Biomechanical Signals of Varied Modality and Location Contribute Differently to Recognition of Transient Locomotion
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Data Collection
2.2. Signal Processing and Recognition Performance
3. Results
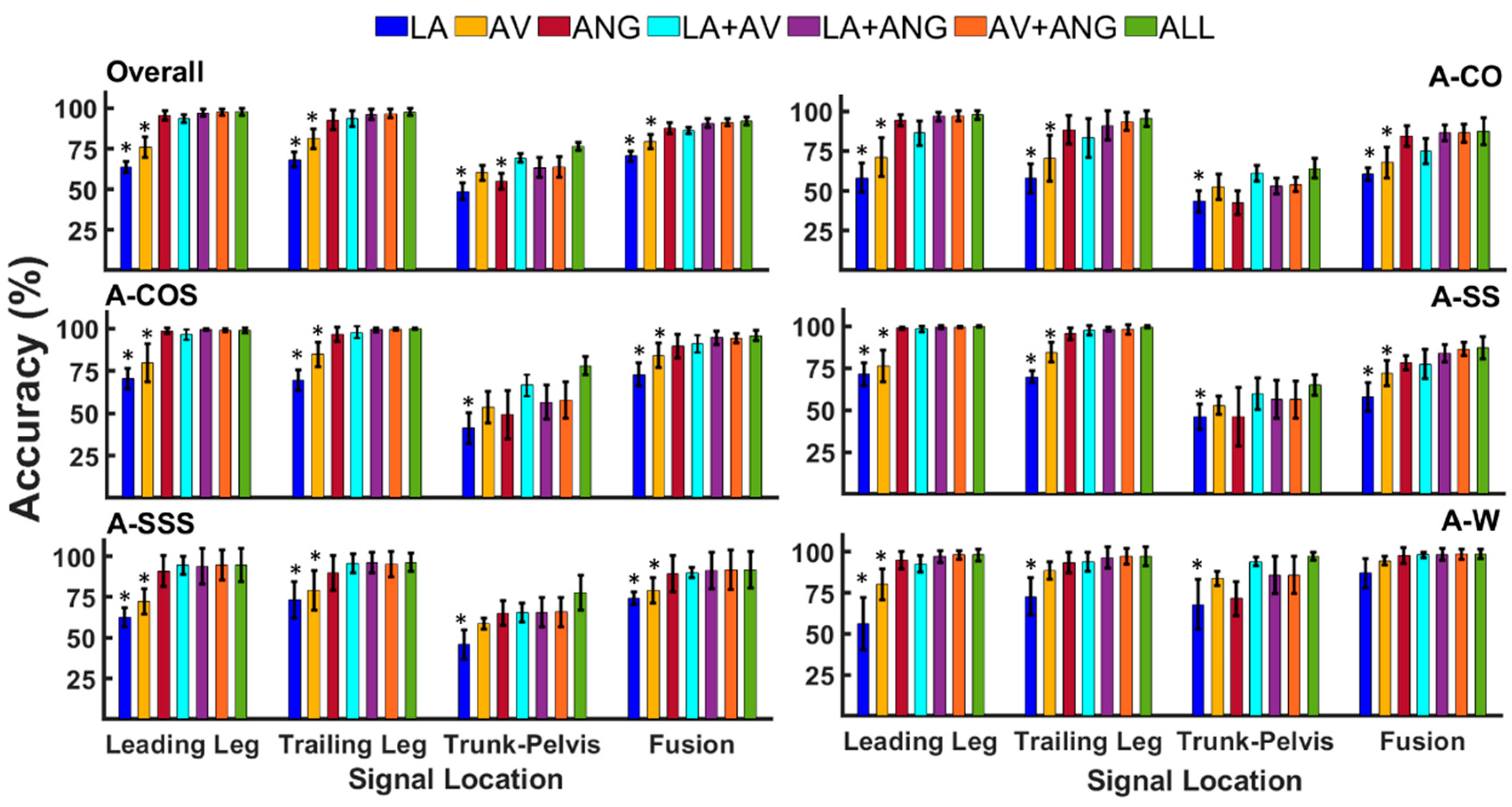
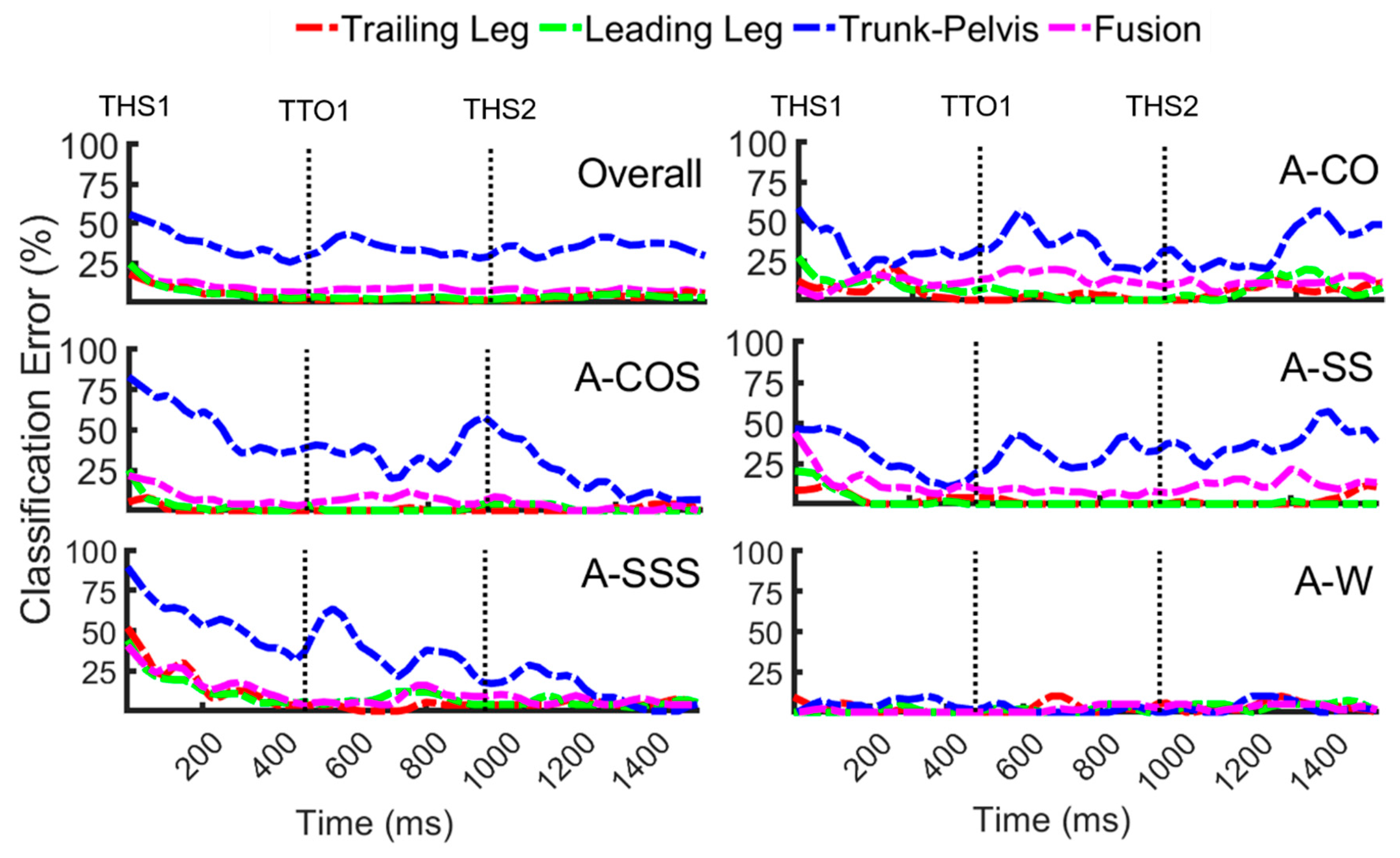
3.1. Classification of Anticipated Locomotor Tasks
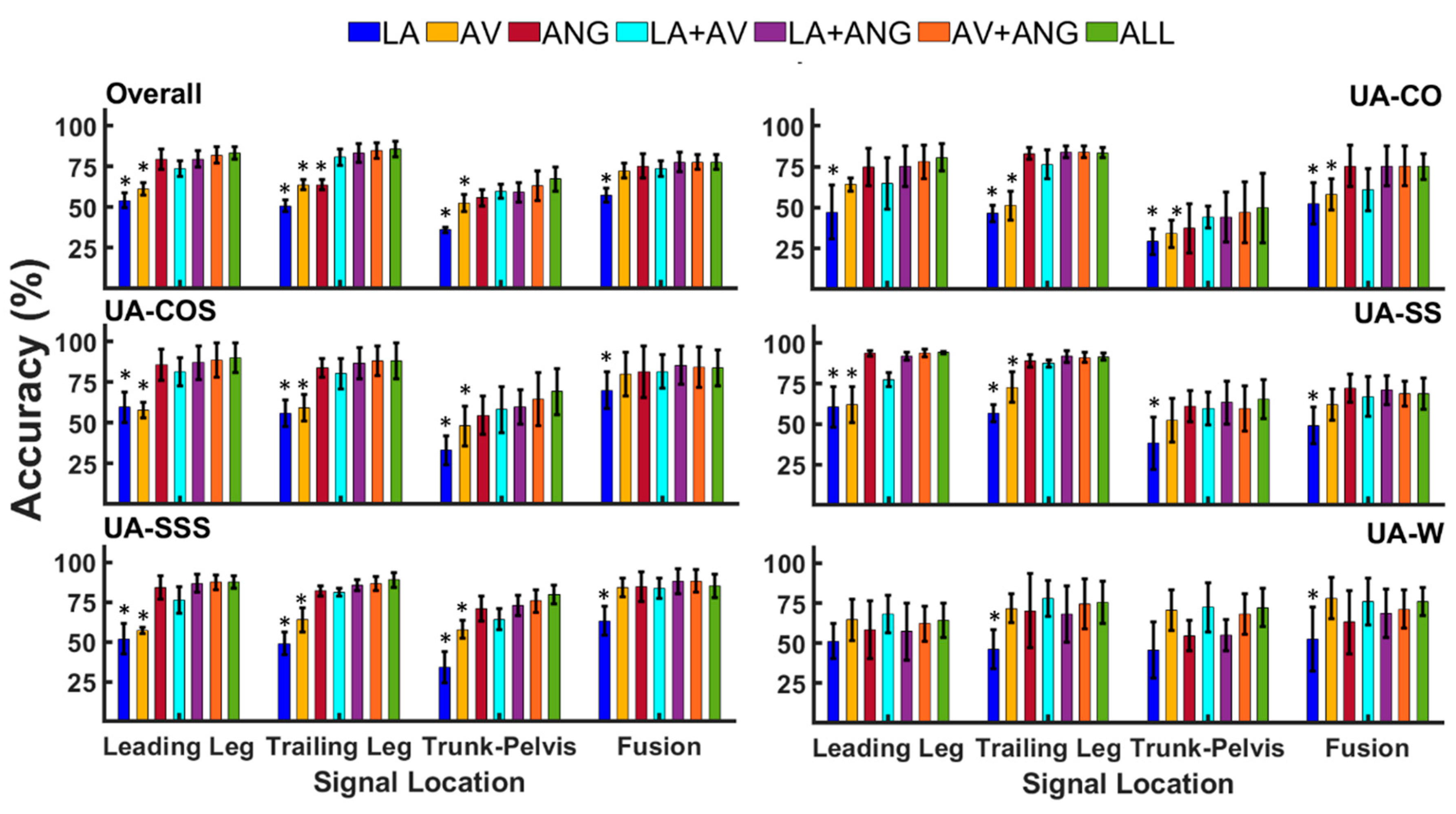
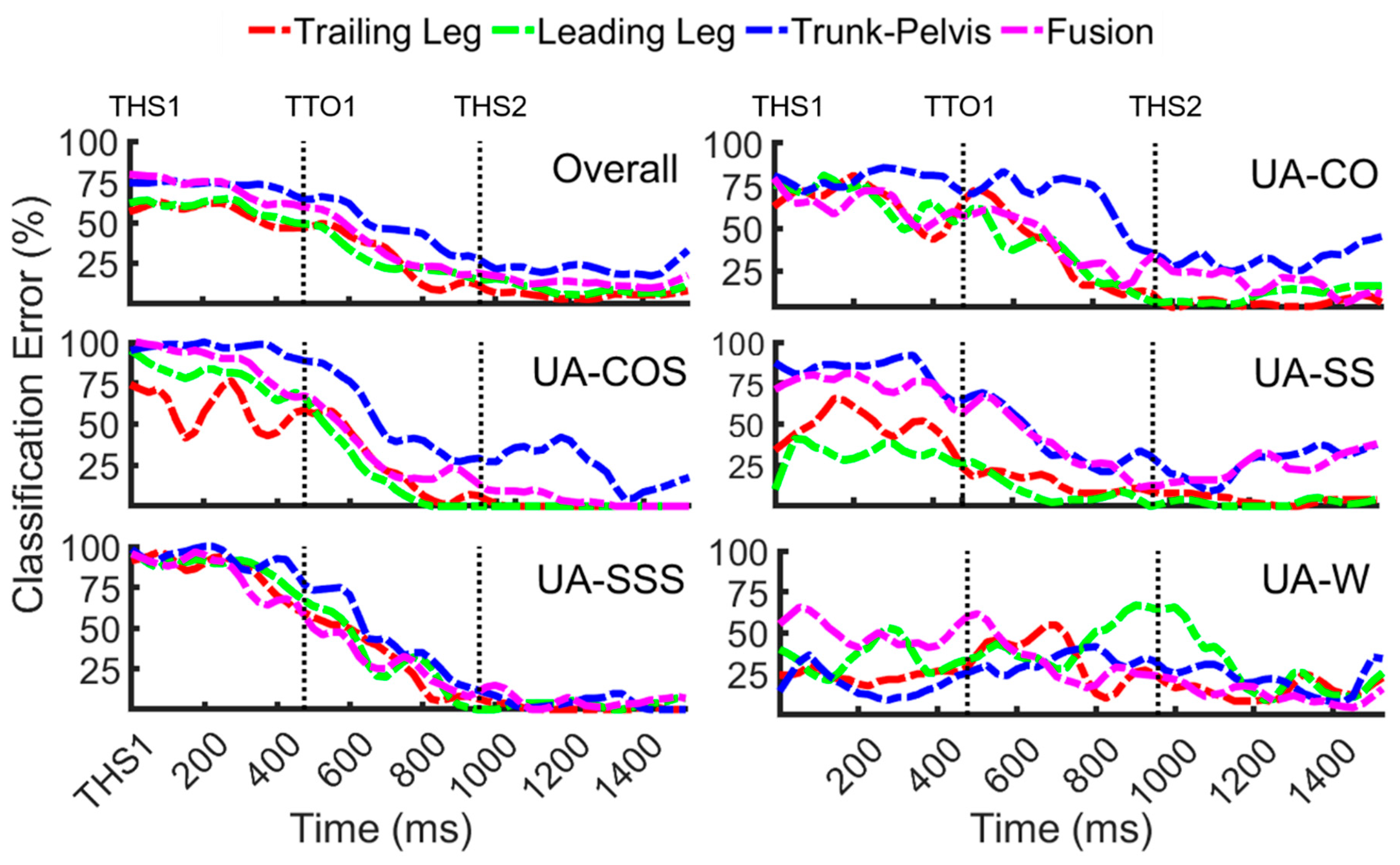
3.2. Classification of Unanticipated Locomotor Tasks
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dietz, V. Spinal cord pattern generators for locomotion. Clin. Neurophysiol. 2003, 114, 1379–1389. [Google Scholar] [CrossRef]
- Mayo, N.E.; Wood-Dauphinee, S.; Ahmed, S.; Gordon, C.; Higgins, J.; McEwen, S.; Salbach, N. Disablement following stroke. Disabil. Rehabil. 1999, 21, 258–268. [Google Scholar] [CrossRef]
- Fuhrer, M.J.; Rintala, D.H.; Hart, K.A.; Clearman, R.; Young, M.E. Relationship of life satisfaction to impairment, disability, and handicap among persons with spinal cord injury living in the community. Arch. Phys. Med. Rehabil. 1992, 73, 552–557. [Google Scholar]
- Davies, B.; Datta, D. Mobility outcome following unilateral lower limb amputation. Prosthet. Orthot. Int. 2003, 27, 186–190. [Google Scholar] [CrossRef]
- Young, A.J.; Ferris, D.P. Analysis of State of the Art and Future Directions for Robotic Exoskeletons. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 171–182. [Google Scholar] [CrossRef]
- Farris, R.J.; Quintero, H.A.; Goldfarb, M. Preliminary evaluation of a powered lower limb orthosis to aid walking in paraplegic individuals. IEEE Trans. Neural Syst. Rehabil. Eng. 2011, 19, 652–659. [Google Scholar] [CrossRef]
- Varol, H.A.; Sup, F.; Goldfarb, M. Multiclass real-time intent recognition of a powered lower limb prosthesis. IEEE Trans. Biomed. Eng. 2010, 57, 542–551. [Google Scholar] [CrossRef]
- Jiménez-Fabián, R.; Verlinden, O. Review of control algorithms for robotic ankle systems in lower-limb orthoses, prostheses, and exoskeletons. Med. Eng. Phys. 2012, 34, 397–408. [Google Scholar] [CrossRef]
- Hargrove, L.J.; Young, A.J.; Simon, A.M.; Fey, N.P.; Lipschutz, R.D.; Finucane, S.B.; Halsne, E.G.; Ingraham, K.A.; Kuiken, T.A. Intuitive Control of a Powered Prosthetic Leg During Ambulation. JAMA 2015, 313, 2244. [Google Scholar] [CrossRef]
- Young, A.J.; Simon, A.M.; Fey, N.P.; Hargrove, L.J. Intent recognition in a powered lower limb prosthesis using time history information. Ann. Biomed. Eng. 2014, 42, 631–641. [Google Scholar] [CrossRef]
- Kawamoto, H.; Sankai, Y. Power assist method based on Phase Sequence and muscle force condition for HAL. Adv. Robot. 2005, 19, 717–734. [Google Scholar] [CrossRef]
- Ottobock. C-Leg: Instructions for Use. 2015. Available online: https://shop.ottobock.us/media/pdf/647G1375-INT-02-1803w_en.pdf (accessed on 20 November 2019).
- Ottobock. C-Brace: Instructions for Use. 2016. Available online: https://shop.ottobock.us/media/pdf/647G631-EN-08-1602.pdf (accessed on 20 November 2019).
- Vallery, H.; van Asseldonk, E.H.F.; Buss, M.; van der Kooij, H. Reference Trajectory Generation for Rehabilitation Robots: Complementary Limb Motion Estimation. IEEE Trans. Neural Syst. Rehabil. Eng. 2009, 17, 23–30. [Google Scholar] [CrossRef]
- Vallery, H.; Burgkart, R.; Hartmann, C.; Mitternacht, J.; Riener, R.; Buss, M. Complementary limb motion estimation for the control of active knee prostheses. Biomed. Tech. 2011, 56, 45–51. [Google Scholar] [CrossRef][Green Version]
- Patla, A.E. Strategies for Dynamic Stability During Adaptive Human Locomotion. IEEE Eng. Med. Biol. Mag. 2003, 22, 48–52. [Google Scholar] [CrossRef]
- Houck, J. Muscle activation patterns of selected lower extremity muscles during stepping and cutting tasks. J. Electromyogr. Kinesiol. 2003, 13, 545–554. [Google Scholar] [CrossRef]
- Meinerz, C.M.; Malloy, P.; Geiser, C.F.; Kipp, K. Anticipatory effects on lower extremity neuromechanics during a cutting task. J. Athl. Train. 2015, 50, 905–913. [Google Scholar] [CrossRef]
- Li, W.; Pickle, N.T.; Fey, N.P. Time evolution of frontal plane dynamic balance during locomotor transitions of altered anticipation and complexity. J. NeuroEngineering Rehabil. 2020, 17, 100. [Google Scholar] [CrossRef]
- Wu, M.; Matsubara, J.H.; Gordon, K.E.; Reddy, H. General and specific strategies used to facilitate locomotor maneuvers. PLoS ONE 2015, 10, e0132707. [Google Scholar] [CrossRef]
- Peng, J.; Fey, N.P.; Kuiken, T.A.; Hargrove, L.J. Anticipatory kinematics and muscle activity preceding transitions from level-ground walking to stair ascent and descent. J. Biomech. 2016, 49, 528–536. [Google Scholar] [CrossRef]
- Young, A.J.; Simon, A.M.; Hargrove, L.J. A training method for locomotion mode prediction using powered lower limb prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2014, 22, 671–677. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, F.; Hargrove, L.J.; Dou, Z.; Rogers, D.R.; Englehart, K.B. Continuous Locomotion-Mode Identification for Prosthetic Legs Based on Neuromuscular–Mechanical Fusion. IEEE Trans. Biomed. Eng. 2011, 58, 2867–2875. [Google Scholar] [CrossRef]
- Kazemimoghadam, M.; Li, W.; Fey, N.P. Continuous Classification of Locomotor Transitions Performed Under Altered Cutting Style, Complexity and Anticipation. In Proceedings of the 2018 7th IEEE International Conference on Biomedical Robotics and Biomechatronics (Biorob), Enschede, The Netherlands, 26–29 August 2018; pp. 972–977. [Google Scholar]
- Lakens, D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013, 4, 1–12. [Google Scholar] [CrossRef]
- Chung, S.; Lim, J.; Noh, K.J.; Kim, G.; Jeong, H. Sensor data acquisition and multimodal sensor fusion for human activity recognition using deep learning. Sensors 2019, 19, 1716. [Google Scholar] [CrossRef]
- Lester, J.; Choudhury, T.; Borriello, G. A practical approach to recognizing physical activities. In Proceedings of the International Conference on Pervasive Computing, Dublin, Ireland, 7–10 May 2006; pp. 1–16. [Google Scholar]
- Hu, B.; Rouse, E.; Hargrove, L. Fusion of Bilateral Lower-Limb Neuromechanical Signals Improves Prediction of Locomotor Activities. Front. Robot AI 2018, 5, 1–16. [Google Scholar] [CrossRef]
- Jain, A.K.; Duin, R.P.W.; Mao, J. Statistical pattern recognition: A review. IEEE Trans. Pattern Anal. Mach. Intell. 2000, 22, 4–37. [Google Scholar] [CrossRef]
- Mathie, M.J.; Celler, B.G.; Lovell, N.H.; Coster, A.C.F. Classification of basic daily movements using a triaxial accelerometer. Med. Biol. Eng. Comput. 2004, 42, 679–687. [Google Scholar] [CrossRef]
- Ravi, N.; Dandekar, N.; Mysore, P.; Littman, M.L. Activity recognition from accelerometer data. In Proceedings of the Seventeenth Conference on Innovative Applications of Artificial Intelligence, San Francisco, CA, USA, 6–9 February 2005; pp. 1541–1546. [Google Scholar]
- Rábago, C.A.; Wilken, J.M. The Prevalence of Gait Deviations in Individuals With Transtibial Amputation. Mil. Med. 2016, 181, 30–37. [Google Scholar] [CrossRef]
- Theodoridis, S.; Koutroumbas, K. Pattern Recognition, 4th ed.; Academic Press, Inc.: Orlando, FL, USA, 2008. [Google Scholar]
- Hak, L.; Houdijk, H.; Steenbrink, F.; Mert, A.; van der Wurff, P.; Beek, P.J.; van Dieën, J.H. Stepping strategies for regulating gait adaptability and stability. J. Biomech. 2013, 46, 905–911. [Google Scholar] [CrossRef]
- Hak, L.; Houdijk, H.; van der Wurff, P.; Prins, M.R.; Mert, A.; Beek, P.J.; Van Dieën, J.H. Stepping strategies used by post-stroke individuals to maintain margins of stability during walking. Clin. Biomech. 2013, 28, 1041–1048. [Google Scholar] [CrossRef]
- Mayagoitia, R.E.; Nene, A.V.; Veltink, P.H. Accelerometer and rate gyroscope measurement of kinematics: An inexpensive alternative to optical motion analysis systems. J. Biomech. 2002, 35, 537–542. [Google Scholar] [CrossRef]
- Aziz, O.; Park, E.J.; Mori, G.; Robinovitch, S.N. Distinguishing the causes of falls in humans using an array of wearable tri-axial accelerometers. Gait Posture 2014, 39, 506–512. [Google Scholar] [CrossRef]
- Hansson, G.A.; Balogh, I.; Ohlsson, K.; Skerfving, S. Measurements of wrist and forearm positions and movements: Effect of, and compensation for, goniometer crosstalk. J. Electromyogr. Kinesiol. 2004, 14, 355–367. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z.Q.; Wu, J.K.; Wong, W.C.; Yu, H. Self-contained pedestrian tracking during normal walking using an inertial/magnetic sensor module. IEEE Trans. Biomed. Eng. 2014, 61, 892–899. [Google Scholar] [CrossRef]

| Signal Location | LA | AV | ANG | LA+AV | LA+ANG | AV+ANG | ALL | |
|---|---|---|---|---|---|---|---|---|
| Overall | Leading Leg | 63.8 (3.5) | 75.9 (6.3) | 95.5 (2.8) | 93.6 (2.4) | 97.2 (2.3) | 97.6 (2.05) | 97.8 (2.3) |
| Trailing Leg | 68.4 (4.6) | 81.1 (6.1) | 92.7 (6.1) | 93.5 (4.7) | 96.2 (3.1) | 96.7 (2.8) | 97.5 (2.2) | |
| Trunk-Pelvis | 48.8 (5) | 60.2 (4.5) | 54.8 (4.9) | 69.4 (2.5) | 63.4 (6.1) | 63.9 (6.2) | 76.4 (2.5) | |
| Fusion | 70.4 (3.2) | 79.4 (4.4) | 87.8 (3.4) | 86.3 (1.9) | 90.8 (2.6) | 91.3 (2.3) | 92 (2.4) | |
| A-CO | Leading Leg | 58.2 (9) | 71.1 (11.9) | 94.5 (3.5) | 86.2 (7.6) | 96.7 (2.6) | 97 (3.2) | 97.5 (2.8) |
| Trailing Leg | 57.7 (9) | 70.5 (14.6) | 88.3 (8.8) | 83.1 (12.1) | 91 (9) | 93.5 (5.6) | 95.2 (5) | |
| Trunk-Pelvis | 43.3 (6.7) | 52.3 (8) | 42.4 (7.5) | 61.1 (5) | 53 (5) | 54.1 (4.5) | 64 (6.2) | |
| Fusion | 60.4 (4) | 67.7 (9.5) | 84.4 (6.3) | 74.8 (7.8) | 86.2 (4.8) | 86.2 (5.6) | 87.2 (8.4) | |
| A-COS | Leading Leg | 70.5 (6.2) | 79.8 (11.2) | 98.7 (1.8) | 96.5 (3) | 99.3 (0.4) | 98.8 (0.9) | 99.1 (1.3) |
| Trailing Leg | 69.6 (6) | 84.9 (7.2) | 96.5 (4.1) | 97.7 (3.5) | 99.2 (1) | 99.6 (0.7) | 99.7 (0.4) | |
| Trunk-Pelvis | 41.3 (9) | 53.7 (9.3) | 49.1 (14) | 66.6 (6.2) | 56.5 (10) | 57.8 (10.5) | 78.1 (5.4) | |
| Fusion | 72.9 (6.7) | 84.1 (7.1) | 89.8 (7) | 91.1 (5.2) | 94.6 (4) | 94.3 (2.8) | 95.8 (3.1) | |
| A-SS | Leading Leg | 71.4 (6.8) | 76.4 (9.4) | 98.7 (0.7) | 98.3 (1.6) | 99.4 (0.8) | 99.4 (0.4) | 99.7 (0.5) |
| Trailing Leg | 69.8 (3.4) | 84.4 (5.9) | 95.5 (3.6) | 97.4 (2.7) | 98.1 (1.2) | 97.9 (2.6) | 99.4 (0.7) | |
| Trunk-Pelvis | 45.9 (7.4) | 52.7 (5.4) | 45.9 (17) | 59.7 (9.5) | 56.5 (11) | 56.2 (11) | 64.9 (6.2) | |
| Fusion | 57.7 (8.5) | 72.04 (7.5) | 78 (4.2) | 77.3 (8.6) | 83.6 (5.1) | 86.2 (4.1) | 86.9 (6.5) | |
| A-SSS | Leading Leg | 62.5 (5.6) | 72.1 (7.7) | 91 (9.6) | 94.4 (5.3) | 93.9 (10.9) | 94.7 (9) | 94.6 (10) |
| Trailing Leg | 73.16(11) | 79.16 (12) | 89.8 (10) | 95.7 (5.7) | 96.2 (6.1) | 95.2 (7.8) | 96.2 (5.6) | |
| Trunk-Pelvis | 45.7 (9.1) | 58.6 (3.5) | 65.2 (7.7) | 65.5 (5.8) | 65.5 (9) | 65.7 (9) | 77.6 (10) | |
| Fusion | 74 (3.9) | 78.9 (7.8) | 89.2 (11) | 90 (3) | 91.3 (11) | 91.5 (12) | 91.7 (11) | |
| A-W | Leading Leg | 56.2 (16) | 80.2 (9.4) | 94.8 (5.3) | 92.6 (4.8) | 97 (3.6) | 98 (2.7) | 98 (3.4) |
| Trailing Leg | 72.6 (11) | 88.3 (5.2) | 93.2 (6.2) | 93.7 (5.7) | 96.4 (6.4) | 97.3 (4.7) | 97.1 (5.7) | |
| Trunk-Pelvis | 68 (15) | 83.5 (4.2) | 71.4 (10) | 94 (2.7) | 85.7 (11) | 85.7 (11) | 97.3 (2.3) | |
| Fusion | 86.8 (9) | 94.5 (2.6) | 97.4 (4.8) | 98.15 (1.6) | 98.4 (3.5) | 98.5 (3.2) | 98.5 (3) |
| Signal Location | LA | AV | ANG | LA+AV | LA+ANG | AV+ANG | ALL | |
|---|---|---|---|---|---|---|---|---|
| Overall | Leading Leg | 54.1 (4.5) | 61.2 (3.7) | 79.4 (6.1) | 73.7 (4.7) | 79.6 (5.1) | 81.9 (5.1) | 83.3 (3.6) |
| Trailing Leg | 50.8 (3.5) | 63.8 (3.1) | 63.8 (3.1) | 80.7 (4.8) | 83.3 (5.7) | 84.6 (4.7) | 85.5 (4.7) | |
| Trunk-Pelvis | 36.1 (1.6) | 52.6 (5.2) | 55.8 (5.1) | 59.8 (4.5) | 59.1 (6) | 63 (9.1) | 67.2 (7.2) | |
| Fusion | 57.4 (4.3) | 72.4 (4.6) | 75.3 (7.4) | 73.8 (4.8) | 77.7 (5.8) | 77.6 (4.6) | 77.7 (4.4) | |
| UA-CO | Leading Leg | 47.2 (16) | 64.1 (3.8) | 75 (11.5) | 64.9 (15.7) | 75.2 (12.3) | 78 (10.1) | 80.7 (8.2) |
| Trailing Leg | 46.4 (5) | 51.4 (8.8) | 83 (3.5) | 76.5 (8.7) | 84 (3.5) | 84.1 (3.6) | 83.6 (3.1) | |
| Trunk-Pelvis | 29.1 (7.8) | 33.9 (8.3) | 37.4 (15) | 44.3 (6.8) | 44.3 (15) | 47 (18) | 49.8 (21) | |
| Fusion | 52.4 (12) | 57.9 (9.5) | 75.4 (12) | 60.9 (13) | 75.5 (12) | 75.4 (12) | 75.2 (7) | |
| UA-COS | Leading Leg | 59.6 (9.2) | 57.8 (4.6) | 85.8 (9.5) | 81.3 (8.7) | 87 (10.3) | 88.6 (10.6) | 89.8 (9.1) |
| Trailing Leg | 55.9 (8) | 59.2 (8.2) | 83.7 (5.8) | 80.4 (9.3) | 86.7 (9.5) | 88.1 (8.9) | 88.1 (11) | |
| Trunk-Pelvis | 33.3 (9) | 48.1 (12) | 54.6 (11) | 58.3 (14) | 59.7 (10) | 64.5 (16) | 69.2 (14) | |
| Fusion | 70 (11) | 79.9 (13) | 81.3 (15) | 81.6 (10) | 85.3 (11) | 84.4 (12) | 83.7 (11) | |
| UA-SS | Leading Leg | 60.5 (12) | 62.16 (11) | 93.6 (1.7) | 77.5 (4.3) | 92 (2.3) | 93.6 (2.3) | 94.1 (0.8) |
| Trailing Leg | 56.7 (5.3) | 72.7 (9.3) | 89.2 (3.8) | 87.4 (2) | 91.7 (3.7) | 91 (3.1) | 91.5 (2) | |
| Trunk-Pelvis | 38.2 (16) | 52.2 (13) | 61 (9.5) | 59.7 (10) | 63.2 (13) | 59.5 (13) | 65.3 (11) | |
| Fusion | 49.1 (11) | 61.9 (9.5) | 72.2 (8.6) | 66.9 (12) | 71 (8.8) | 68.8 (7.7) | 68.6 (9.5) | |
| UA-SSS | Leading Leg | 51.9 (9.7) | 57.2 (2) | 84.1 (7.5) | 76.3 (8.1) | 86.9 (5.5) | 87.4 (4.4) | 87.7 (4) |
| Trailing Leg | 49 (7) | 63.8 (7.7) | 82.2 (3.2) | 81.3 (2.5) | 85.6 (3.4) | 86.5 (4.4) | 89 (4.6) | |
| Trunk-Pelvis | 34.1 (9.9) | 57.8 (5.6) | 71.1 (7.9) | 64.2 (6.6) | 73 (6.3) | 75.6 (6.9) | 79.8 (5.9) | |
| Fusion | 63.1 (9) | 84.1 (5.8) | 84.7 (9.3) | 83.58 (6.3) | 88.15 (7.9) | 88.2 (7.1) | 85.2 (7.5) | |
| UA-W | Leading Leg | 51.2 (10) | 64.5 (12) | 58.4 (17) | 68.3 (11) | 57.3 (17) | 62.1 (11) | 64.2 (10) |
| Trailing Leg | 46.2 (12) | 71.7 (9) | 70.3 (23) | 77.8 (11) | 68.2 (17) | 74.5 (15) | 75.3 (13) | |
| Trunk-Pelvis | 45.7 (17) | 70.8 (12) | 54.7 (9.7) | 72.4 (15.5) | 55.1 (9.7) | 68.1(12.8) | 72.2 (11.8) | |
| Fusion | 52.5 (20) | 78 (13) | 63.1 (20) | 76 (15) | 68.7 (15) | 71.2 (12) | 75.8 (9) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazemimoghadam, M.; Fey, N.P. Biomechanical Signals of Varied Modality and Location Contribute Differently to Recognition of Transient Locomotion. Sensors 2020, 20, 5390. https://doi.org/10.3390/s20185390
Kazemimoghadam M, Fey NP. Biomechanical Signals of Varied Modality and Location Contribute Differently to Recognition of Transient Locomotion. Sensors. 2020; 20(18):5390. https://doi.org/10.3390/s20185390
Chicago/Turabian StyleKazemimoghadam, Mahdieh, and Nicholas P. Fey. 2020. "Biomechanical Signals of Varied Modality and Location Contribute Differently to Recognition of Transient Locomotion" Sensors 20, no. 18: 5390. https://doi.org/10.3390/s20185390
APA StyleKazemimoghadam, M., & Fey, N. P. (2020). Biomechanical Signals of Varied Modality and Location Contribute Differently to Recognition of Transient Locomotion. Sensors, 20(18), 5390. https://doi.org/10.3390/s20185390






