Silver Nanoplates for Colorimetric Determination of Xanthine in Human Plasma and in Fish Meat via Etching/Aggregation/Fusion Steps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrument
2.3. Preparation of Ag Nanoplates
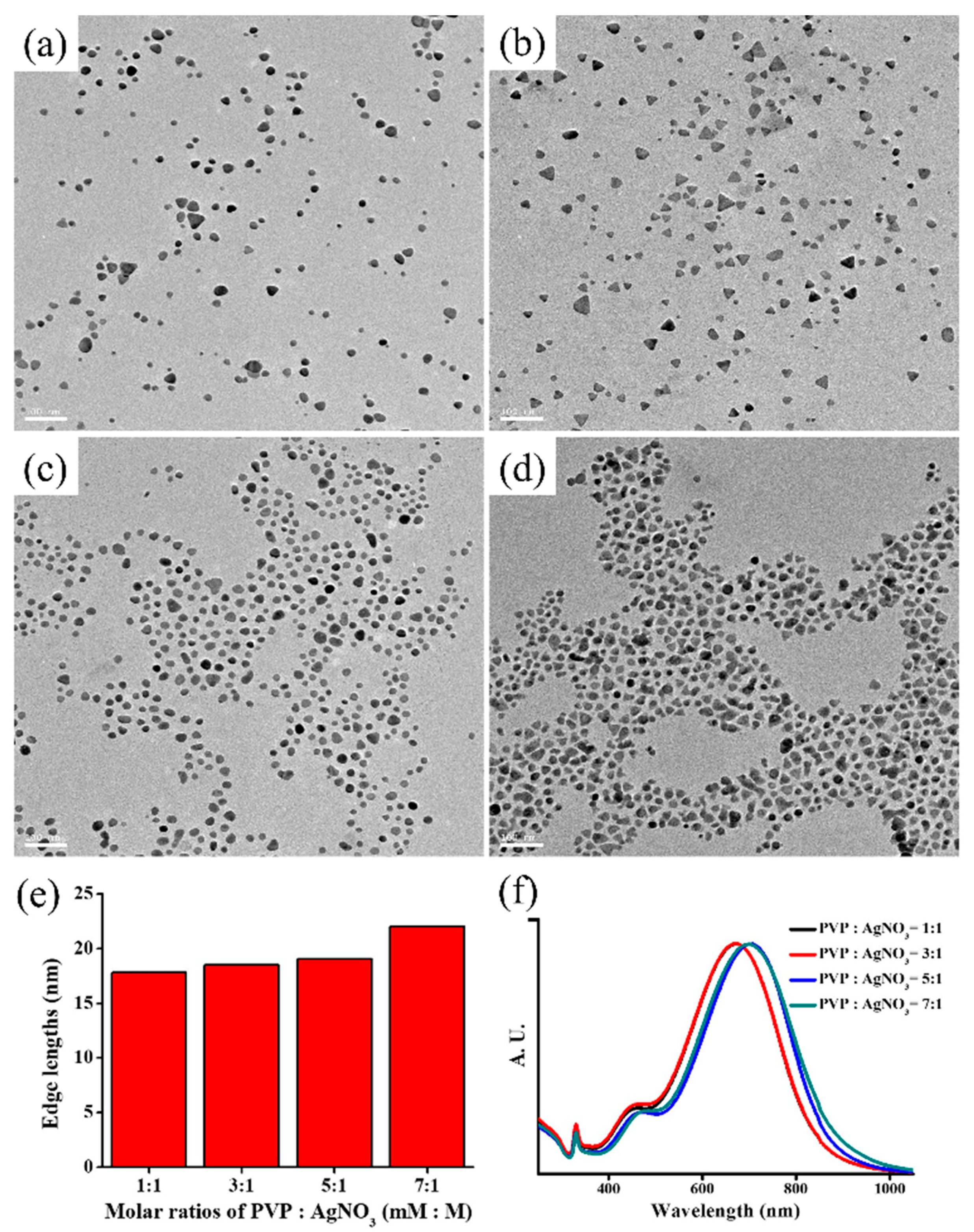
2.4. The Optimization of PVP in the Synthesis of AgP
2.5. Analytical Procedures
2.6. Determination of Xan in Fish Meat
2.7. Effects of Interfering Compounds
2.8. Xan Determination in Human Plasma
3. Results and Discussion
3.1. The Role of PVP Role in AgP Synthesis
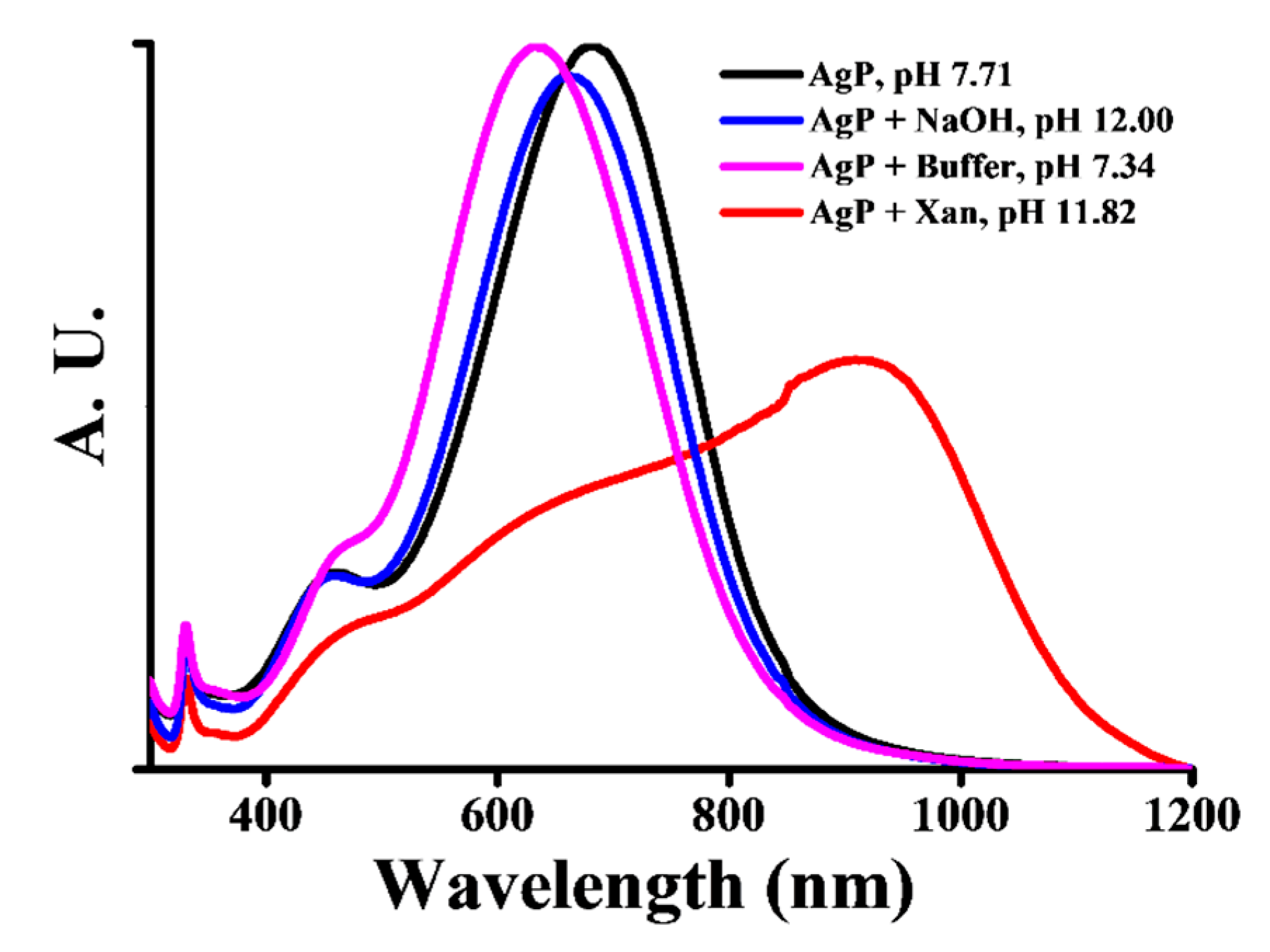
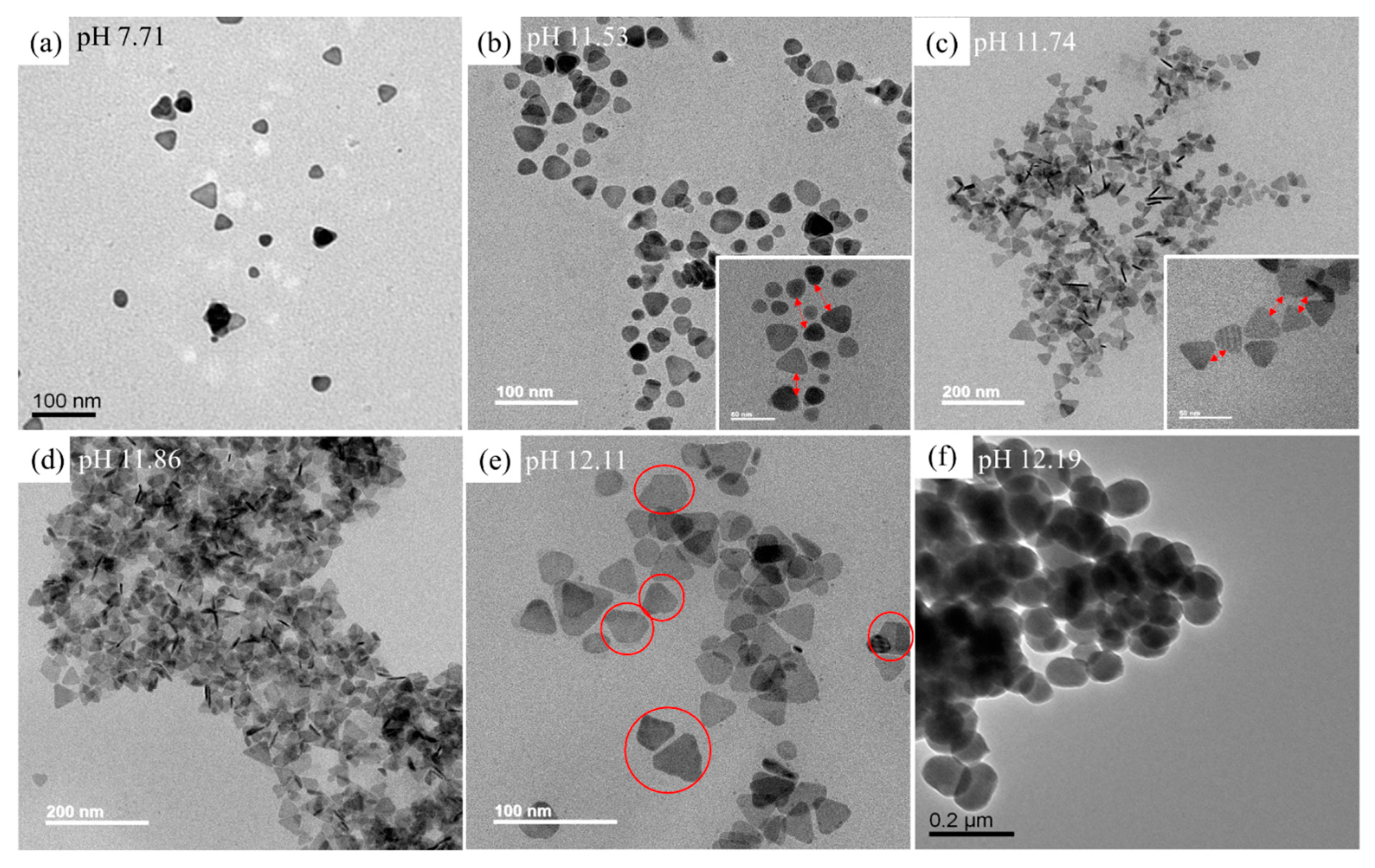
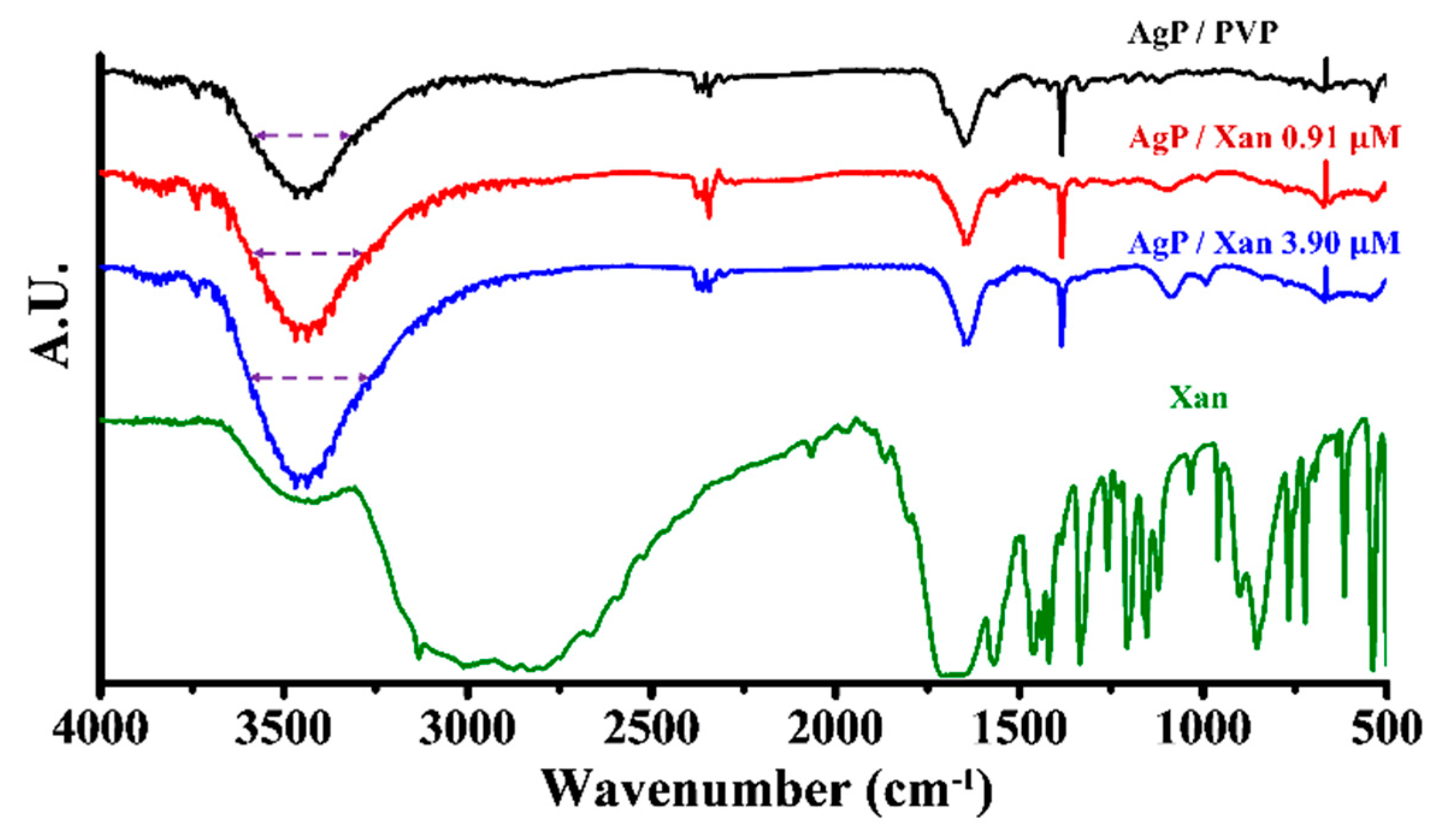
3.2. Sensing Principle for Xan
3.3. Absorption Titration Curve of Xan
3.4. Xan Determination in Fish Meat
3.5. Effects of Interfering Compounds in Plasma
3.6. Xan Determination in Human Plasma
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Devi, R.; Yadav, S.; Pundir, C. Amperometric determination of xanthine in fish meat by zinc oxide nanoparticle/chitosan/multiwalled carbon nanotube/polyaniline composite film bound xanthine oxidase. Analyst 2012, 137, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.S.; England, E.M. Postmortem glycolysis and glycogenolysis: Insights from species comparisons. Meat Sci. 2018, 144, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Regenstein, J.M.; Luo, Y. The Importance of ATP-related Compounds for the Freshness and Flavor of Post-mortem Fish and Shellfish Muscle: A Review. Crit. Rev. Food Sci. 2017, 57, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Dervisevic, M.; Custiuc, E.; Çevik, E.; Şenel, M. Construction of novel xanthine biosensor by using polymeric mediator/MWCNT nanocomposite layer for fish freshness detection. Food Chem. 2015, 181, 277–283. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Liu, Y.; Zhang, Q.; Liu, X.; Tang, Y.; Li, J. Construction of a novel xanthine biosensor using zinc oxide (ZnO) and the biotemplate method for detection of fish freshness. Anal. Methods 2019, 11, 1021–1026. [Google Scholar] [CrossRef]
- Kalimuthu, P.; Leimkühler, S.; Bernhardt, P.V. Low-Potential Amperometric Enzyme Biosensor for Xanthine and Hypoxanthine. Anal. Chem. 2012, 84, 10359–10365. [Google Scholar] [CrossRef]
- Wu, S.; Jia, S.; Dong, X. Study on detection methods for xanthine in food and biological samples. Int. J. Curr. Res. Chem. Pharm. Sci. 2016, 3, 1–5. [Google Scholar]
- Li, Z.; Liu, X.; Liang, X.-H.; Zhong, J.; Guo, L.; Fu, F. Colorimetric determination of xanthine in urine based on peroxidase-like activity of WO3 nanosheets. Talanta 2019, 204, 278–284. [Google Scholar] [CrossRef]
- Pu, W.; Zhao, H.; Wu, L.; Zhao, X. A colorimetric method for the determination of xanthine based on the aggregation of gold nanoparticles. Microchim. Acta 2015, 182, 395–400. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Q.; Shan, Z.; Huang, Q.-M. BSA-stabilized Au clusters as peroxidase mimetics for use in xanthine detection. Biosens. Bioelectron. 2011, 26, 3614–3619. [Google Scholar] [CrossRef]
- Elsner, C.; Prager, A.; Sobottka, A.; Lotnyk, A.; Abel, B. Coated triangular Ag nanoprisms as optical sensors: Control of stability and spectral response with a thermo-responsive polymer. Anal. Methods 2017, 9, 4663–4672. [Google Scholar] [CrossRef]
- Yakoh, A.; Rattanarat, P.; Siangproh, W.; Chailapakul, O. Simple and selective paper-based colorimetric sensor for determination of chloride ion in environmental samples using label-free silver nanoprisms. Talanta 2018, 178, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Hesketh, A.V.; Kelly, T.L. Chemical stability and degradation mechanisms of triangular Ag, Ag@Au, and Au nanoprisms. Phys. Chem. Chem. Phys. 2014, 16, 12407–12414. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Liu, W.; Ding, F.; Zhao, Q.; Zou, P.; Wang, X.; Rao, H. A fluorometric aptasensor for bisphenol a based on the inner filter effect of gold nanoparticles on the fluorescence of nitrogen-doped carbon dots. Microchim. Acta 2018, 185, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Meetraux, G.S.; Mirkin, C.C. Supported Lyotropic Liquid-Crystal Polymer Membranes: Promising Materials for Molecular-Size-Selective Aqueous Nanofiltration. Adv. Mater. 2005, 17, 412–415. [Google Scholar]
- Geng, X.; Leng, W.; Carter, N.A.; Vikesland, P.J.; Grove, T.Z. Protein-aided formation of triangular silver nanoprisms with enhanced SERS performance. J. Mater. Chem. B 2016, 4, 4182–4190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albelda, J.A.V.; Uzunoglu, A.; Santos, G.N.C.; Stanciu, L.A. Graphene-titanium dioxide nanocomposite based hypoxanthine sensor for assessment of meat freshness. Biosens. Bioelectron. 2017, 89, 518–524. [Google Scholar] [CrossRef]
- Li, C.; Hao, J.; Wu, K. Triethylamine-controlled Cu-BTC frameworks for electrochemical sensing fish freshness. Anal. Chim. Acta 2019, 1085, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Mayers, B.; Herricks, T.; Xia, Y. Crystalline Silver Nanowires by Soft Solution Processing. Nano. Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Mandal, A.; Bhattacharya, M.; Kuznetsov, D.V.; Ghosh, T.; Das Chakraborty, S.; Satpati, B.; Mazov, V.; Senapati, D. Decoupled in-plane Dipole Resonance Modulated Colorimetric Assay-Based Optical Ruler for Ultra-Trace Gold (Au) Detection. Sci. Rep. 2018, 8, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, H.; Zhan, P.; Wang, Z.; Ming, N. Au-Induced Polyvinylpyrrolidone Aggregates with Bound W. Adv. Funct. Mater. 2007, 17, 1558–1566. [Google Scholar] [CrossRef]
- Yu, P.; Huang, J.; Tang, J. Observation of Coalescence Process of Silver Nanospheres during Shape Transformation to Nanoprisms. Nanoscale Res. Lett. 2011, 6, 46–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, H.; Anwer, S.; Bharath, G.; Iqbal, S.; Chen, L. Nanoporous Ag-Au Bimetallic Triangular Nanoprisms Synthesized by Galvanic Replacement for Plasmonic Applications. J. Nanomater. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Xue, B.; Wang, D.; Zuo, J.; Kong, X.; Zhang, Y.; Liu, X.; Tu, L.; Chang, Y.; Li, C.; Wu, F.; et al. Towards high quality triangular silver nanoprisms: Improved synthesis, six-tip based hot spots and ultra-high local surface plasmon resonance sensitivity. Nanoscale 2015, 7, 8048–8057. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Sankari, G.; Ponnusamy, S. Vibrational spectral investigation on xanthine and its derivatives—Theophylline, caffeine and theobromine. Spectrochim Acta A 2005, 61, 117–127. [Google Scholar] [CrossRef]
- Qiao, F.; Wang, J.; Ai, S.; Li, L. As a new peroxidase mimetics: The synthesis of selenium doped graphitic carbon nitride nanosheets and applications on colorimetric detection of H2O2 and xanthine. Sens. Actuators B Chem. 2015, 216, 418–427. [Google Scholar] [CrossRef]
- Yan, Z.; Niu, Q.; Mou, M.; Wu, Y.; Liu, X.; Liao, S. A novel colorimetric method based on copper nanoclusters with intrinsic peroxidase-like for detecting xanthine in serum samples. J. Nanopart. Res. 2017, 19, 235–246. [Google Scholar] [CrossRef]
- Su, L.; Cai, Y.; Wang, L.; Dong, W.; Mao, G.; Li, Y.; Zhao, M.; Ma, Y.; Zhang, H. Hemin@carbon dot hybrid nanozymes with peroxidase mimicking properties for dual (colorimetric and fluorometric) sensing of hydrogen peroxide, glucose and xanthine. Microchim. Acta 2020, 187, 132–142. [Google Scholar] [CrossRef]
- Ko, Y.-C.; Lin, T.-L.; Yeh, C.-T.; Sun, N.-K.; Shyue, J.-J.; Liu, G.-Y.; Chou, S.-W.; Liu, Y.-C.; Hsu, C.-H.; Ho, M.-L. Silver nanoprism-based paper as a ratiometric sensor for extending biothiol detection in serum. New J. Chem. 2017, 41, 15120–15126. [Google Scholar] [CrossRef]
- Zare, D.; Khoshnevisan, K.; Barkhi, M.; Tahami, H.V.; Tahami, S.H.V. Fabrication of capped gold nanoparticles by using various amino acids. J. Exp. Nanosci. 2014, 9, 957–965. [Google Scholar] [CrossRef]
- Pleskacova, A.; Brejcha, S.; Pacal, L.; Kankova, K.; Tomandl, J. Simultaneous Determination of Uric Acid, Xanthine and Hypoxanthine in Human Plasma and Serum by HPLC–UV: Uric Acid Metabolism Tracking. Chromatographia 2017, 80, 529–536. [Google Scholar] [CrossRef]



| Material | Linear Range (μM) | Limit of Detection (μM) | Ref. |
|---|---|---|---|
| BSA−Au clusters a | 1.0−200 | 0.5 | [11] |
| Se−g−C3N4 b | 0.16–40 | 0.016 | [27] |
| AuNPs c | 0.125–6.0 | 0.023 | [9] |
| BSA−DTT−CuNCs d (nanoclusters) | 0.50–100 | 0.38 | [28] |
| WO3 nanosheets−TMB e | 25–200 | 1.24 | [8] |
| Hemin@CDs f | 0.17−33 | 0.11 | [29] |
| AgP | 0.15–0.60 0.61–3.00 | 0.001 | This work |
| Xan Added (M) b | Commercial Assay Kit (M) a | Our Proposed Method (M) | Recovery (%) | R.S.D. (%) c |
|---|---|---|---|---|
| 0.0 | 5.76 ± 0.54 | 5.47 ± 0.49 | / | 8.96 |
| 31.3 | 33.7 ± 3.98 | 33.8 ± 2.47 | 107.9 | 7.30 |
| 61.5 | 56.8 ± 6.96 | 59.6 ± 5.93 | 97.0 | 9.94 |
| 80.0 | 80.2 ± 4.97 | 82.4 ± 5.51 | 103.0 | 6.68 |
| 123.0 | 111.7 ± 10.56 | 128.3 ± 11.1 | 104.3 | 8.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, H.-C.; Liao, P.-W.; Lee, H.-T.; Liu, W.-C.; Ho, M.-L. Silver Nanoplates for Colorimetric Determination of Xanthine in Human Plasma and in Fish Meat via Etching/Aggregation/Fusion Steps. Sensors 2020, 20, 5739. https://doi.org/10.3390/s20205739
Hsu H-C, Liao P-W, Lee H-T, Liu W-C, Ho M-L. Silver Nanoplates for Colorimetric Determination of Xanthine in Human Plasma and in Fish Meat via Etching/Aggregation/Fusion Steps. Sensors. 2020; 20(20):5739. https://doi.org/10.3390/s20205739
Chicago/Turabian StyleHsu, Hung-Cheng, Pei-Wen Liao, Hsiang-Tzu Lee, Wei-Chen Liu, and Mei-Lin Ho. 2020. "Silver Nanoplates for Colorimetric Determination of Xanthine in Human Plasma and in Fish Meat via Etching/Aggregation/Fusion Steps" Sensors 20, no. 20: 5739. https://doi.org/10.3390/s20205739
APA StyleHsu, H.-C., Liao, P.-W., Lee, H.-T., Liu, W.-C., & Ho, M.-L. (2020). Silver Nanoplates for Colorimetric Determination of Xanthine in Human Plasma and in Fish Meat via Etching/Aggregation/Fusion Steps. Sensors, 20(20), 5739. https://doi.org/10.3390/s20205739




