3D-Printed Bubble-Free Perfusion Cartridge System for Live-Cell Imaging
Abstract
:1. Introduction
2. Materials and Methods
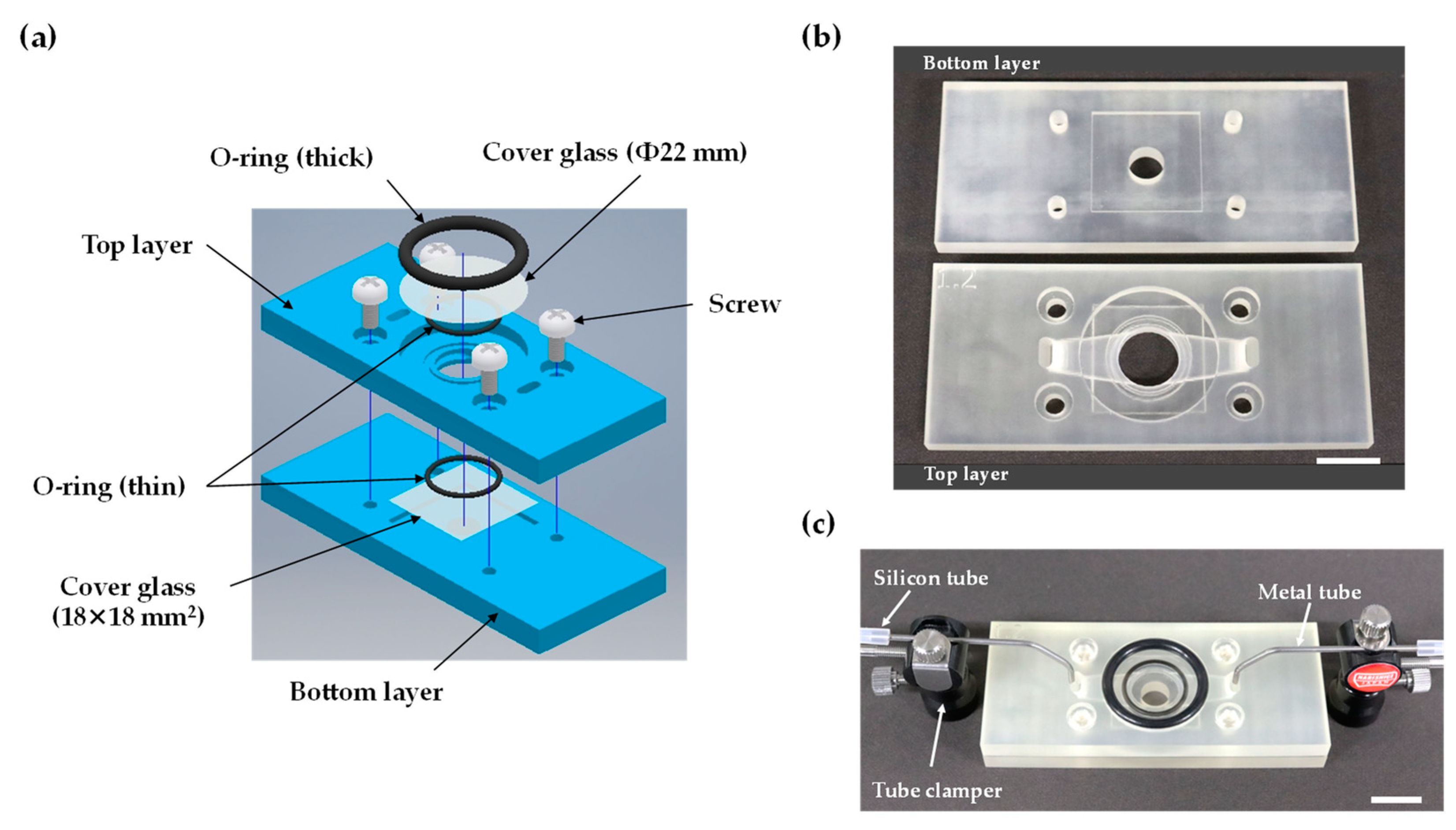
2.1. Cartridge Design and Fabrication
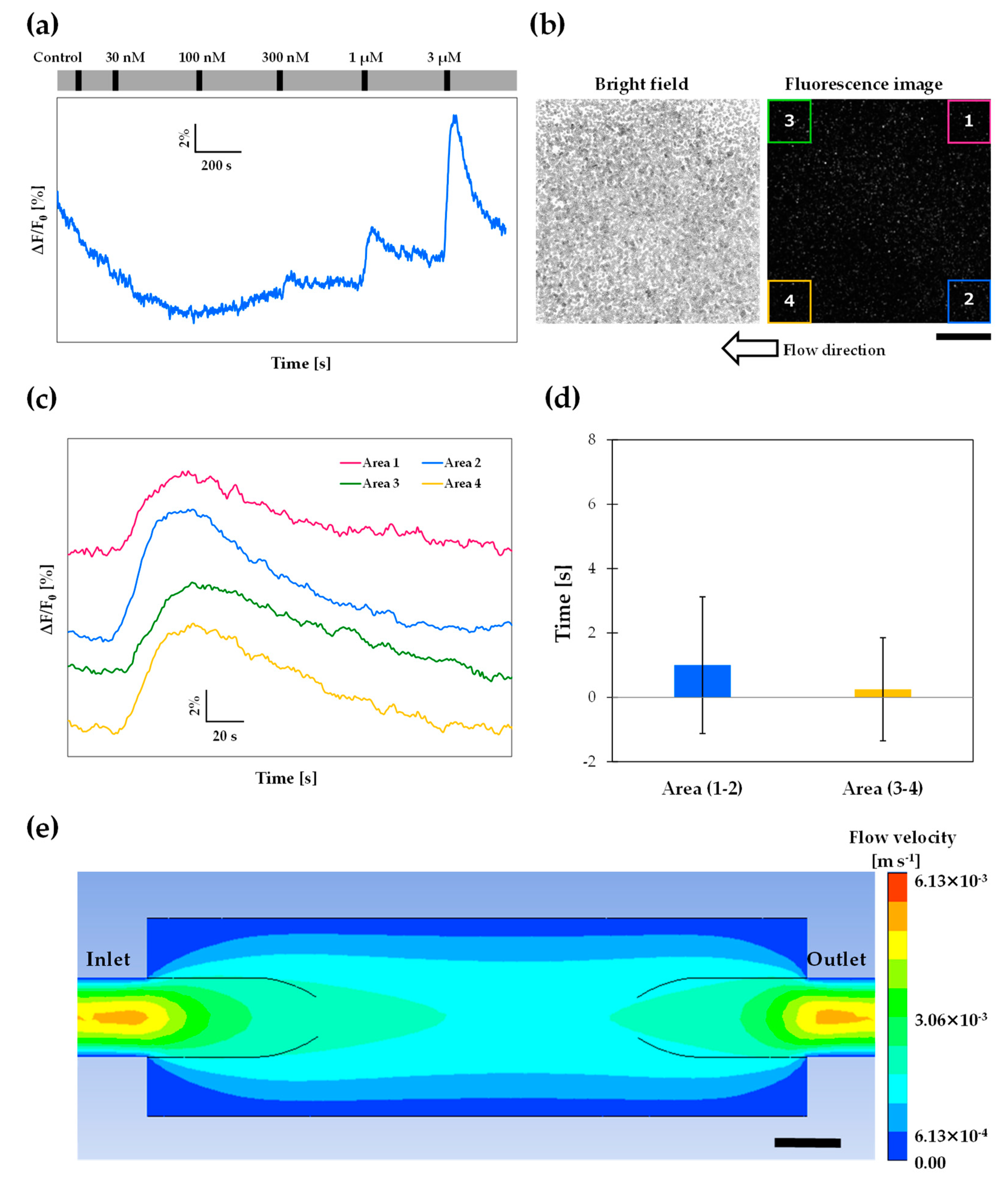
2.2. Computational Fluid Dynamics (CFD) Simulation
2.3. Cells and Odorants
2.4. Device Assembly and Cell Seeding
2.5. Calcium Imaging
3. Results
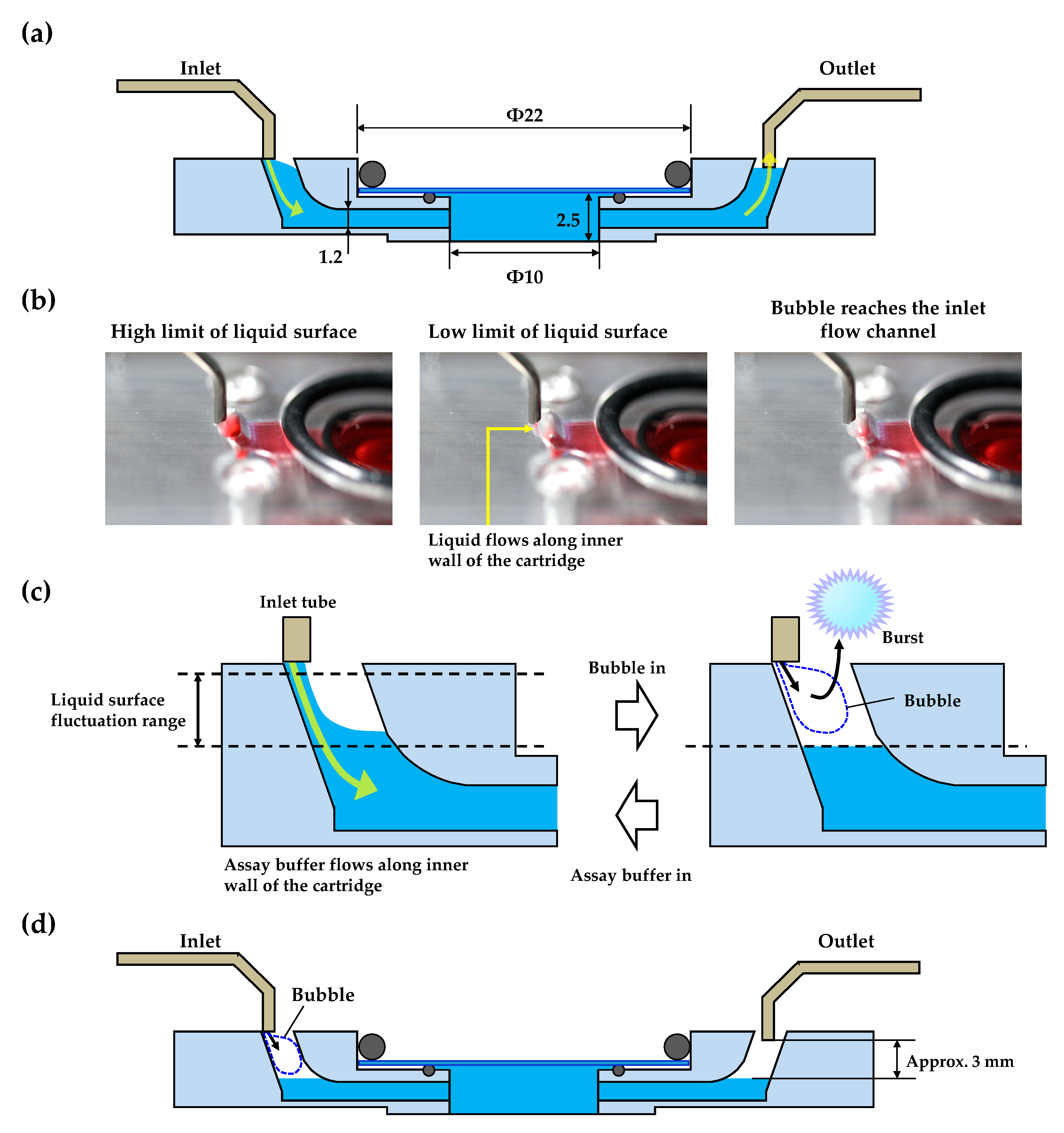
3.1. Design and Operation of the 3D-Printed Perfusion Cartridges
3.2. Bubble Removal Ability of the 3D-Printed Perfusion Cartridge
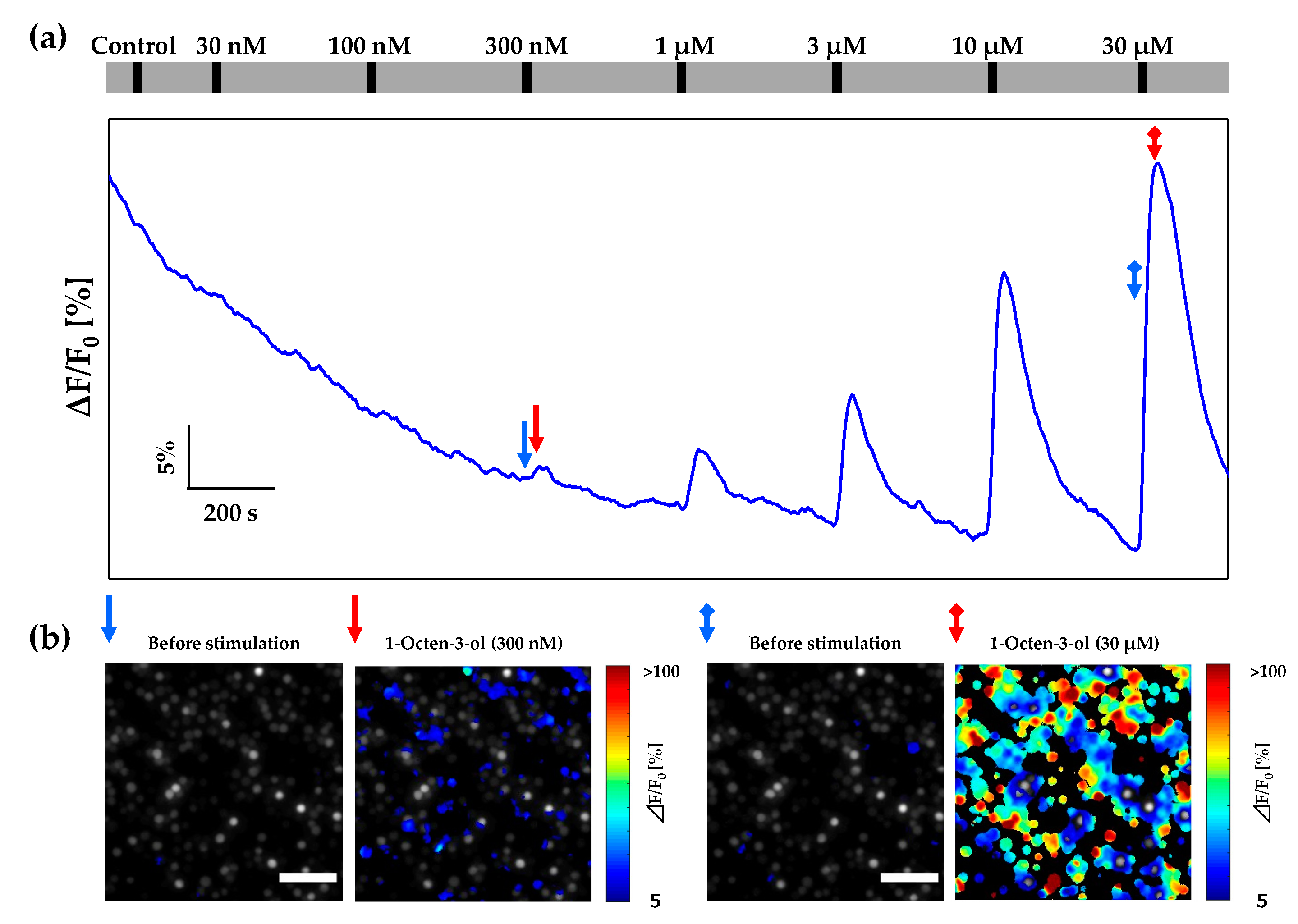
3.3. Calcium Imaging of Or13a Cells using the Designed Cartridge
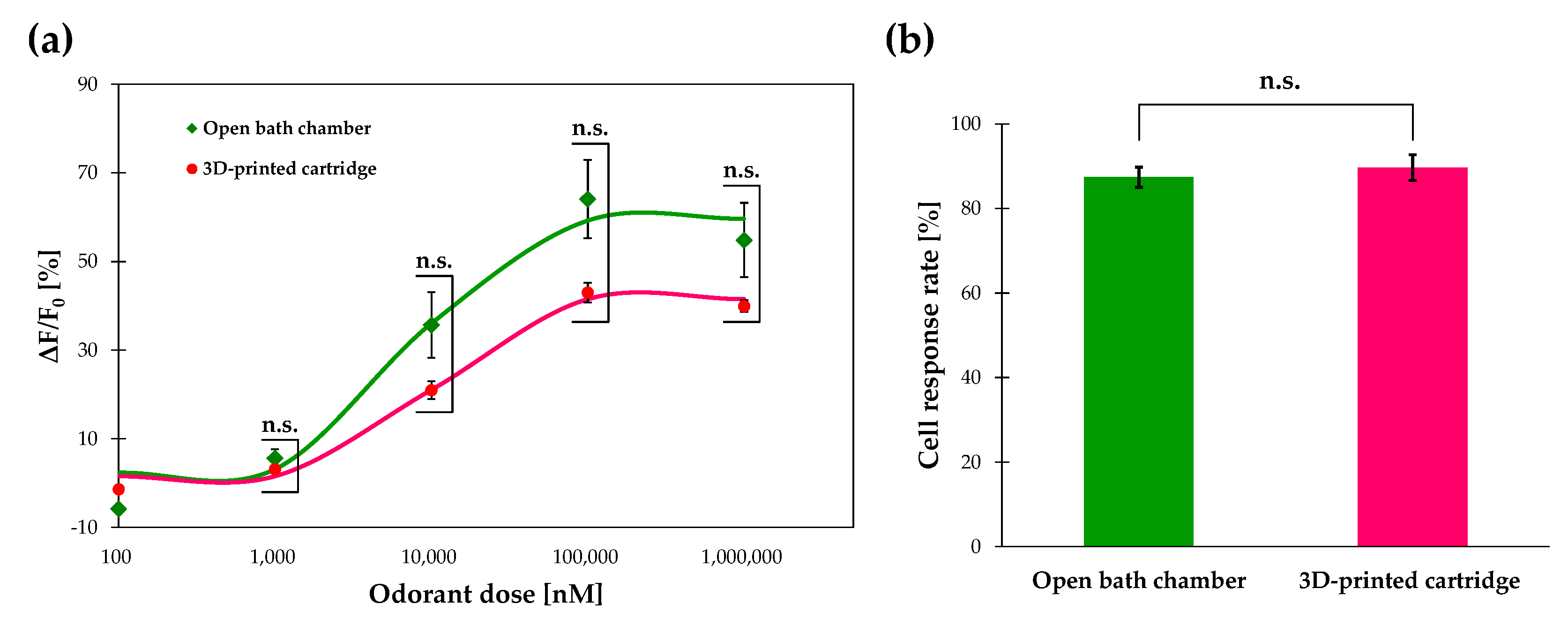
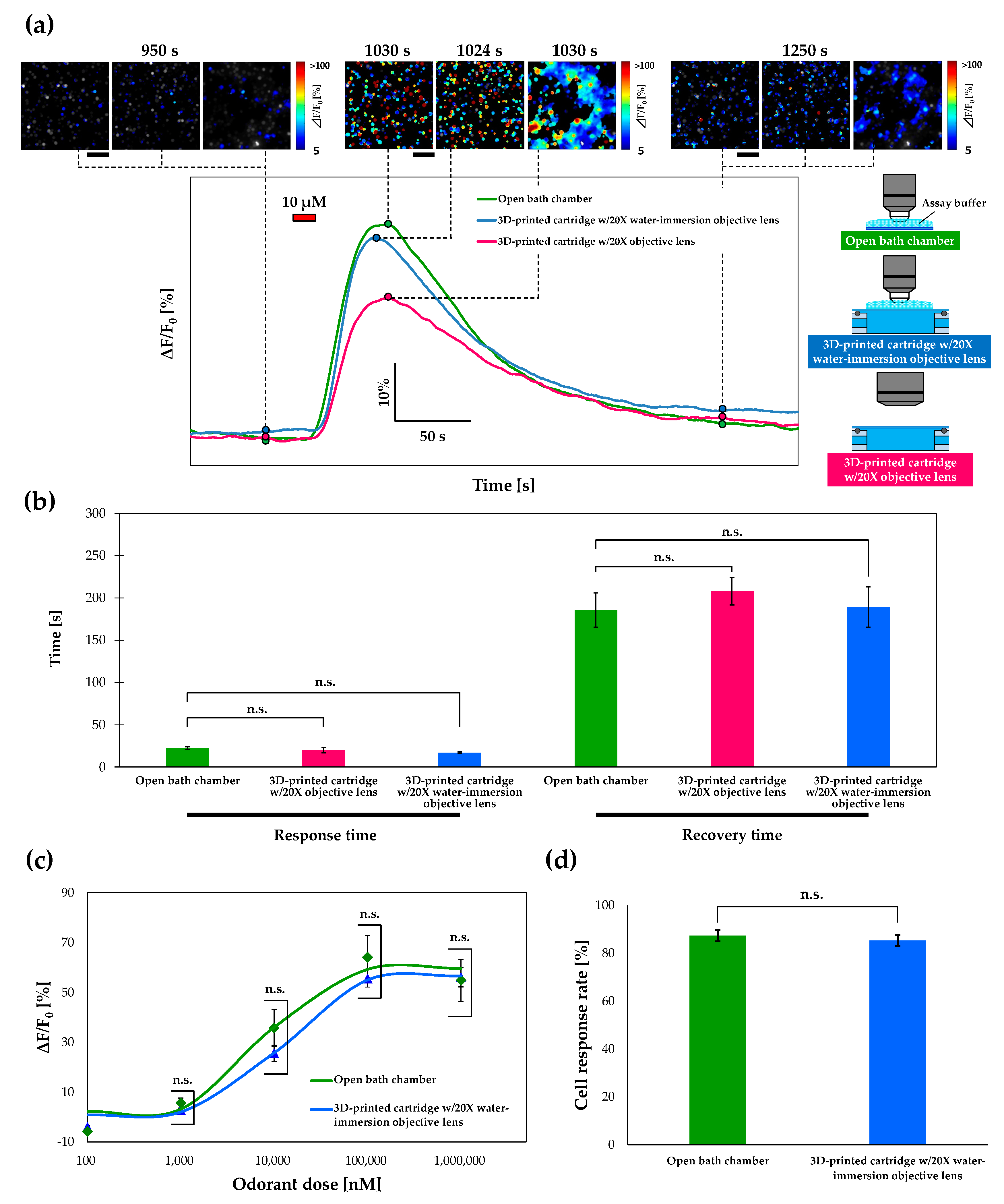
3.4. Calcium Imaging of Or13a Cells using a Commercial Open Chamber
3.5. Response Time and Recovery Time
3.6. Wide-Area Observation
3.7. 3D-Printed Cartridge as a Chemical Reactor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Munaz, A.; Vadivelu, R.K.; St. John, J.; Barton, M.; Kamble, H.; Nguyen, N.-T. Three-dimensional printing of biological matters. J. Sci. Adv. Mater. Devices 2016, 1, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A review of 3D printing technology for medical applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Frigault, M.M.; Lacoste, J.; Swift, J.L.; Brown, C.M. Live-cell microscopy—Tips and tools. J. Cell Sci. 2009, 122, 753–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ettinger, A.; Wittmann, T. Fluorescence live cell imaging. Methods Cell Biol. 2014, 123, 77–94. [Google Scholar] [PubMed] [Green Version]
- Mitsuno, H.; Sakurai, T.; Namiki, S.; Mitsuhashi, H.; Kanzaki, R. Novel cell-based odorant sensor elements based on insect odorant receptors. Biosens. Bioelectron. 2015, 65, 287–294. [Google Scholar] [CrossRef]
- Termtanasombat, M.; Mitsuno, H.; Misawa, N.; Yamahira, S.; Sakurai, T.; Yamaguchi, S.; Nagamune, T.; Kanzaki, R. Cell-based odorant sensor array for odor discrimination based on insect odorant receptors. J. Chem. Ecol. 2016, 42, 716–724. [Google Scholar] [CrossRef]
- Terutsuki, D.; Mitsuno, H.; Sakurai, T.; Okamoto, Y.; Tixier-Mita, A.; Toshiyoshi, H.; Mita, Y.; Kanzaki, R. Increasing cell-device adherence using cultured insect cells for receptor-based biosensors. R. Soc. Open Sci. 2018, 5, 172366. [Google Scholar] [CrossRef] [Green Version]
- Betzenhauser, M.J.; Won, J.H.; Park, H.; Yule, D.I. Measurement of intracellular calcium concentration in pancreatic acini. Pancreapedia 2011. [Google Scholar] [CrossRef]
- Stucki, J.D.; Guenat, O.T. A microfluidic bubble trap and oscillator. Lab Chip 2015, 15, 4393–4397. [Google Scholar] [CrossRef] [Green Version]
- Pappas, D. Practical Cell Analysis; John Wiley & Sons, Ltd: Chichester, UK, 2010. [Google Scholar] [CrossRef]
- Sung, J.H.; Shuler, M.L. Prevention of air bubble formation in a microfluidic perfusion cell culture system using a microscale bubble trap. Biomed. Microdevices 2009, 11, 731–738. [Google Scholar] [CrossRef]
- Skelley, A.M.; Voldman, J. An active bubble trap and debubbler for microfluidic systems. Lab Chip 2008, 8, 1733–1737. [Google Scholar] [CrossRef]
- Lochovsky, C.; Yasotharan, S.; Günther, A. Bubbles no more: In-plane trapping and removal of bubbles in microfluidic devices. Lab Chip 2012, 12, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Geng, Y.; Yuan, Z.; Ren, S.; Liu, M.; Meng, Z.; Pan, D. A bubble-free microfluidic device for easy-to-operate immobilization, culturing and monitoring of zebrafish embryos. Micromachines 2019, 10, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, F.; Deng, R.; Tong, W.H.; Huan, L.; Way, N.C.; IslamBadhan, A.; Iliescu, C.; Yu, H. A perfusion incubator liver chip for 3D cell culture with application on chronic hepatotoxicity testing. Sci. Rep. 2017, 7, 14528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lee, D.; Zhang, L.; Jeon, H.; Mendoza-Elias, J.E.; Harvat, T.A.; Hassan, S.Z.; Zhou, A.; Eddington, D.T.; Oberholzer, J. Systematic prevention of bubble formation and accumulation for long-term culture of pancreatic islet cells in microfluidic device. Biomed. Microdevices 2012, 14, 419–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Thompson, J.A.; Bau, H.H. A membrane-based, high-efficiency, microfluidic debubbler. Lab Chip 2011, 11, 1688–1693. [Google Scholar] [CrossRef]
- Friend, J.; Yeo, L. Fabrication of microfluidic devices using polydimethylsiloxane. Biomicrofluidics 2010, 4, 026502. [Google Scholar] [CrossRef] [Green Version]
- Babic, J.; Griscom, L.; Cramer, J.; Coudreuse, D. An easy-to-build and re-usable microfluidic system for live-cell imaging. BMC Cell Biol. 2018, 19, 8. [Google Scholar] [CrossRef]
- Amin, R.; Knowlton, S.; Hart, A.; Yenilmez, B.; Ghaderinezhad, F.; Katebifar, S.; Messina, M.; Khademhosseini, A.; Tasoglu, S. 3D-printed microfluidic devices. Biofabrication 2016, 8, 022001. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D printed microfluidic devices: Enablers and barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [Green Version]
- Kitson, P.J.; Rosnes, M.H.; Sans, V.; Dragone, V.; Cronin, L. Configurable 3D-printed millifluidic and microfluidic ‘lab on a chip’ reactionware devices. Lab Chip 2012, 12, 3267–3271. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, S.; Jaffery, H.; Doran, D.; Hezwani, M.; Robbins, P.J.; Yoshida, M.; Cronin, L. Customizable 3D printed ‘plug and play’ millifluidic devices for programmable fluidics. PLoS ONE 2015, 10, e0141640. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sanampudi, A.; Raji Reddy, V.; Biswas, S.; Nandakumar, K.; Yemane, D.; Goettert, J.; Kumar, C.S. Size evolution of gold nanoparticles in a millifluidic reactor. Chem. Phys. Chem. 2012, 13, 177–182. [Google Scholar] [CrossRef]
- Au, A.K.; Huynh, W.; Horowitz, L.F.; Folch, A. 3D-printed microfluidics. Angew. Chem. Int. Ed. 2016, 55, 3862–3881. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.J.; Nordin, G.P.; Woolley, A.T. Moving from millifluidic to truly Microfluidic sub-100-μm cross-section 3D printed devices. Anal. Bioanal. Chem. 2017, 409, 4311–4319. [Google Scholar] [CrossRef]
- Bhattacharjee, N.; Urrios, A.; Kang, S.; Folch, A. The upcoming 3D-printing revolution in microfluidics. Lab Chip 2016, 16, 1720–1742. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Arai, F. Circulation type blood vessel simulator made by microfabrication. In Advanced Applications of Rapid Prototyping Technology in Modern Engineering; Hoque, M.E., Ed.; IntechOpen: London, UK, 2011; pp. 41–60. [Google Scholar]
- Lynn, D.E. Methods for maintaining insect cell cultures. J. Insect Sci. 2002, 2, 9. [Google Scholar]
- Atherton, T.J.; Kerbyson, D.J. Size invariant circle detection. Image Vis. Comput. 1999, 17, 795–803. [Google Scholar] [CrossRef]
- Yuen, H.K.; Princen, J.; Illingworth, J.; Kittler, J. Comparative study of Hough Transform methods for circle finding. Image Vis. Comput. 1990, 8, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Ong, L.J.Y.; Islam, A.; DasGupta, R.; Iyer, N.G.; Leo, H.L.; Toh, Y.C. A 3D printed microfluidic perfusion device for multicellular spheroid cultures. Biofabrication 2017, 9, 045005. [Google Scholar] [CrossRef]
- Jacob, S.W.; de la Torre, J.C. Pharmacology of dimethyl sulfoxide in cardiac and CNS damage. Pharmacol. Rep. 2009, 61, 225–235. [Google Scholar] [CrossRef]
- Yuan, C.; Gao, J.; Guo, J.; Bai, L.; Marshall, C.; Cai, Z.; Wang, L.; Xiao, M. Dimethyl sulfoxide damages mitochondrial integrity and membrane potential in cultured astrocytes. PLoS ONE 2014, 9, e107447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terutsuki, D.; Mitsuno, H.; Sakurai, T.; Okamoto, Y.; Tixier-Mita, A.; Toshiyoshi, H.; Mita, Y.; Kanzaki, R. Odor-sensitive field effect transistor (OSFET) based on insect cells expressing insect odorant receptors. Proc. IEEE MEMS 2017, 394, 7. [Google Scholar]
- Nagata, S.; Kameshiro, N.; Terutsuki, D.; Mitsuno, H.; Sakurai, T.; Niitsu, K.; Nakazato, K.; Kanzaki, R.; Ando, M. A sensor array chip for parallel electrical detection of odorant response of cells expressing odorant receptors. Proc. IEEE MEMS 2018, 282, 5. [Google Scholar]
- Brattoli, M.; de Gennaro, G.; de Pinto, V.; Loiotile, A.D.; Lovascio, S.; Penza, M. Odour detection methods: Olfactometry and chemical sensors. Sensors 2011, 11, 5290–5322. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Jiang, X. Why microfluidics? Merits and trends in chemical synthesis. Lab Chip 2017, 17, 3960–3978. [Google Scholar] [CrossRef]
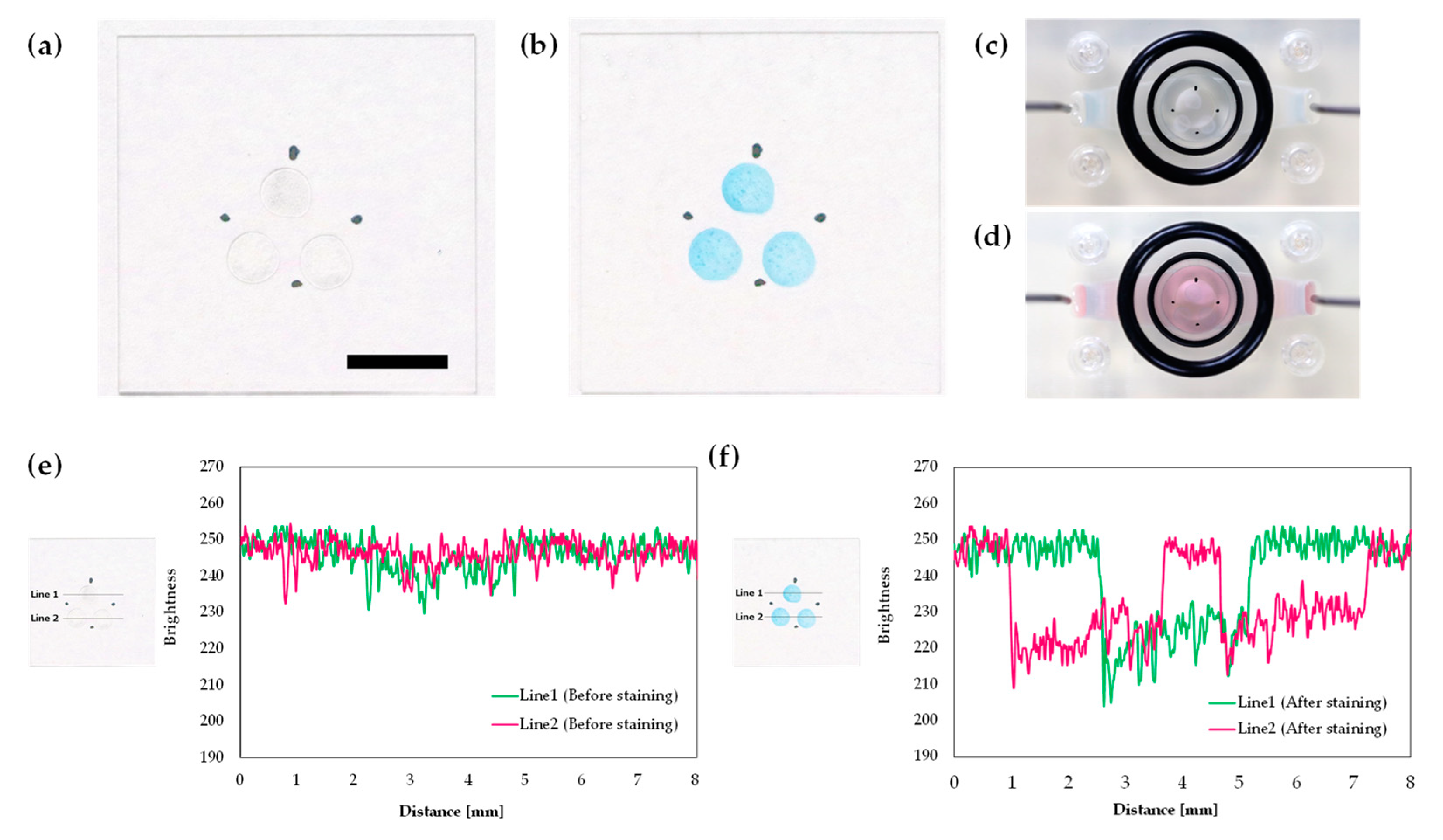
| Bubble Volume [µL] | Number of Bubbles | Removing Results |
|---|---|---|
| 0.39–0.79 | Over 100 | Removed |
| 25 | 30 | Removed |
| 250 | 5 | Removed |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terutsuki, D.; Mitsuno, H.; Kanzaki, R. 3D-Printed Bubble-Free Perfusion Cartridge System for Live-Cell Imaging. Sensors 2020, 20, 5779. https://doi.org/10.3390/s20205779
Terutsuki D, Mitsuno H, Kanzaki R. 3D-Printed Bubble-Free Perfusion Cartridge System for Live-Cell Imaging. Sensors. 2020; 20(20):5779. https://doi.org/10.3390/s20205779
Chicago/Turabian StyleTerutsuki, Daigo, Hidefumi Mitsuno, and Ryohei Kanzaki. 2020. "3D-Printed Bubble-Free Perfusion Cartridge System for Live-Cell Imaging" Sensors 20, no. 20: 5779. https://doi.org/10.3390/s20205779
APA StyleTerutsuki, D., Mitsuno, H., & Kanzaki, R. (2020). 3D-Printed Bubble-Free Perfusion Cartridge System for Live-Cell Imaging. Sensors, 20(20), 5779. https://doi.org/10.3390/s20205779





