulse electrical measurements. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; Volume 2012, pp. 2575–2578. [Google Scholar] [CrossRef]
- Xu, M.L.; McCanna, D.J.; Sivak, J.G. Use of the viability reagent PrestoBlue in comparison with alamarBlue and MTT to assess the viability of human corneal epithelial cells. J. Pharmacol. Toxicol. Methods 2015, 71, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Napotnik, T.B.; Miklavcic, D. In vitro electroporation detection methods—An overview. Bioelectrochemistry 2018, 120, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, F.; Tesei, A.; Arienti, C.; Bevilacqua, A. Cell Counting and Viability Assessment of 2D and 3D Cell Cultures: Expected Reliability of the Trypan Blue Assay. Biol. Proced. Online 2017, 19. [Google Scholar] [CrossRef]
- Chang, A.-Y.; Liu, X.; Tian, H.; Hua, L.; Yang, Z.; Wang, S. Microfluidic Electroporation Coupling Pulses of Nanoseconds and Milliseconds to Facilitate Rapid Uptake and Enhanced Expression of DNA in Cell Therapy. Sci. Rep. 2020, 10, 6061. [Google Scholar] [CrossRef]
- Sel, D.; Cukjati, D.; Batiuskaite, D.; Slivnik, T.; Mir, L.M.; Miklavcic, D. Sequential finite element model of tissue electropermeabilization. IEEE Trans. Biomed. Eng. 2005, 52, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Rems, L.; Miklavcic, D. Tutorial: Electroporation of cells in complex materials and tissue. J. Appl. Phys. 2016, 119. [Google Scholar] [CrossRef]
- Ivorra, A.; Rubinsky, B. In vivo electrical impedance measurements during and after electroporation of rat liver. Bioelectrochemistry 2007, 70, 287–295. [Google Scholar] [CrossRef]
- Ivorra, A.; Al-Sakere, B.; Rubinsky, B.; Mir, L.M. In vivo electrical conductivity measurements during and after tumor electroporation: Conductivity changes reflect the treatment outcome. Phys. Med. Biol. 2009, 54, 5949–5963. [Google Scholar] [CrossRef]
- Langus, J.; Kranjc, M.; Kos, B.; Sustar, T.; Miklavcic, D. Dynamic finite-element model for efficient modelling of electric currents in electroporated tissue. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Hasgall, P.A.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Lloyd, B.; Gosselin, M.C.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues. Available online: https://itis.swiss/virtual-population/tissue-properties/database/database-summary/ (accessed on 15 May 2018).
- Paulides, M.M.; Vossen, S.; Zwamborn, A.P.M.; Van Rhoon, G.C. Theoretical investigation into the feasibility to deposit RF energy centrally in the head-and-neck region. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 634–642. [Google Scholar] [CrossRef]
- Kos, B.; Voigt, P.; Miklavcic, D.; Moche, M. Careful treatment planning enables safe ablation of liver tumors adjacent to major blood vessels by percutaneous irreversible electroporation (IRE). Radiol. Oncol. 2015, 49, 234–241. [Google Scholar] [CrossRef]
- Zupanic, A.; Kos, B.; Miklavcic, D. Treatment planning of electroporation-based medical interventions: Electrochemotherapy, gene electrotransfer and irreversible electroporation. Phys. Med. Biol. 2012, 57, 5425–5440. [Google Scholar] [CrossRef]
- Van der Zee, J.; Gonzalez, D.G.; van Rhoon, G.C.; van Dijk, J.D.P.; van Putten, W.L.J.; Hart, A.A.M. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- Cho, C.H.; Wust, P.; Hildebrandt, B.; Issels, R.D.; Sehouli, J.; Kerner, T.; Deja, M.; Budach, V.; Gellermann, J. Regional hyperthermia of the abdomen in conjunction with chemotherapy for peritoneal carcinomatosis: Evaluation of two annular-phased-array applicators. Int. J. Hyperth. 2008, 24, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, S.A. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, E990–E1005. [Google Scholar] [CrossRef]
- Ware, M.J.; Tinger, S.; Colbert, K.L.; Corr, S.J.; Rees, P.; Koshkina, N.; Curley, S.; Summers, H.D.; Godin, B. Radiofrequency treatment alters cancer cell phenotype. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Curley, S.A.; Palalon, F.; Lu, X.L.; Koshkina, N.V. Noninvasive Radiofrequency Treatment Effect on Mitochondria in Pancreatic Cancer Cells. Cancer 2014, 120, 3418–3425. [Google Scholar] [CrossRef]
- Wust, P.; Kortum, B.; Strauss, U.; Nadobny, J.; Zschaeck, S.; Beck, M.; Stein, U.; Ghadjar, P. Non-thermal effects of radiofrequency electromagnetic fields. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]


| Ttarget [°C] | Temperature of H-Container [°C] | Temperature of C-Container [°C] |
|---|---|---|
| 37 | 37.5 | 32.4 |
| 46 | 46.1 | 44.2 |
| Ttarget [°C] | E [V·cm−1] | |
|---|---|---|
| 500 | 1250 | |
| 37 | 36.75 ± 0.58 | 36.86 ± 0.34 |
| 46 | 46.44 ± 0.82 | 46.66 ± 0.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnass, P.; Rodermond, H.M.; Zweije, R.; Sijbrands, J.; Vogel, J.A.; van Lienden, K.P.; van Gulik, T.M.; van Veldhuisen, E.; Franken, N.A.P.; Oei, A.L.; et al. HyCHEED System for Maintaining Stable Temperature Control during Preclinical Irreversible Electroporation Experiments at Clinically Relevant Temperature and Pulse Settings. Sensors 2020, 20, 6227. https://doi.org/10.3390/s20216227
Agnass P, Rodermond HM, Zweije R, Sijbrands J, Vogel JA, van Lienden KP, van Gulik TM, van Veldhuisen E, Franken NAP, Oei AL, et al. HyCHEED System for Maintaining Stable Temperature Control during Preclinical Irreversible Electroporation Experiments at Clinically Relevant Temperature and Pulse Settings. Sensors. 2020; 20(21):6227. https://doi.org/10.3390/s20216227
Chicago/Turabian StyleAgnass, Pierre, Hans M. Rodermond, Remko Zweije, Jan Sijbrands, Jantien A. Vogel, Krijn P. van Lienden, Thomas M. van Gulik, Eran van Veldhuisen, Nicolaas A. P. Franken, Arlene L. Oei, and et al. 2020. "HyCHEED System for Maintaining Stable Temperature Control during Preclinical Irreversible Electroporation Experiments at Clinically Relevant Temperature and Pulse Settings" Sensors 20, no. 21: 6227. https://doi.org/10.3390/s20216227
APA StyleAgnass, P., Rodermond, H. M., Zweije, R., Sijbrands, J., Vogel, J. A., van Lienden, K. P., van Gulik, T. M., van Veldhuisen, E., Franken, N. A. P., Oei, A. L., Kok, H. P., Besselink, M. G., & Crezee, J. (2020). HyCHEED System for Maintaining Stable Temperature Control during Preclinical Irreversible Electroporation Experiments at Clinically Relevant Temperature and Pulse Settings. Sensors, 20(21), 6227. https://doi.org/10.3390/s20216227








