Characterization of a Low-Cost Plastic Fiber Array Detector for Proton Beam Dosimetry
Abstract
:1. Introduction
2. Materials and Methods
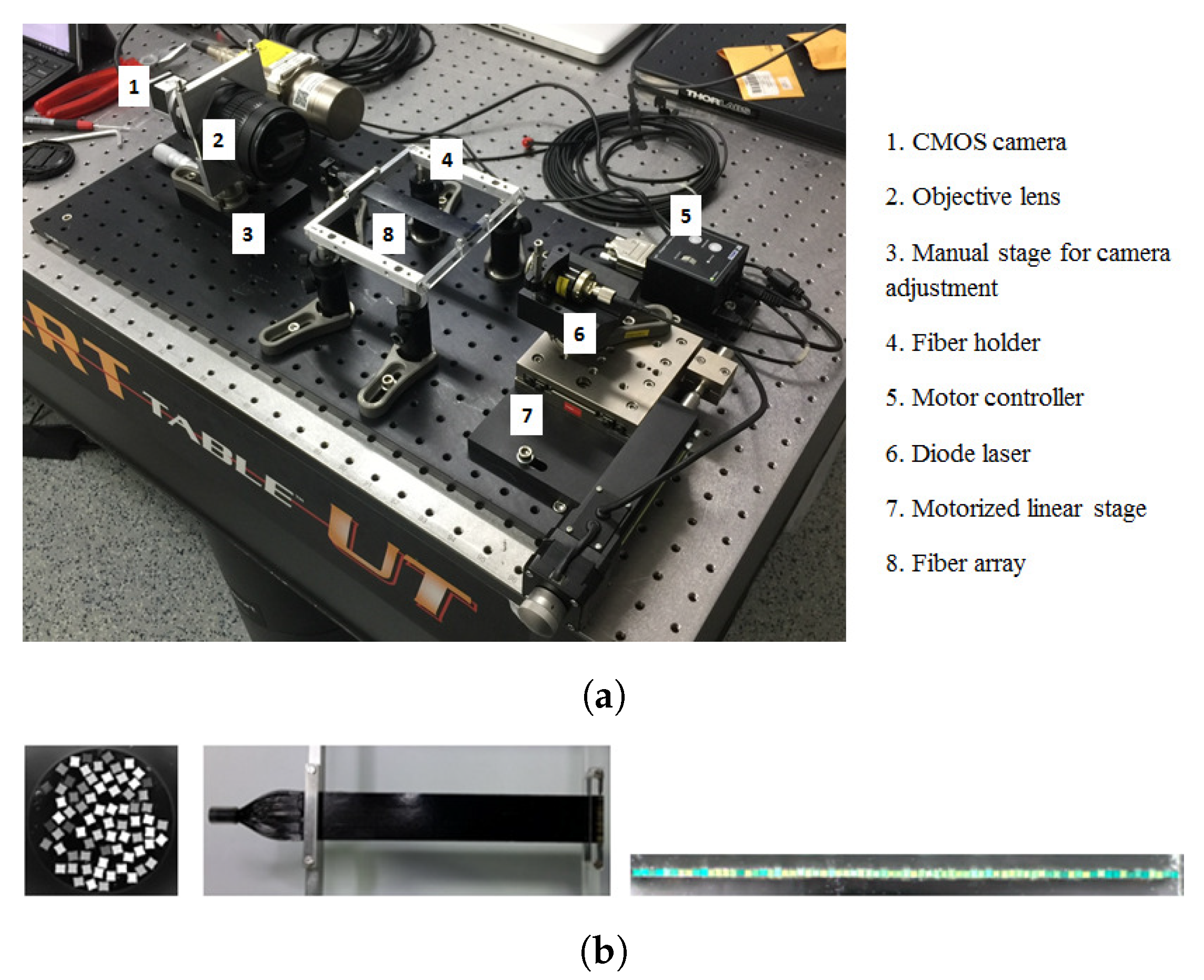
2.1. Setup at the CPT
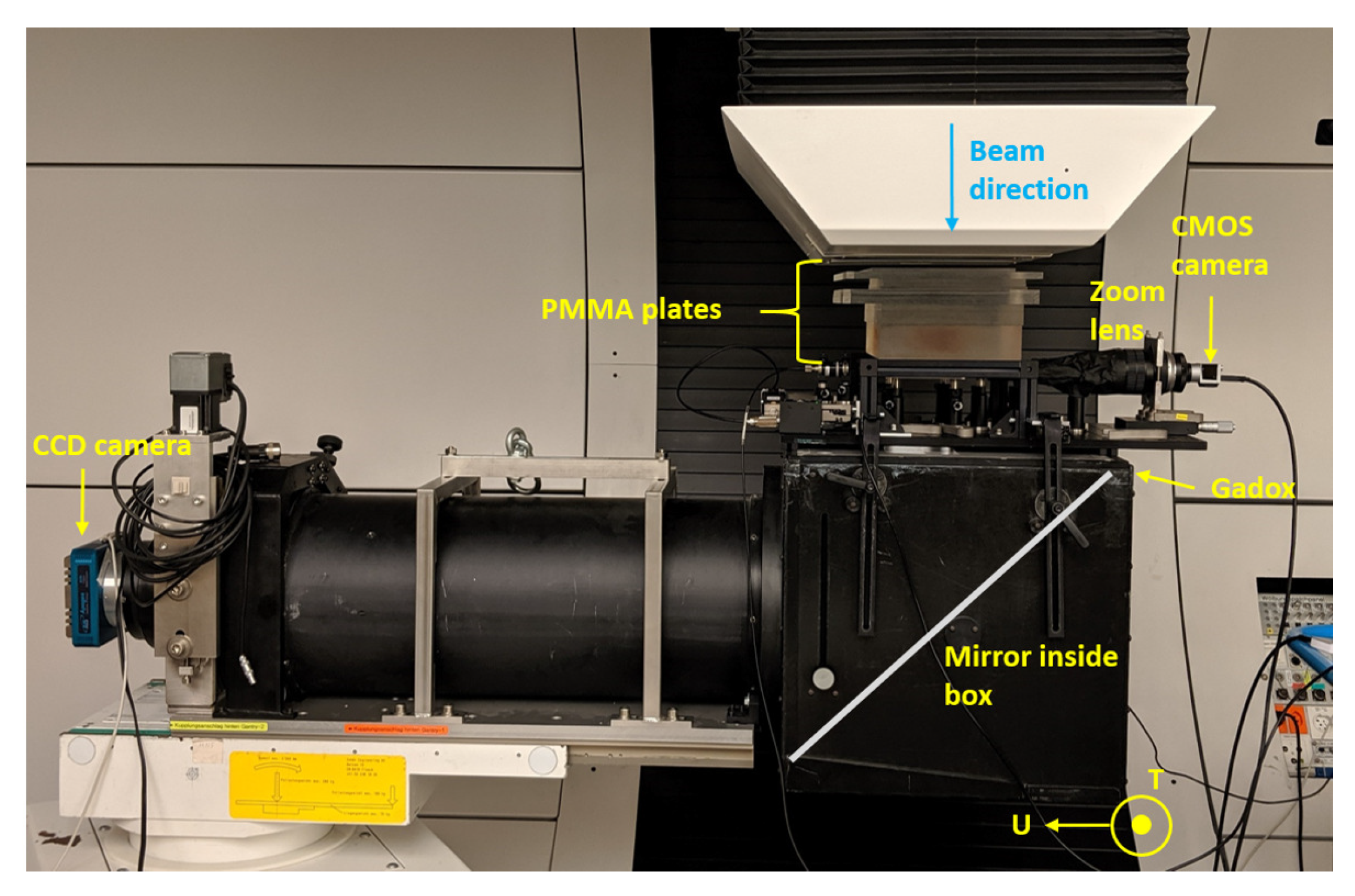
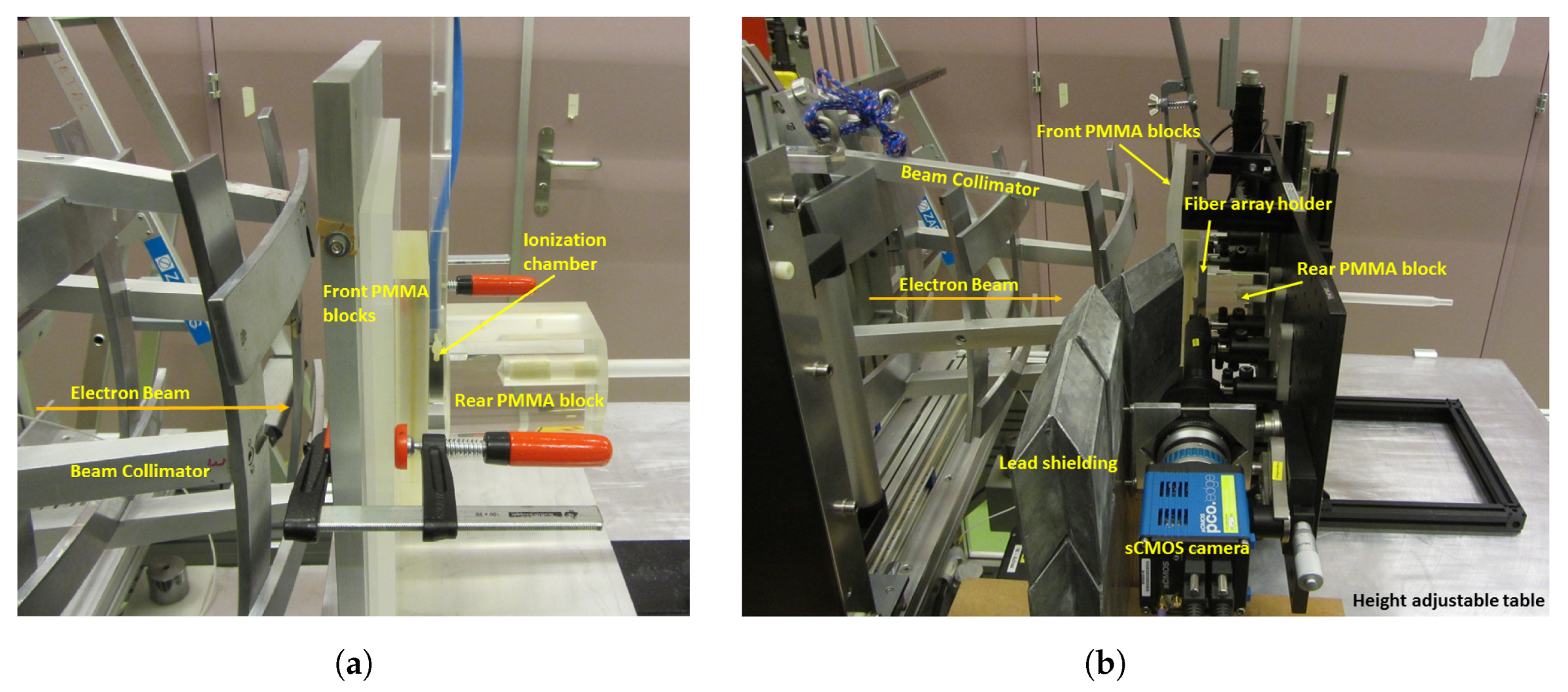
2.2. Setup at METAS
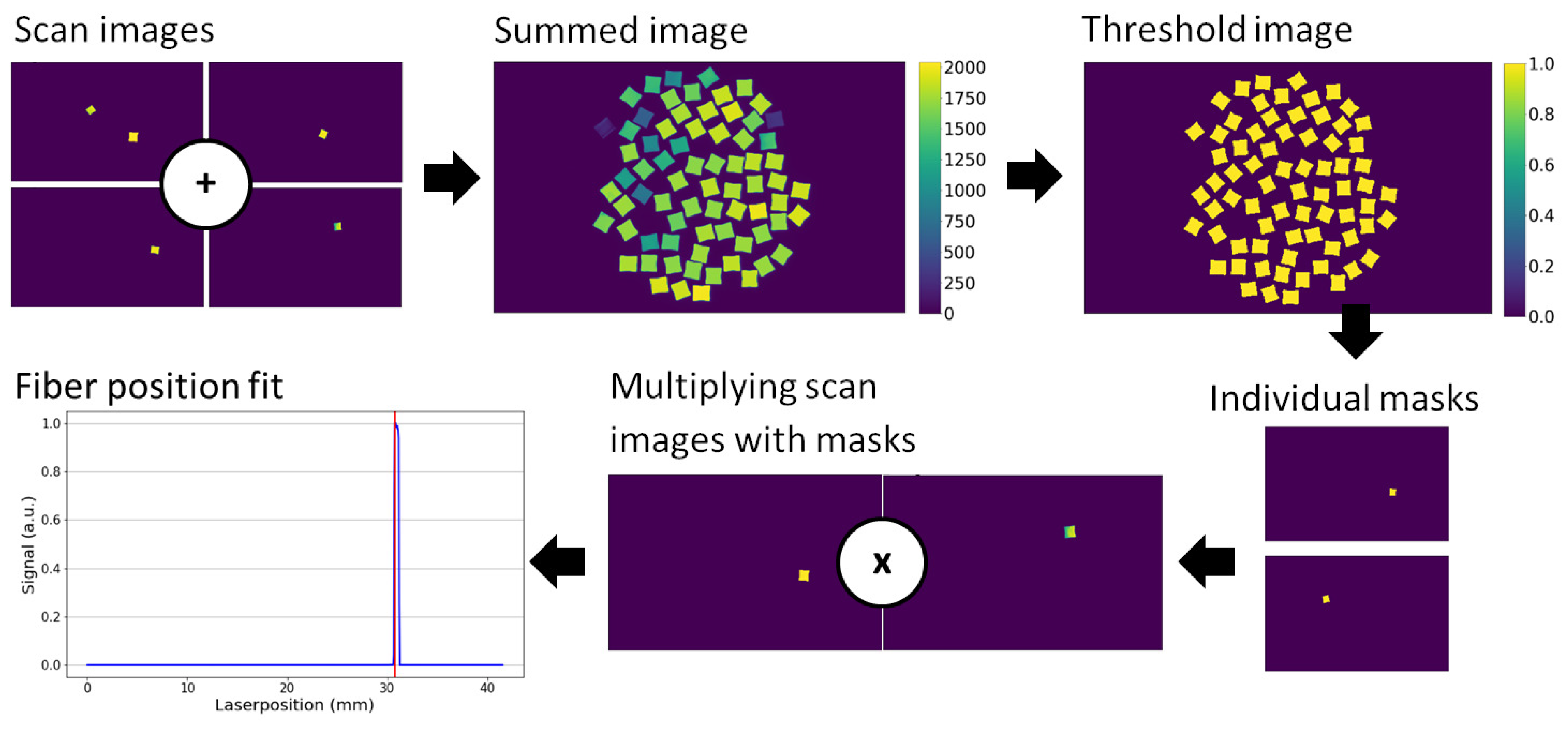
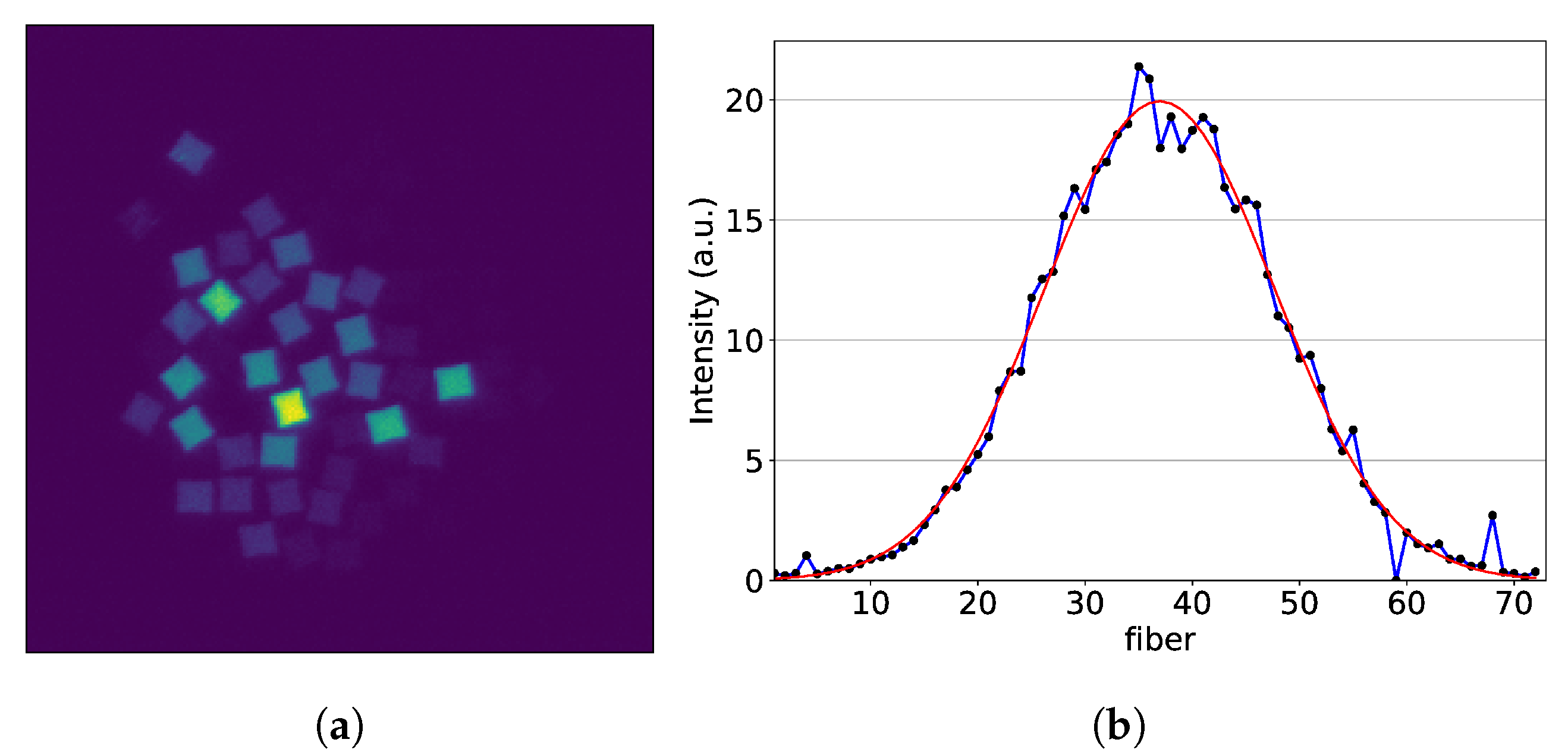
2.3. Image Reconstruction
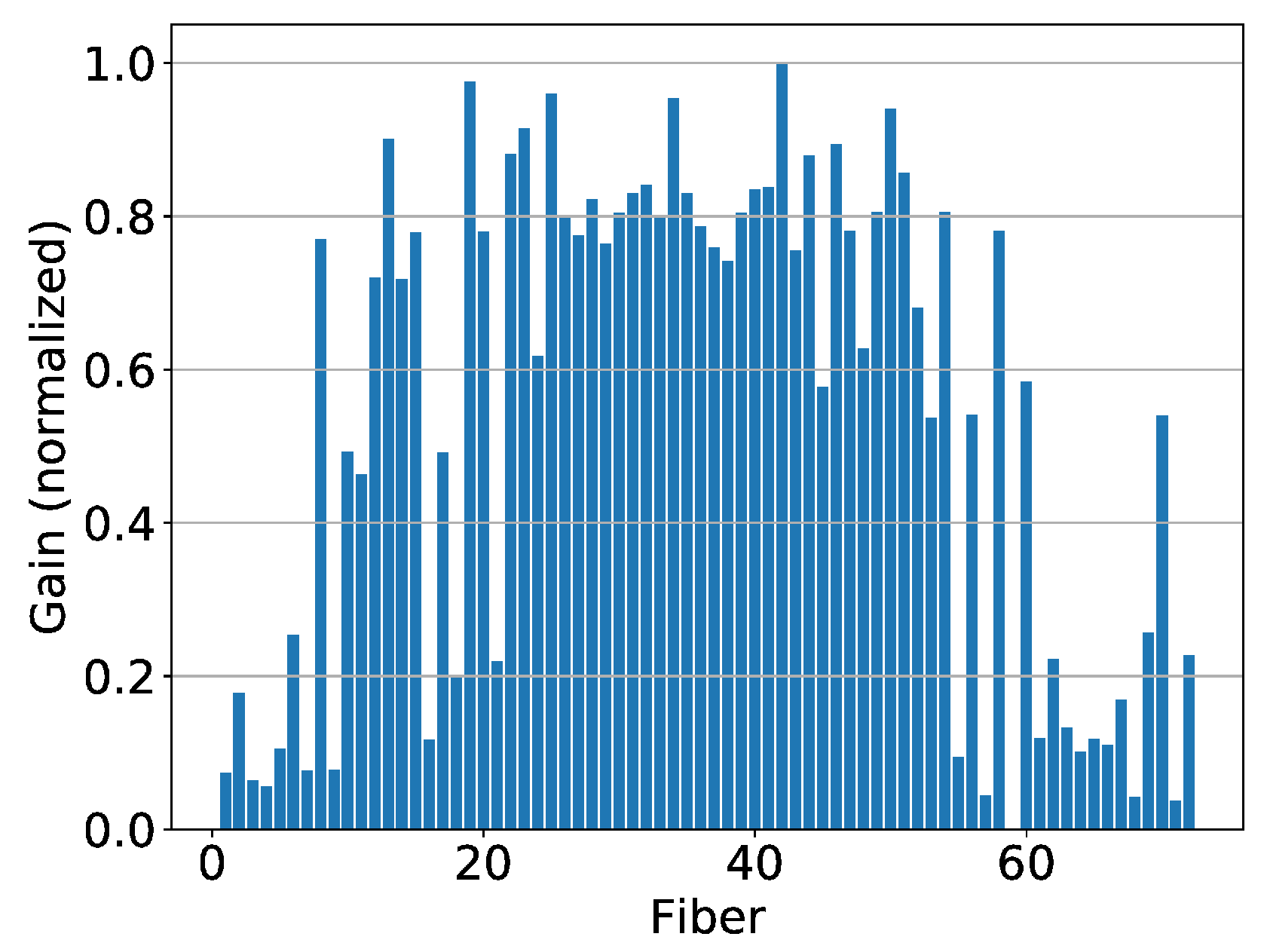
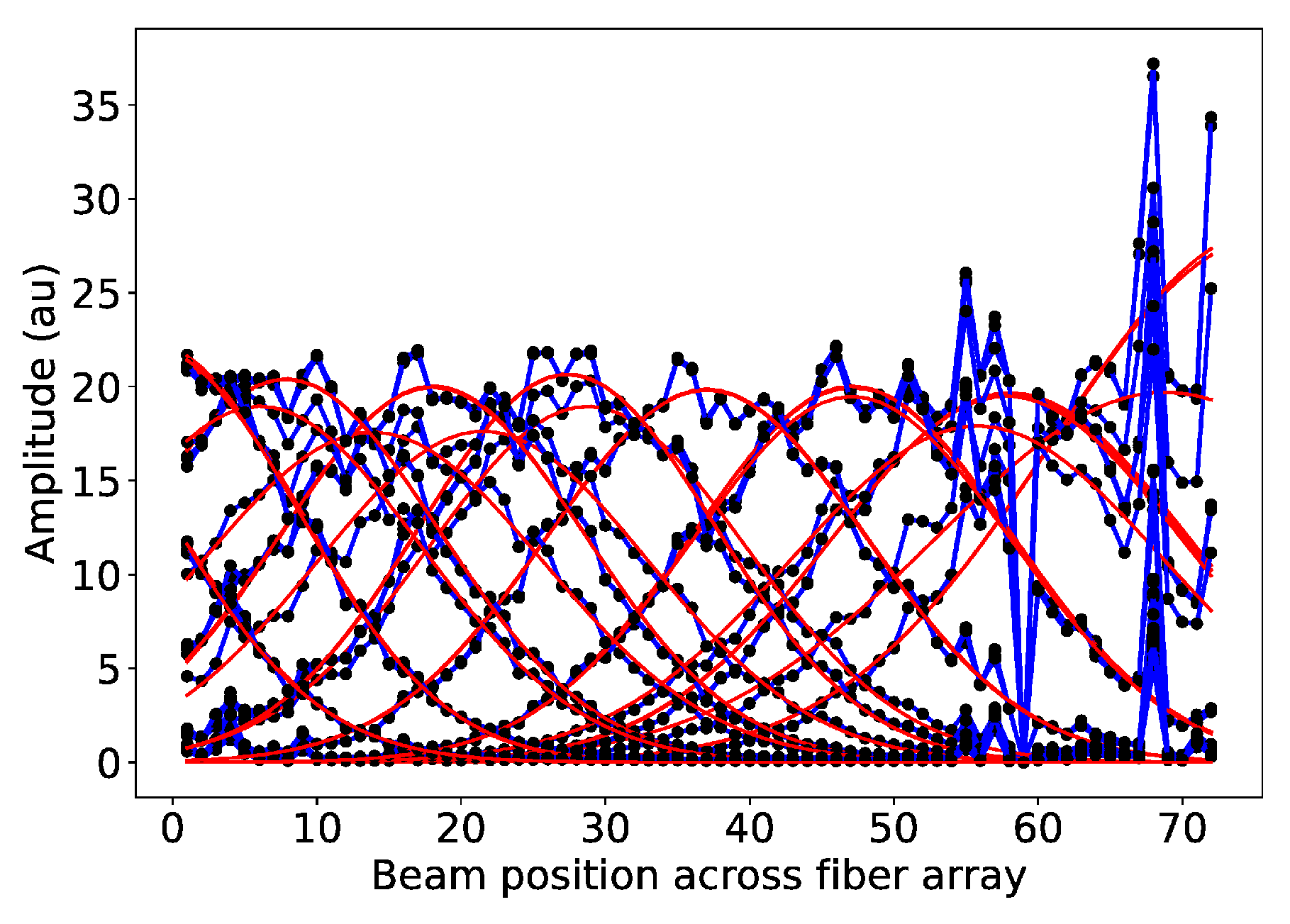
2.4. Position Calibration
3. Results
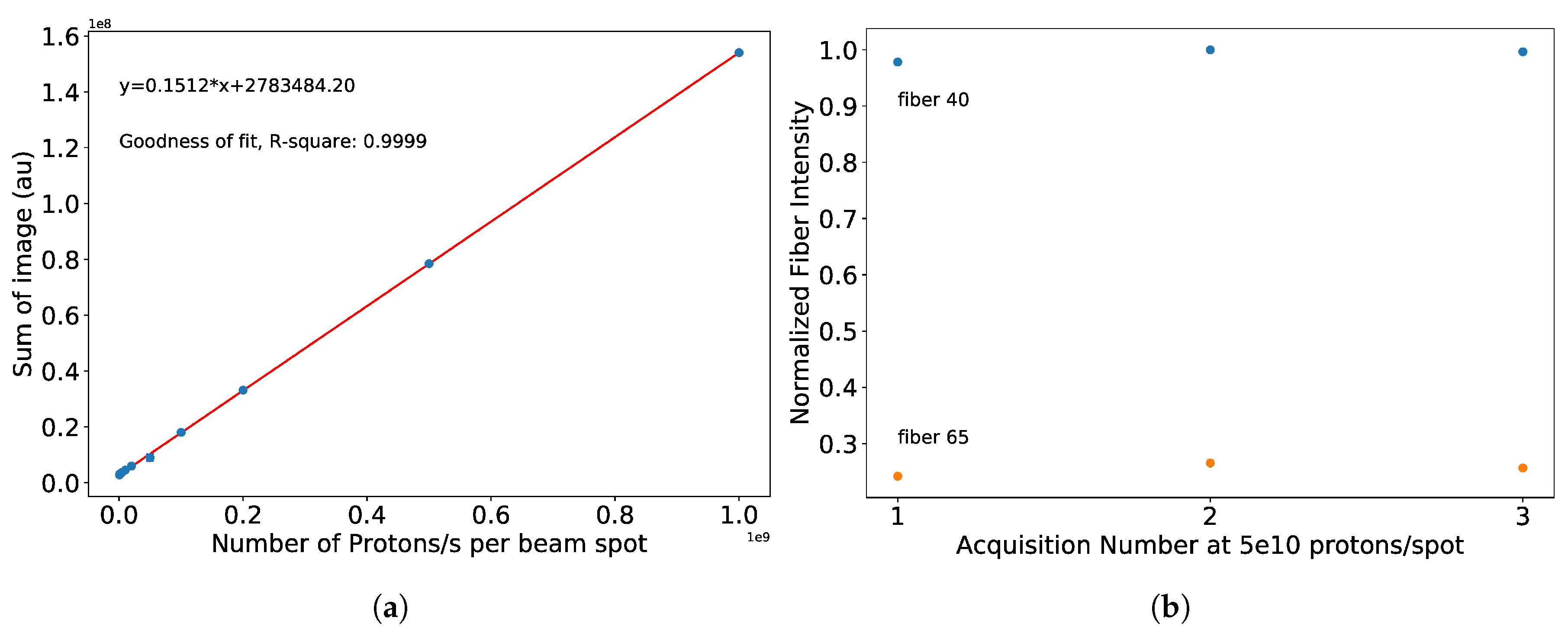
3.1. Results from Measurements at CPT
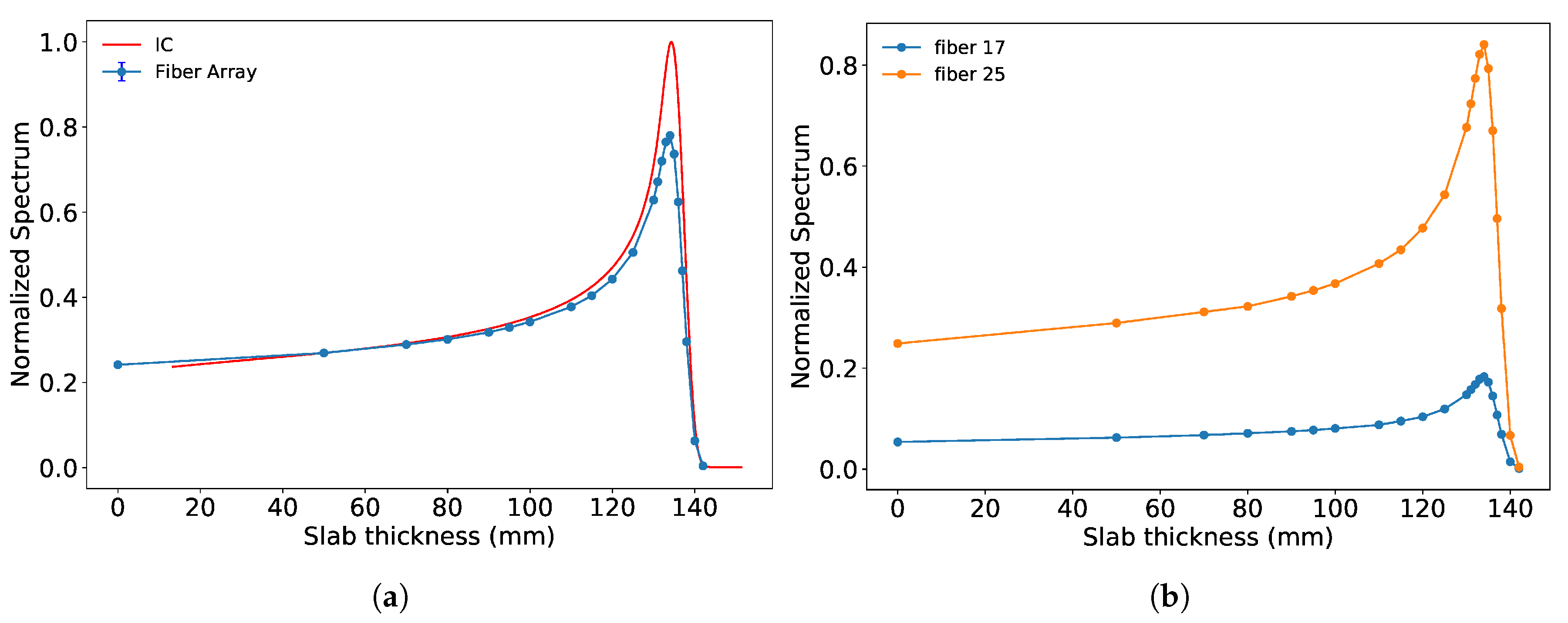
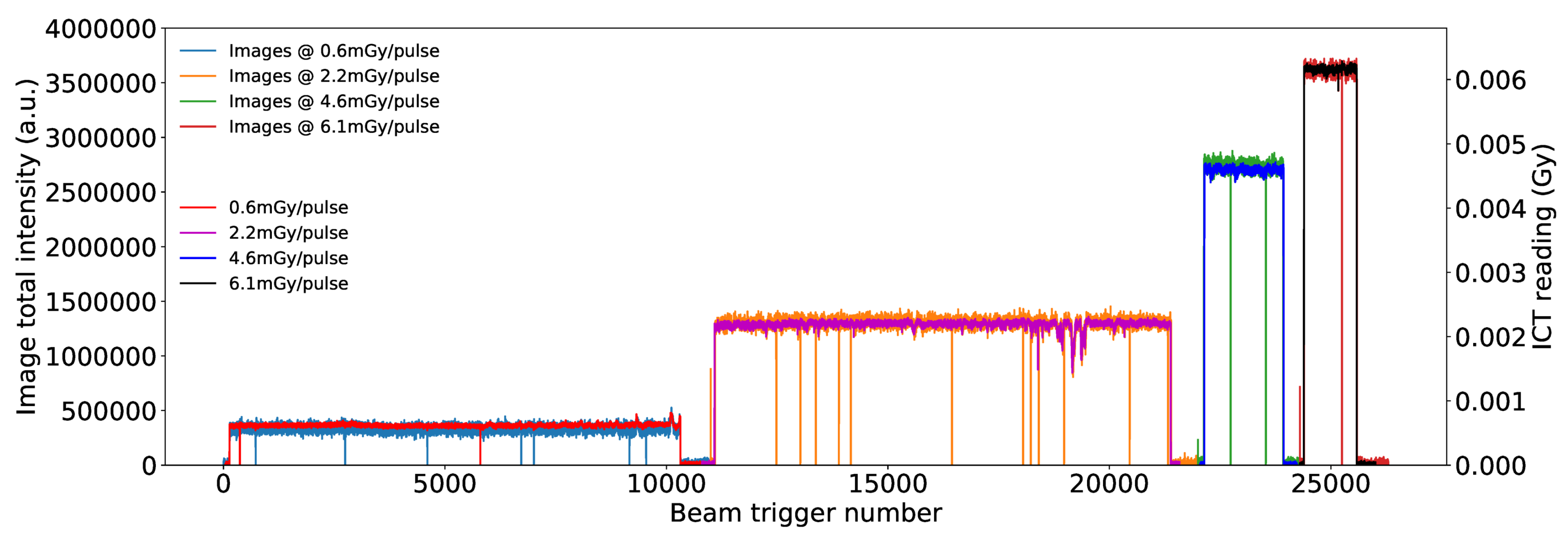
3.2. Results from Measurements at METAS
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pedroni, E.; Bacher, R.; Blattmann, H.; Böhringer, T.; Coray, A.; Lomax, A.; Lin, S.; Munkel, G.; Scheib, S.; Schneider, U.; et al. The 200 MeV proton therapy project at the Paul Scherrer Institute: Conceptual design and practical realization. Med. Phys. 1995, 22, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Lomax, A.J. Intensity modulated methods for proton therapy. Phys. Med. Biol. 1999, 44, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Pedroni, E. The PSI Gantry 2: A second generation proton scanning gantry. Zeitschrift für Medizinische Physik 2004, 14, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Lomax, A.J.; Bortfeld, T.; Goitein, G.; Debus, J.; Dykstra, C.; Tercier, P.A.; Coucke, P.A.; Mirimanoff, R.O. A treatment planning inter-comparison of proton and intensity modulated photon radiotherapy. Radiat. Oncol. 1999, 51, 257–271. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, S.M.; Safai, S.; Trofimov, A.; Wolfgang, J.; Fullerton, B.; Yeap, B.Y.; Bortfeld, T.; Tarbell, N.J.; Yock, T. Proton radiotherapy for childhood ependymoma: Initial clinical outcomes and dose comparisons. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.M.; Pawlicki, T.; Dong, L.; Fourkal, E.; Buyyounouski, M.; Cengel, K.; Plastaras, J.; Bucci, M.K.; Yock, T.I.; Bonilla, L.; et al. An evidence based review of proton beam therapy: The report of ASTRO’s emerging technology committee. Radiat. Oncol. 2012, 103, 8–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beddar, S.; Mackie, T.R.; Attix, F.H. Water-equivalent plastic scintillation detectors for high-energy beam dosimetry: I. Physical characteristics and theoretical considerations. Phys. Med. Biol. 1992, 37, 1883–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Therriault-Proulx, F.; Archambault, L.; Beaulieu, L.; Beddar, S. Development of a novel multi-point plastic scintillation detector with a single optical transmission line for radiation dose measurement. Phys. Med. Biol. 1992, 37, 1883–1900. [Google Scholar] [CrossRef] [Green Version]
- Safai, S.; Lin, S.; Pedroni, E. Development of an inorganic scintillating mixture for proton beam verification dosimetry. Phys. Med. Biol. 2004, 49, 4637–4655. [Google Scholar] [CrossRef]
- Goma, C.; Hofstetter-Boillat, B.; Safai, S.; Vörös, S. Experimental validation of beam quality correction factors for proton beams. Phys. Med. Biol. 2015, 60, 3207–3216. [Google Scholar] [CrossRef]
- Goma, C.; Marinelli, M.; Safai, S.; Verona-Rinati, G.; Würfe, l.J. The role of a microDiamond detector in the dosimetry of proton pencil beams. Med. Phys. 2016, 26, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Guillot, M.; Beaulieu, L.; Archambault, L.; Beddar, S.; Gingras, L. A new water-equivalent 2D plastic scintillation detectors array for the dosimetry of megavoltage energy photon beams in radiation therapy. Med. Phys. 2011, 38, 6763–6774. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.W.; Perles, L.A.; Archambault, L.; Sahoo, N.; Mirkovic, D.; Beddar, S. Determination of the quenching correction factors for plastic scintillation detectors in therapeutic high-energy proton beams. J. Phys. Med. Biol. 2012, 57, 7767–7781. [Google Scholar] [CrossRef] [PubMed]
- Guillot, M. Toward 3D dosimetry of intensity modulated radiation therapy treatments with plastic scintillation detectors. J. Phys. Conf. Ser. 2010, 250, 012006. [Google Scholar] [CrossRef]
- Son, J.; Lee, S.; Lim, Y.; Park, S.; Cho, K.; Yoon, M.; Shin, D. Development of Optical Fiber Based Measurement System for the Verification of Entrance Dose Map in Pencil Beam Scanning Proton Beam. Med. Phys. 2018, 18, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linares Rosales, H.M.; Duguay-Drouin, P.; Archambault, L.; Beddar, S.; Beaulieu, L. Optimization of a multipoint plastic scintillator dosimeter for high dose rate brachytherapy. Med. Phys. 2019, 46, 2412–2421. [Google Scholar] [CrossRef]
- Almurayshid, M.; Helo, Y.; Kacperek, A.; Griffiths, J.; Hebden, J.; Gibson, A. Quality assurance in proton beam therapy using a plastic scintillator and a commercially available digital camera. J. Appl. Clin. Med. Phys. 2017, 18, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Giordanengo, S.; Palmans, H. Dose detectors, sensors, and their applications. Med. Phys. 2018, 45, 1051–1072. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.R.; Pankuch, M.; Hammer, C.G.; DeWerd, L.A.; Culberson, W.S. LET response variability of Gafchromic EBT3 film from a Co calibration in clinical proton beam qualities. Med. Phys. 2019, 46, 2716–2728. [Google Scholar] [CrossRef] [Green Version]
- Arjomandy, B.; Tailor, R.; Anand, A.; Sahoo, N.; Gillin, M.; Prado, K.; Vicic, M. Energy dependence and dose response of Gafchromic EBT2 film over a wide range of photon, electron, and proton beam energies. Med. Phys. 2010, 37, 1942–1947. [Google Scholar] [CrossRef]
- Lacroix, F.; Beaulieu, L.; Archambault, L.; Beddar, S. Simulation of the precision limits of plastic scintillation detectors using optimal component selection. Med. Phys. 2010, 37, 412–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boon, S.N. Dosimetry and Quality Control of Scanning Proton Beams. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, September 1998. [Google Scholar]
- Birks, J.B. The Theory and Practice of Scintillation Counting. In International Series of Monographs in Electronics and Instrumentation; Birks, J.B., Ed.; Elsevier: Amsterdam, The Netherlands, 1964; pp. 15–38. [Google Scholar]
- Baslerweb. acA 1920-50gm. Available online: https://www.baslerweb.com/en/products/cameras/area-scan-cameras/ace/aca1920-50gm/ (accessed on 21 January 2019).
- Saint Gobain. Scintillating Fibers. Available online: https://www.crystals.saint-gobain.com/products/scintillating-fiber (accessed on 18 July 2018).
- Papa, A.; Kettle, P.-R.; Ripiccini, E.; Rutar, G. Scintillating fibres coupled to silicon photomultiplier prototypes for fast beam monitoring and thin timing detectors. Nucl. Instrum. Methods Phys. Res. Sect. A 2016, 824, 128–130. [Google Scholar] [CrossRef]
- Scmos. Pco.edge. Available online: https://www.pco.de/scientific-cameras/pcoedge-55/ (accessed on 13 January 2020).
- León Marroquin, E.Y.; Herrera González, J.A.; Camacho López, M.A.; Villarreal Barajas, J.E.; García-Garduño, O.A. Evaluation of the uncertainty in an EBT3 film dosimetry system utilizing net optical density. J. Appl. Clin. Med. Phys. 2016, 17, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Krzempek, D.; Mianowska, G.; Bassler, N.; Stolarczyk, L.; Kopeć, R.; Sas-Korczyńska, B.; Olko, P. Calibration of Gafchromic EBT3 Film for Dosimetry of Scanning Proton Pencil Beam (PBS). Radiat. Prot. Dosim. 2018, 180, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Zullo, J.R.; Kudchadker, R.J.; Zhu, X.R.; Sahoo, N.; Gillin, M.T. LiF TLD-100 as a Dosimeter in High Energy Proton Beam Therapy—Can It Yield Accurate Results? Med. Dosim. 2010, 35, 63–66. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozkan Loch, C.; Eichenberger, M.A.; Togno, M.; Zinsli, S.P.; Egloff, M.; Papa, A.; Ischebeck, R.; Lomax, A.J.; Peier, P.; Safai, S. Characterization of a Low-Cost Plastic Fiber Array Detector for Proton Beam Dosimetry. Sensors 2020, 20, 5727. https://doi.org/10.3390/s20205727
Ozkan Loch C, Eichenberger MA, Togno M, Zinsli SP, Egloff M, Papa A, Ischebeck R, Lomax AJ, Peier P, Safai S. Characterization of a Low-Cost Plastic Fiber Array Detector for Proton Beam Dosimetry. Sensors. 2020; 20(20):5727. https://doi.org/10.3390/s20205727
Chicago/Turabian StyleOzkan Loch, Cigdem, Michael Alexander Eichenberger, Michele Togno, Simon Pascal Zinsli, Martina Egloff, Angela Papa, Rasmus Ischebeck, Antony John Lomax, Peter Peier, and Sairos Safai. 2020. "Characterization of a Low-Cost Plastic Fiber Array Detector for Proton Beam Dosimetry" Sensors 20, no. 20: 5727. https://doi.org/10.3390/s20205727
APA StyleOzkan Loch, C., Eichenberger, M. A., Togno, M., Zinsli, S. P., Egloff, M., Papa, A., Ischebeck, R., Lomax, A. J., Peier, P., & Safai, S. (2020). Characterization of a Low-Cost Plastic Fiber Array Detector for Proton Beam Dosimetry. Sensors, 20(20), 5727. https://doi.org/10.3390/s20205727





