Multiplexed Immunosensor Based on the Amperometric Transduction for Monitoring of Marine Pollutants in Sea Water
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Instrumentation
2.2. Chemicals and Biochemicals
2.3. Buffers and Solutions
2.4. Biofunctionalization Protocol
2.5. Immunosensor Protocol (Static Mode)
2.6. Immunosensor Protocol (Flow Mode)
2.7. Amperometric Measurement
3. Results and Discussion
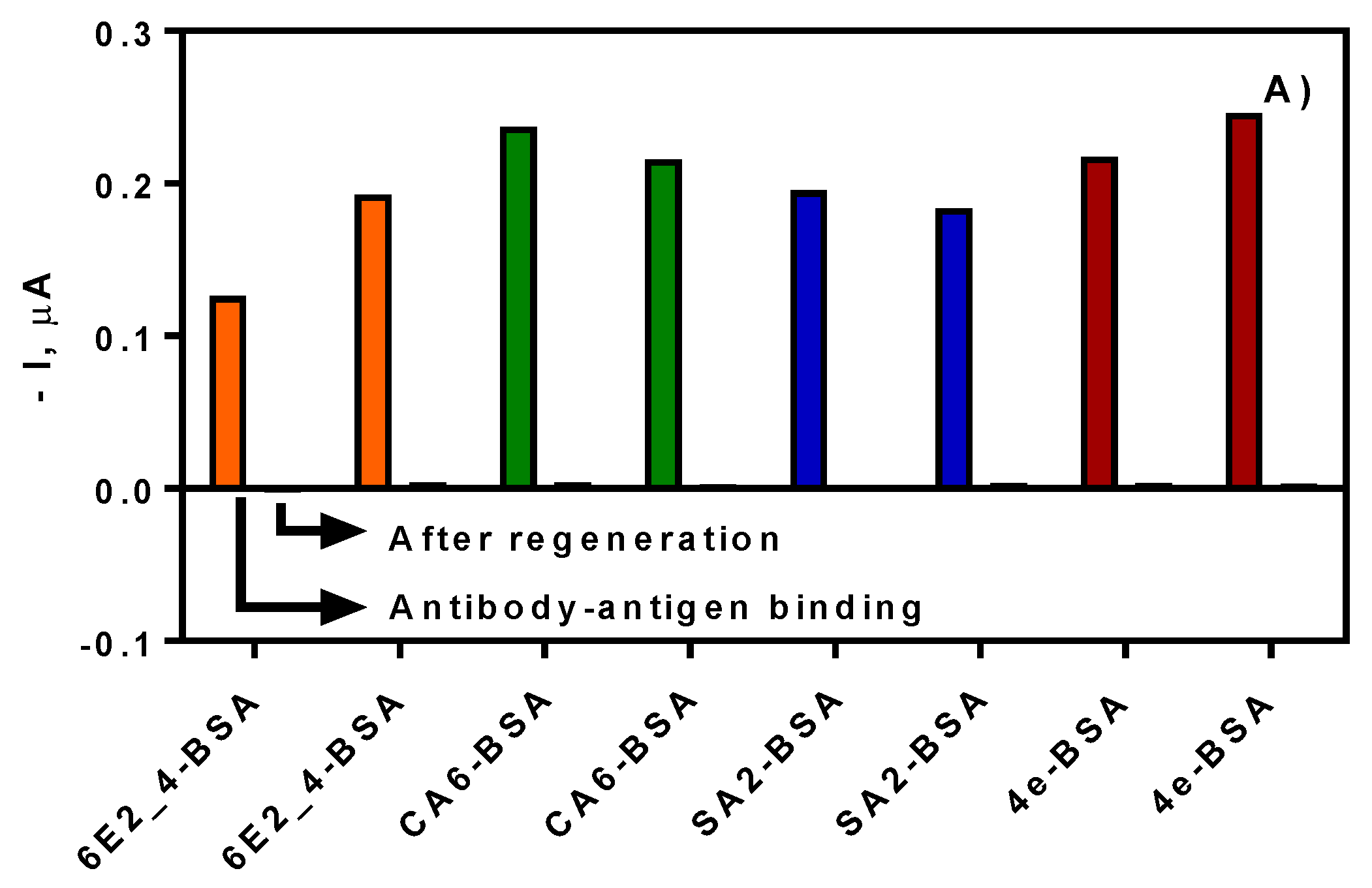
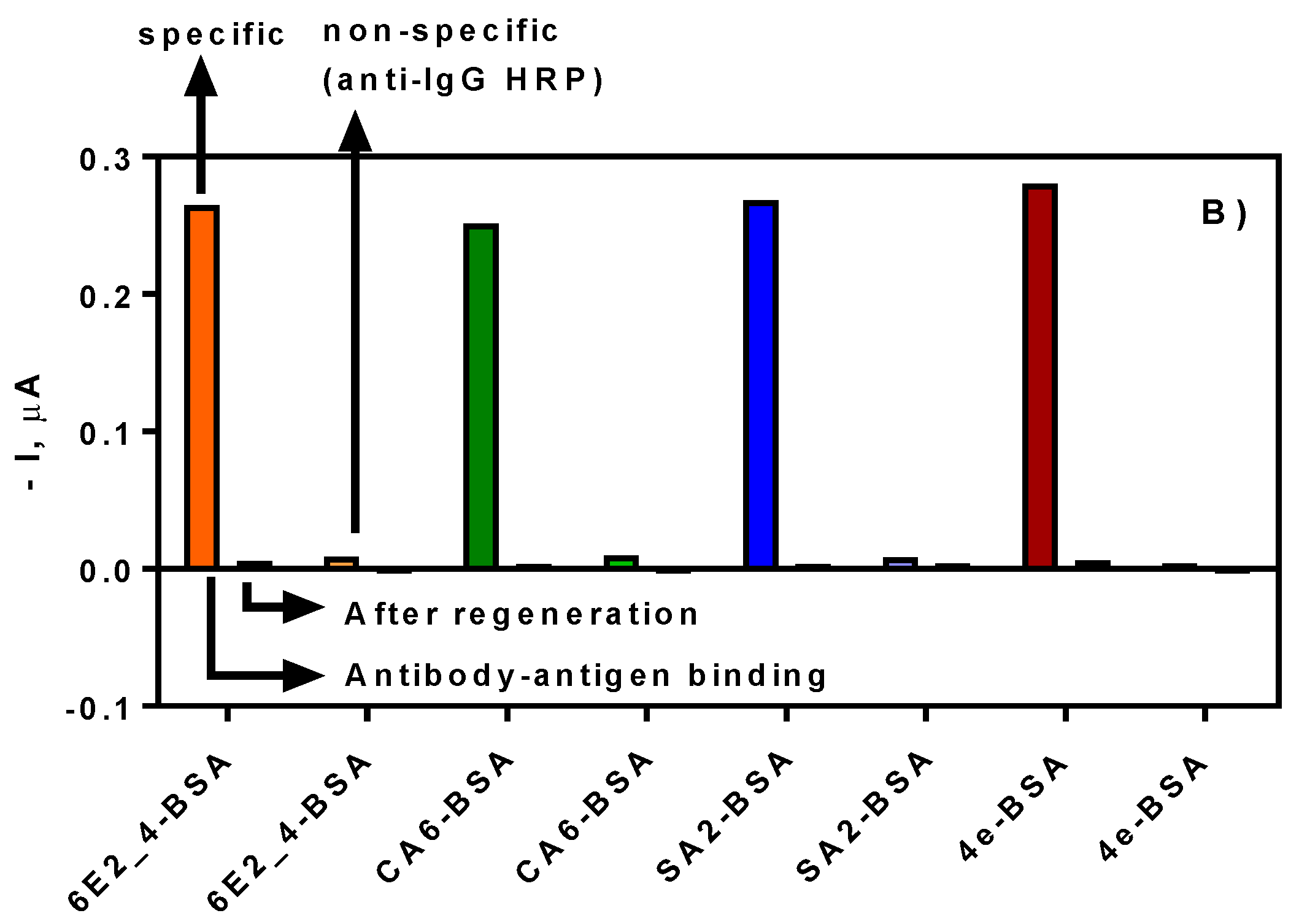
3.1. Biofunctionalization of SPE
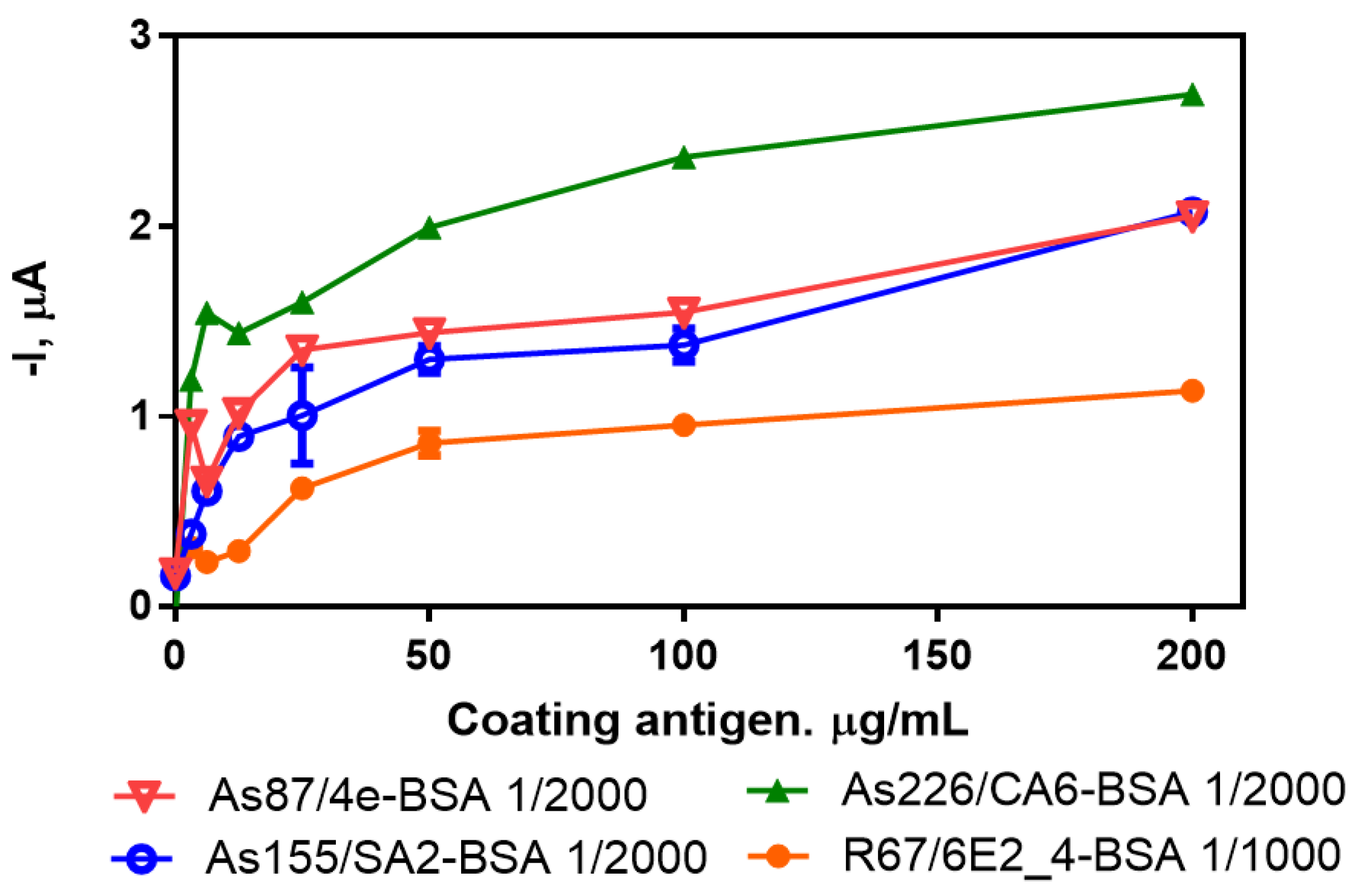
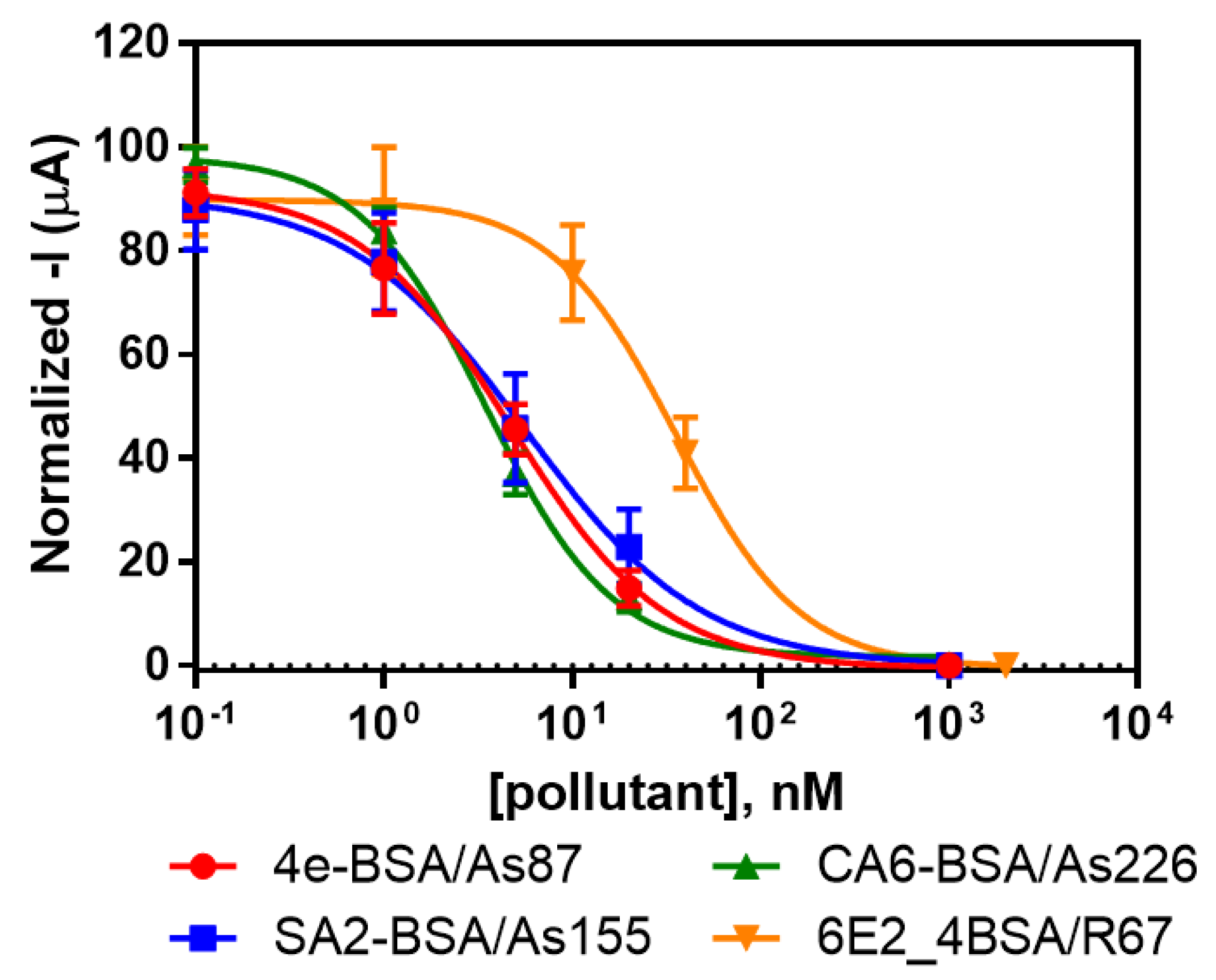
3.2. Individual Immunosensors for the Detection of Irgarol 1051, Sulfapyridine, Chloramphenicol and Estradiol
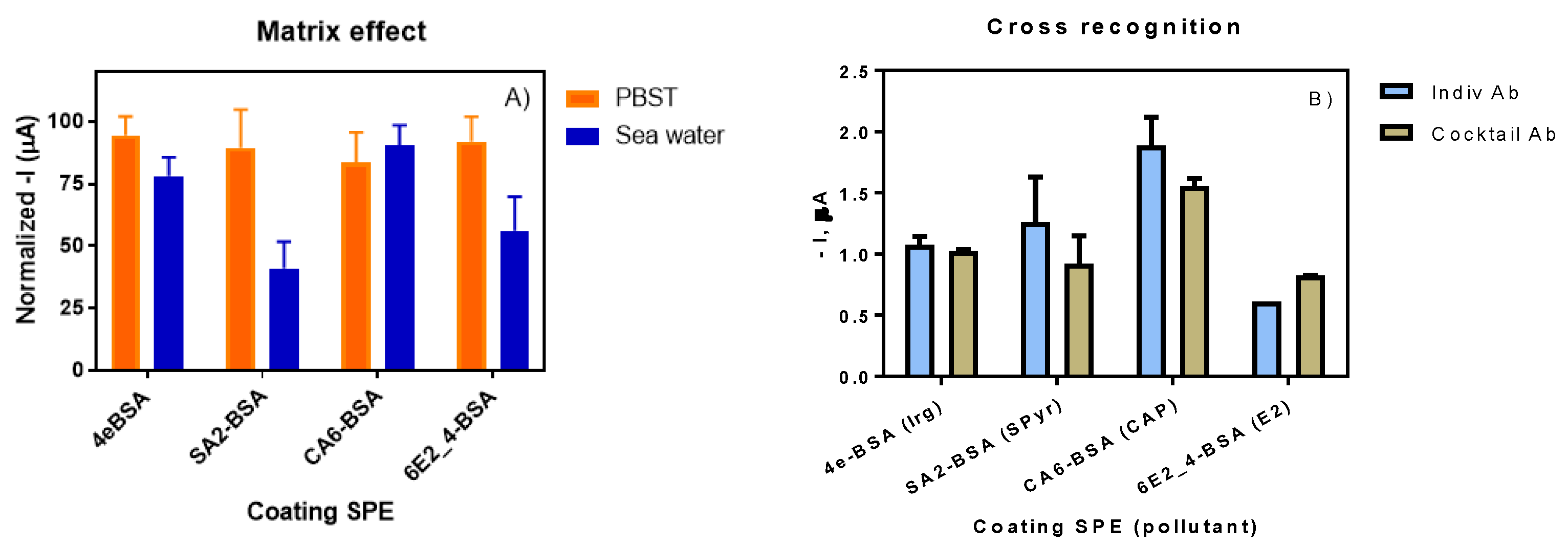
3.3. Multiplexed Immunosensor for the Detection of Irgarol 1051, Sulfapyridine, Chloramphenicol and Estradiol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Net, S.; Sempéré, R.; Delmont, A.; Paluselli, A.; Ouddane, B. Occurrence, Fate, Behavior and Ecotoxicological State of Phthalates in Different Environmental Matrices. Environ. Sci. Technol. 2015, 49, 4019–4035. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Siedlewicz, G.; Borecka, M.; Białk-Bielińska, A.; Sikora, K.; Stepnowski, P.; Pazdro, K. Determination of antibiotic residues in southern Baltic Sea sediments using tandem solid-phase extraction and liquid chromatography coupled with tandem mass spectrometry. Oceanologia 2016, 58, 221–234. [Google Scholar] [CrossRef]
- Ripollés, C.; Ibáñez, M.; Sancho, J.V.; López, F.J.; Hernández, F. Determination of 17β-estradiol and 17α-ethinylestradiol in water at sub-ppt levels by liquid chromatography coupled to tandem mass spectrometry. Anal. Methods 2014, 6, 5028–5037. [Google Scholar] [CrossRef]
- Loos, R.; Tavazzi, S.; Mariani, G.; Suurkuusk, G.; Paracchini, B.; Umlauf, G. Analysis of emerging organic contaminants in water, fish and suspended particulate matter (SPM) in the Joint Danube Survey using solid-phase extraction followed by UHPLC-MS-MS and GC–MS analysis. Sci. Total Environ. 2017, 607–608, 1201–1212. [Google Scholar] [CrossRef]
- Gago-Ferrero, P.; Bletsou, A.A.; Damalas, D.E.; Aalizadeh, R.; Alygizakis, N.A.; Singer, H.P.; Hollender, J.; Thomaidis, N.S. Wide-scope target screening of >2000 emerging contaminants in wastewater samples with UPLC-Q-ToF-HRMS/MS and smart evaluation of its performance through the validation of 195 selected representative analytes. J. Hazard. Mater. 2019, 387, 121712. [Google Scholar] [CrossRef]
- Sanchis, A.; Bosch-Orea, C.; Salvador, J.-P.; Marco, M.-P.; Farré, M. Development and validation of a multianalyte immunoassay for the quantification of environmental pollutants in seawater samples from the Catalonia coastal area. Anal. Bioanal. Chem. 2019, 411, 5897–5907. [Google Scholar] [CrossRef] [PubMed]
- Fatta-Kassinos, D.; Meric, S.; Nikolaou, A. Pharmaceutical residues in environmental waters and wastewater: Current state of knowledge and future research. Anal. Bioanal. Chem. 2011, 399, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Farré, M.; de Alda, M.L.; Perez, S.; Postigo, C.; Köck, M.; Radjenovic, J.; Gros, M.; Barcelo, D. Recent trends in the liquid chromatography–mass spectrometry analysis of organic contaminants in environmental samples. J. Chromatogr. A 2010, 1217, 4004–4017. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.T. Environmental Mass Spectrometry. Annu. Rev. Anal. Chem. 2013, 6, 163–189. [Google Scholar] [CrossRef]
- Bell, R.J.; Davey, N.G.; Martinsen, M.; Collin-Hansen, C.; Krogh, E.T.; Gill, C.G. A Field-Portable Membrane Introduction Mass Spectrometer for Real-time Quantitation and Spatial Mapping of Atmospheric and Aqueous Contaminants. J. Am. Soc. Mass Spectrom. 2015, 26, 212–223. [Google Scholar] [CrossRef]
- Zhang, Z.; Zeng, K.; Liu, J. Immunochemical detection of emerging organic contaminants in environmental waters. Trends Analyt. Chem. 2017, 87, 49–57. [Google Scholar] [CrossRef]
- Piro, B.; Reisberg, S. Recent Advances in Electrochemical Immunosensors. Sensors 2017, 17, 794. [Google Scholar] [CrossRef]
- Raman Suri, C.; Boro, R.; Nangia, Y.; Gandhi, S.; Sharma, P.; Wangoo, N.; Rajesh, K.; Shekhawat, G.S. Immunoanalytical techniques for analyzing pesticides in the environment. Trends Analyt. Chem. 2009, 28, 29–39. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef]
- Tate, J.; Ward, G. Interferences in immunoassay. Clin. Biochem. Rev. 2004, 25, 105–120. [Google Scholar]
- Pla-Roca, M.; Leulmi, R.F.; Tourekhanova, S.; Bergeron, S.; Laforte, V.; Moreau, E.; Gosline, S.J.C.; Bertos, N.; Hallett, M.; Park, M.; et al. Antibody Colocalization Microarray: A Scalable Technology for Multiplex Protein Analysis in Complex Samples. Mol. Cell. Proteomics 2012, 11, M111.011460. [Google Scholar] [CrossRef]
- Sanchis, A.; Salvador, J.P.; Marco, M.P. Multiplexed immunochemical techniques for the detection of pollutants in aquatic environments. Trends Analyt. Chem. 2018, 106, 1–10. [Google Scholar] [CrossRef]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. Trends Analyt. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Bertok, T.; Lorencova, L.; Chocholova, E.; Jane, E.; Vikartovska, A.; Kasak, P.; Tkac, J. Electrochemical Impedance Spectroscopy Based Biosensors: Mechanistic Principles, Analytical Examples and Challenges towards Commercialization for Assays of Protein Cancer Biomarkers. ChemElectroChem. 2019, 6, 989–1003. [Google Scholar] [CrossRef]
- Palmisano, F.; Rizzi, R.; Centonze, D.; Zambonin, P.G. Simultaneous monitoring of glucose and lactate by an interference and cross-talk free dual electrode amperometric biosensor based on electropolymerized thin films. Biosens. Bioelectron. 2000, 15, 531–539. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Povedano, E.; Benedé, S.; Mata, L.; Galán-Malo, P.; Gamella, M.; Reviejo, A.J.; Campuzano, S.; Pingarrón, J.M. Disposable Amperometric Immunosensor for the Detection of Adulteration in Milk through Single or Multiplexed Determination of Bovine, Ovine, or Caprine Immunoglobulins G. Anal. Chem. 2019, 91, 11266–11274. [Google Scholar] [CrossRef]
- Pastucha, M.; Farka, Z.; Lacina, K.; Mikušová, Z.; Skládal, P. Magnetic nanoparticles for smart electrochemical immunoassays: A review on recent developments. Microchimica Acta. 2019, 186, 312. [Google Scholar] [CrossRef]
- Ballesteros, B.; Barceló, D.; Sanchez-Baeza, F.; Camps, F.; Marco, M.-P. Influence of the Hapten Design on the Development of a Competitive ELISA for the Determination of the Antifouling Agent Irgarol 1051 at Trace Levels. Anal. Chem. 1998, 70, 4004–4014. [Google Scholar] [CrossRef]
- Sanvicens, N.; Varela, B.; Ballesteros, B.; Marco, M.P. Development of an immunoassay for terbutryn: Study of the influence of the immunization protocol. Talanta 2012, 89, 310–316. [Google Scholar] [CrossRef]
- Adrian, J.; Font, H.; Diserens, J.-M.; Sánchez-Baeza, F.; Marco, M.P. Generation of Broad Specificity Antibodies for Sulfonamide Antibiotics and Development of an Enzyme-Linked Immunosorbent Assay (ELISA) for the Analysis of Milk Samples. J. Agric. Food Chem. 2009, 57, 385–394. [Google Scholar] [CrossRef]
- Sanchis, A.; Salvador, J.P.; Campbell, K.; Elliott, C.T.; Shelver, W.L.; Li, Q.X.; Marco, M.P. Fluorescent microarray for multiplexed quantification of environmental contaminants in seawater samples. Talanta 2018, 184, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.P.; Marco, M.P. Amperometric Biosensor for Continuous Monitoring Irgarol 1051 in Sea Water. Electroanalysis 2016, 28, 1833–1838. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Escamilla-Gómez, V.; Campuzano, S.; Pedrero, M.; Salvador, J.P.; Marco, M.P.; Pingarrón, J.M. Ultrasensitive amperometric magnetoimmunosensor for human C-reactive protein quantification in serum. Sens. Actuators B Chem. 2013, 188, 212–220. [Google Scholar] [CrossRef]
- Lowe, S.; O’Brien-Simpson, N.M.; Connal, L.A. Antibiofouling polymer interfaces: Poly(ethylene glycol) and other promising candidates. Polym. Chem. 2015, 6, 198–212. [Google Scholar] [CrossRef]
- Fernández, F.; Pinacho, D.G.; Sánchez-Baeza, F.; Marco, M.P. Portable Surface Plasmon Resonance Immunosensor for the Detection of Fluoroquinolone Antibiotic Residues in Milk. J. Agric. Food Chem. 2011, 59, 5036–5043. [Google Scholar] [CrossRef]
- Fruhmann, P.; Sanchis, A.; Mayerhuber, L.; Vanka, T.; Kleber, C.; Salvador, J.-P.; Marco, M.-P. Immunoassay and amperometric biosensor approaches for the detection of deltamethrin in seawater. Anal. Bioanal. Chem. 2018, 410, 5923–5930. [Google Scholar] [CrossRef]
- Marco, M.P.; Gee, S.; Hammock, B.D. Immunochemical techniques for environmental-analysis II. antibody-production and immunoassay development. TRAC-Trends Anal. Chem. 1995, 14, 463. [Google Scholar] [CrossRef]
- Crowther, J. The ELISA Guidebook; Humana Press: Totowa, NJ, USA, 2009; pp. 203–204. [Google Scholar]
- Adrian, J.; Pasche, S.; Diserens, J.-M.; Sánchez-Baeza, F.; Gao, H.; Marco, M.P.; Voirin, G. Waveguide interrogated optical immunosensor (WIOS) for detection of sulfonamide antibiotics in milk. Biosens. Bioelectron. 2009, 24, 3340–3346. [Google Scholar] [CrossRef]
- Muriano, A.; Pinacho, D.-G.; Chabottaux, V.; Diserens, J.-M.; Granier, B.; Stead, S.; Sanchez Baeza, F.; Pividori, M.I.; Marco, M.-P. A portable electrochemical magnetoimmunosensor for detection of sulfonamide antimicrobials in honey. Anal. Bioanal. 2013, 405, 7885–7895. [Google Scholar] [CrossRef]
- Chocarro-Ruiz, B.; Herranz, S.; Fernández Gavela, A.; Sanchís, J.; Farré, M.; Marco, M.P.; Lechuga, L.M. Interferometric nanoimmunosensor for label-free and real-time monitoring of Irgarol 1051 in seawater. Biosens. Bioelectron. 2018, 117, 47–52. [Google Scholar] [CrossRef]
- Wu, D.; Rios-Aguirre, D.; Chounlakone, M.; Camacho-Leon, S.; Voldman, J. Sequentially multiplexed amperometry for electrochemical biosensors. Biosens. Bioelectron. 2018, 117, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Kanso, H.; Begoña González García, M.; Ma, S.; Ludwig, R.; Fanjul Bolado, P.; Hernández Santos, D. Dual Biosensor for Simultaneous Monitoring of Lactate and Glucose Based on Thin-layer Flow Cell Screen-printed Electrode. Electroanalysis 2017, 29, 87–92. [Google Scholar] [CrossRef]


| Analyte | Irgarol 1051 | Sulfapyridine | Chloramphenicol | Estradiol |
|---|---|---|---|---|
| Structure | 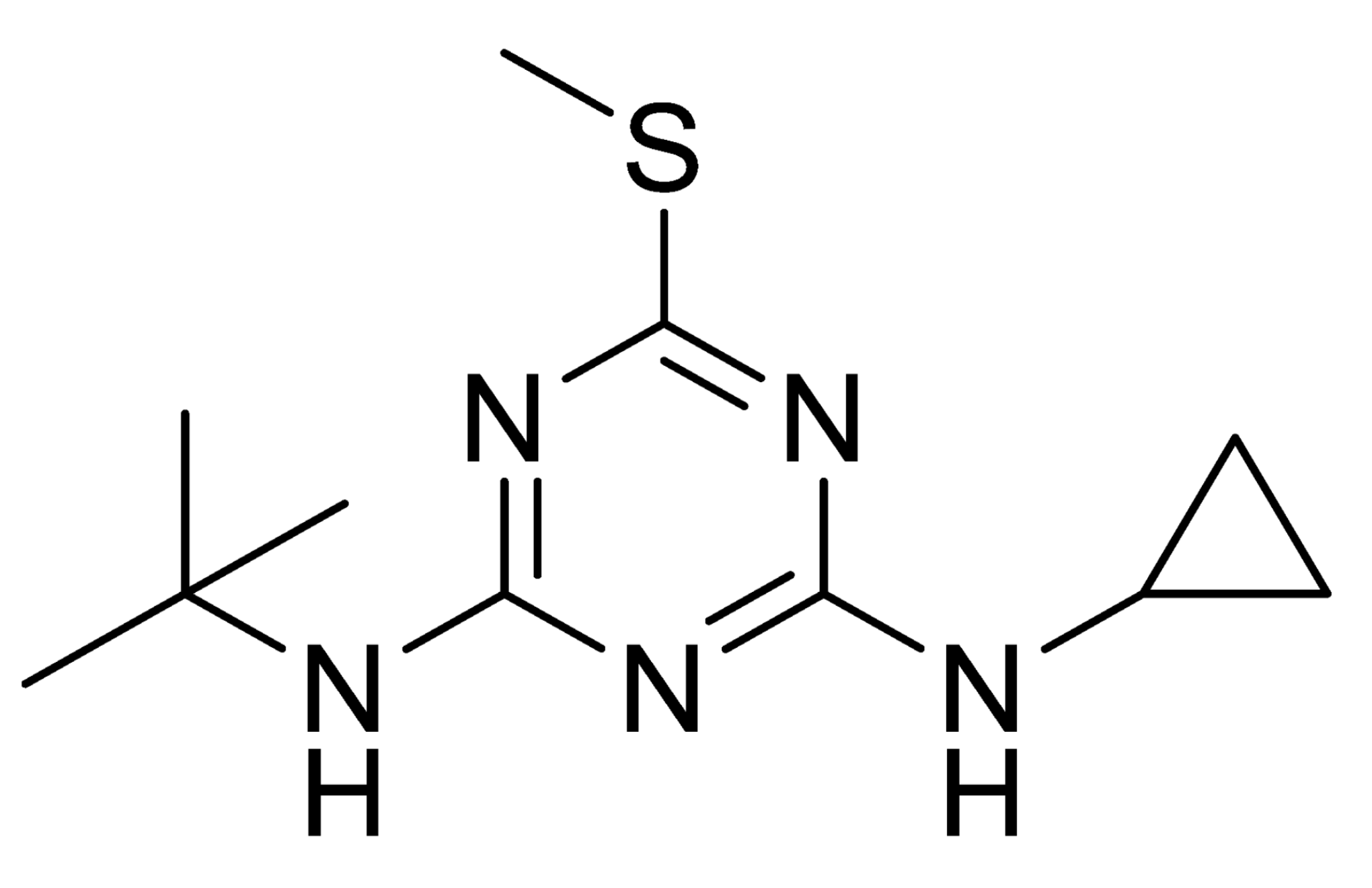 | 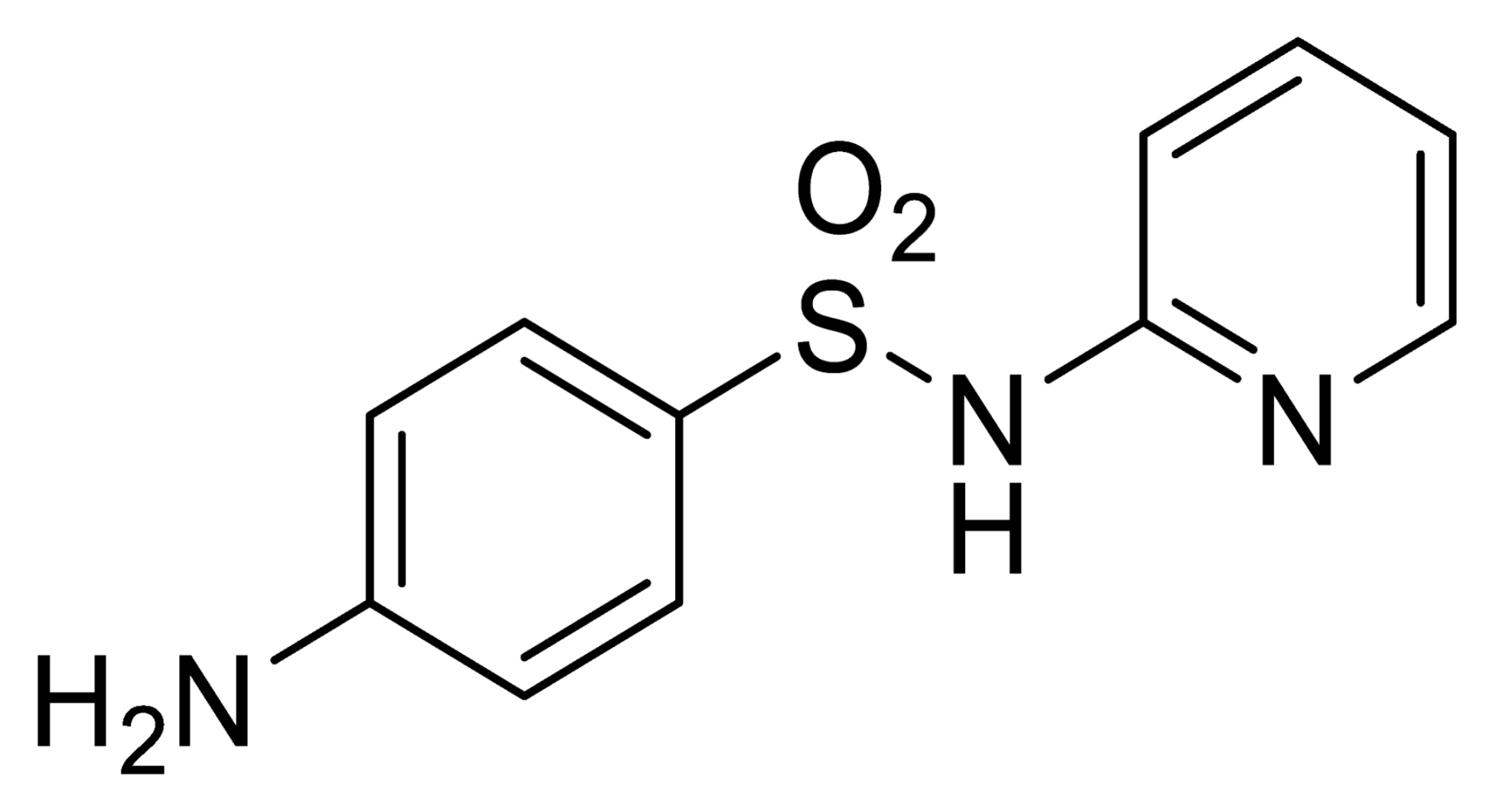 |  | 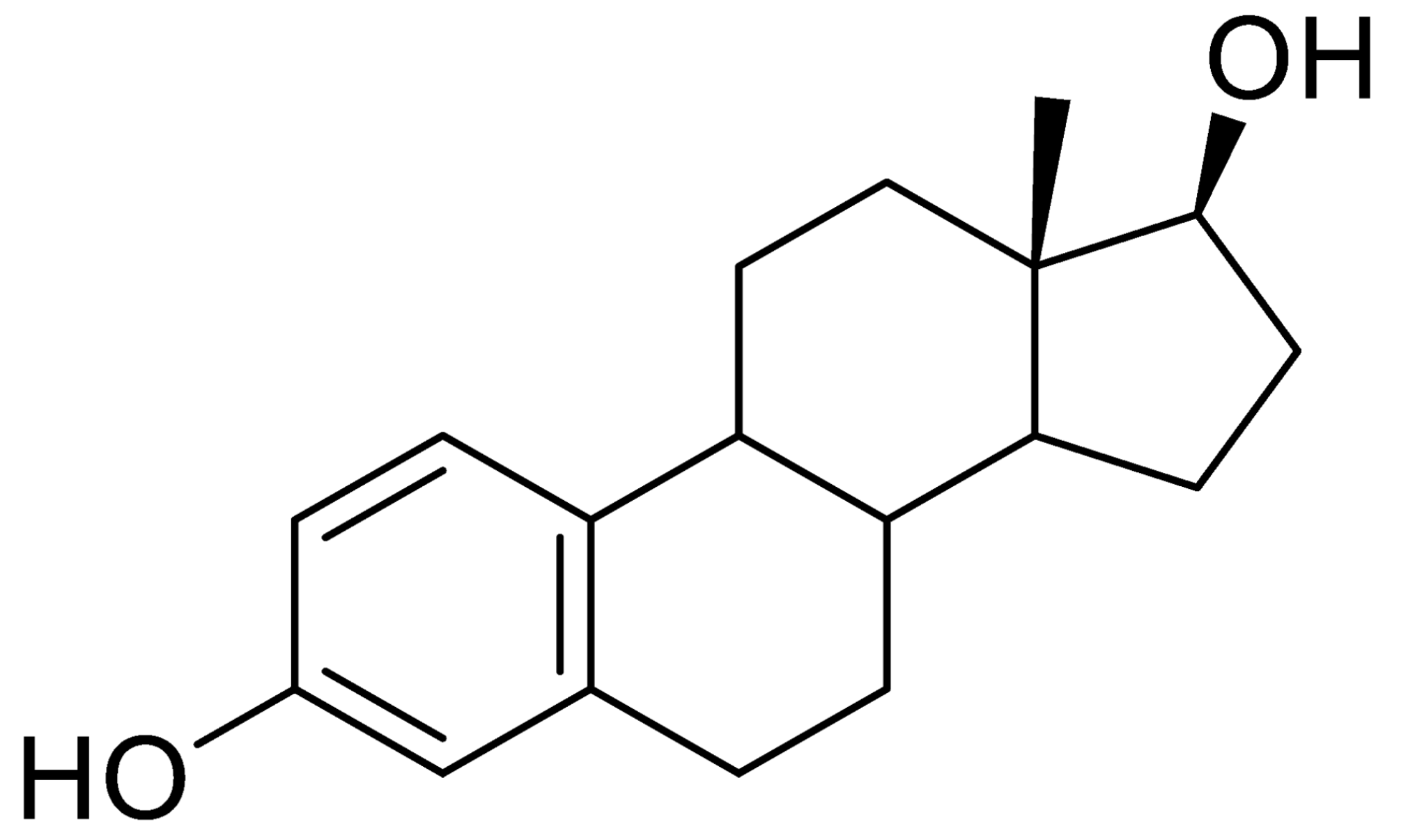 |
| Anti-serum | As87 | As155 | As226 | R64 |
| As dilution | 1/2000 | 1/2000 | 1/2000 | 1/1000 |
| Coating antigen (CA) | 4e-BSA | SA2-BSA | CA6-BSA | 6E2_4-BSA |
| (CA), µg/mL | 10 | 25 | 10 | 50 |
| Reference | [28] | [30] | - | - |
| ELISA [11] *1 | Microarray [31] *1 | SPE 1x Format [32] *2 | SPE 8× Format *1 | Multianalyte SPE 8× Format *2 | ELISA [11] | Microarray [31] | Individual SPE 8× Format | Multianalyte SPE 8× Format | |
|---|---|---|---|---|---|---|---|---|---|
| Irgarol 1051 | Sulfapyridine | ||||||||
| Signalmax | 100.4 ± 0.4 | 100.6 ± 2.1 | 95.42 ± 4.40 | 92.2 ± 6.0 | 97.3 ± 6.5 | 100.4 ± 0.6 | 97.1 ± 5.1 | 90.9 ± 10.0 | 91.3 ± 4.3 |
| Signalmin | 2.9 ± 0.4 | 3.1 ± 1.9 | −2.45 ± 4.78 | −0.6 ± 5.2 | 23.3 ± 8.3 | 2.7 ± 1.2 | −2.5 ± 6.4 | −0.22 ± 8.4 | 9.9 ± 7.0 |
| Slope | −0.98 ± 0.22 | −1.45 ± 0.19 | −0.81 ± 0.16 | −1.07 ± 0.26 | −5.01 ± 4.9 | −0.784 ± 0.09 | −1.14 ± 0.36 | −0.93 ± 0.37 | −3.50 ± 1.51 |
| IC50, nM | 0.58 ± 0.19 | 2.29 ± 1.11 | 4.12 ± 1.31 | 4.77 ± 1.27 | 19.89 ± 1.16 | 6.58 ± 1.73 | 15.23 ± 1.34 | 5.65 ± 1.57 | 13.85 ± 1.20 |
| IC50 µg/L | 0.145 ± 0.05 | 0.579 ± 0.28 | 1.04 ± 0.33 | 1.20 ± 0.23 | 5.04 ± 0.29 | 1.43 ± 0.23 | 3.79 ± 0.33 | 1.40 ± 0.39 | 3.45 ± 0.29 |
| LOD µg/L | 0.012 ± 0.007 | 0.135 | 0.038 ± 0.022 | 0.037 | 3.24 | 0.08 ± 0.02 | 0.397 | 0.009 | 1.08 |
| LOQ µg/L | - | - | 0.24 ± 0.23 | 0.207 | 3.97 | - | - | 0.16 | 2.05 |
| R2 | 0.999 | 0.997 | 0.98 | 0.85 | 0.77 | 0.999 | 0.983 | 0.77 | 0.89 |
| Chloramphenicol | Estradiol | ||||||||
| Signalmax | 102.6 ± 8.8 | 98.8 ± 2.8 | - | 98.2 ± 3.8 | 97.2 ± 6.5 | 99.8 ± 0.5 | 97.7 ± 1.7 | 89.9 ± 6.7 | 96.1 ± 4.3 |
| Signalmin | 8.8 ± 1.2 | 28.7 ± 3.9 | - | 1.5 ± 3.4 | 20.0 ± 10.5 | 0.6 ± 0.7 | −0.05 ± 2.39 | 0.45 ± 8.2 | 20.4 ± 6.5 |
| Slope | −0.60 ± 0.05 | −1.18 ± 0.32 | - | −1.30 ± 0.19 | −2.86 ± 1.99 | −1.01 ± 0.07 | −1.23 ± 0.15 | −1.32 ± 0.62 | −9.56 ± 4.3 |
| IC50, nM | 0.59 ± 0.05 | 10.25 ± 1.28 | - | 3.47 ± 1.15 | 12.91 ± 1.36 | 4.01 ± 0.74 | 9.67 ± 1.11 | 35.4 ± 1.36 | 21.83 ± 1.05 |
| IC50 µg/L | 0.192 ± 0.09 | 3.00 ± 0.41 | - | 1.12 ± 0.37 | 4.17 ± 0.44 | 1.09 ± 0.20 | 2.63 ± 0.30 | 9.72 ± 0.37 | 5.94 ± 0.28 |
| LOD µg/L | 0.004 ± 0.01 | 0.453 | - | 0.18 | 1.89 | 0.11 ± 0.03 | 0.362 | 0.41 | 4.60 |
| LOQ µg/L | - | - | - | 0.36 | 2.70 | - | - | 1.93 | 5.18 |
| R2 | 0.997 | 0.988 | - | 0.94 | 0.72 | 0.999 | 0.998 | 0.90 | 0.82 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador, J.-P.; Kopper, K.; Miti, A.; Sanchis, A.; Marco, M.-P. Multiplexed Immunosensor Based on the Amperometric Transduction for Monitoring of Marine Pollutants in Sea Water. Sensors 2020, 20, 5532. https://doi.org/10.3390/s20195532
Salvador J-P, Kopper K, Miti A, Sanchis A, Marco M-P. Multiplexed Immunosensor Based on the Amperometric Transduction for Monitoring of Marine Pollutants in Sea Water. Sensors. 2020; 20(19):5532. https://doi.org/10.3390/s20195532
Chicago/Turabian StyleSalvador, J.-Pablo, Klaudia Kopper, Andrea Miti, Ana Sanchis, and M.-Pilar Marco. 2020. "Multiplexed Immunosensor Based on the Amperometric Transduction for Monitoring of Marine Pollutants in Sea Water" Sensors 20, no. 19: 5532. https://doi.org/10.3390/s20195532
APA StyleSalvador, J.-P., Kopper, K., Miti, A., Sanchis, A., & Marco, M.-P. (2020). Multiplexed Immunosensor Based on the Amperometric Transduction for Monitoring of Marine Pollutants in Sea Water. Sensors, 20(19), 5532. https://doi.org/10.3390/s20195532







