Analysis of Cattle Social Transitional Behaviour: Attraction and Repulsion
Abstract
1. Introduction
2. Experimental Design and Data Capture
2.1. Ethical Statement
2.2. Animals and Experimental Protocol
2.3. The Wireless Tracking Platform
3. Data Analyses
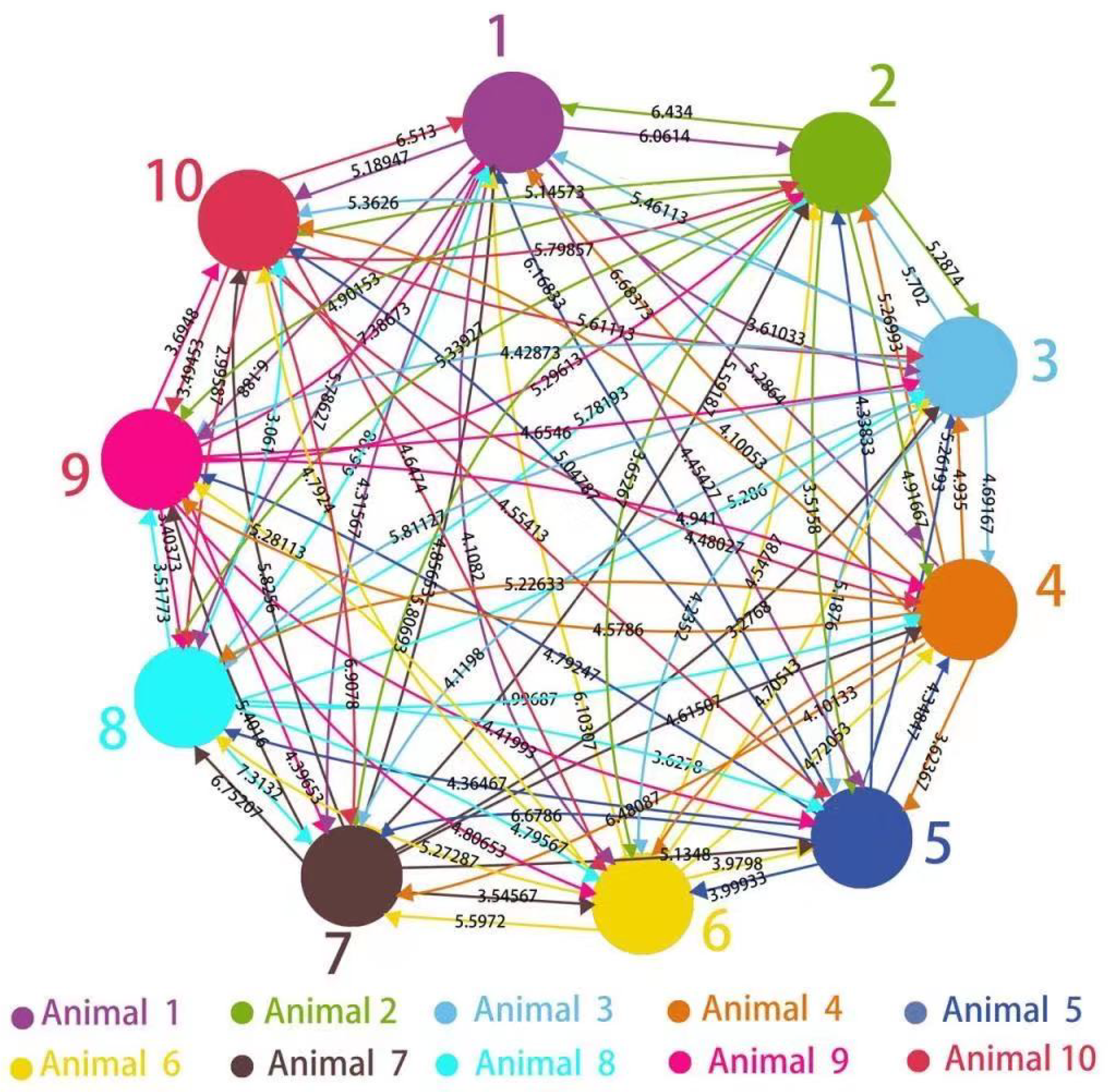
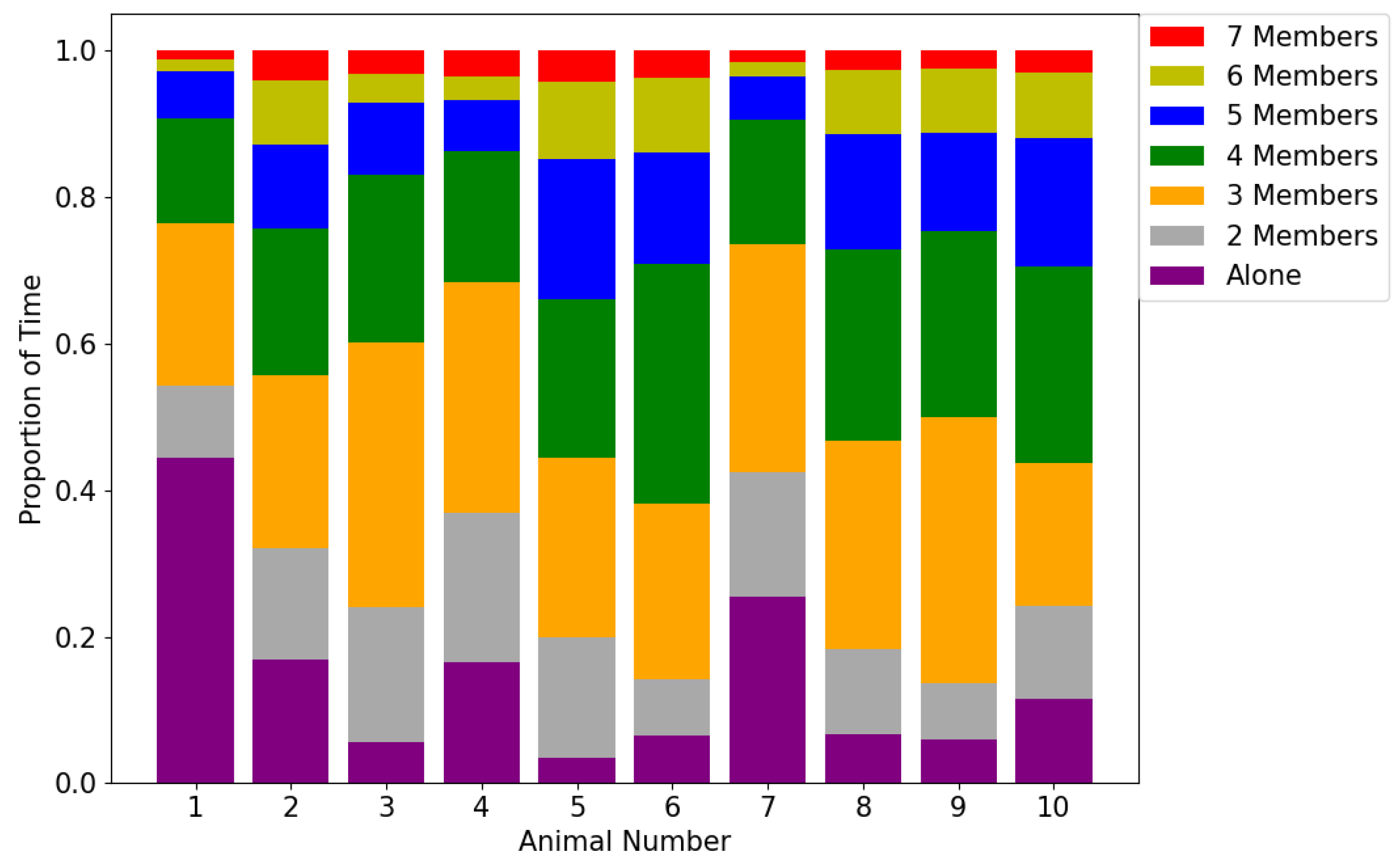
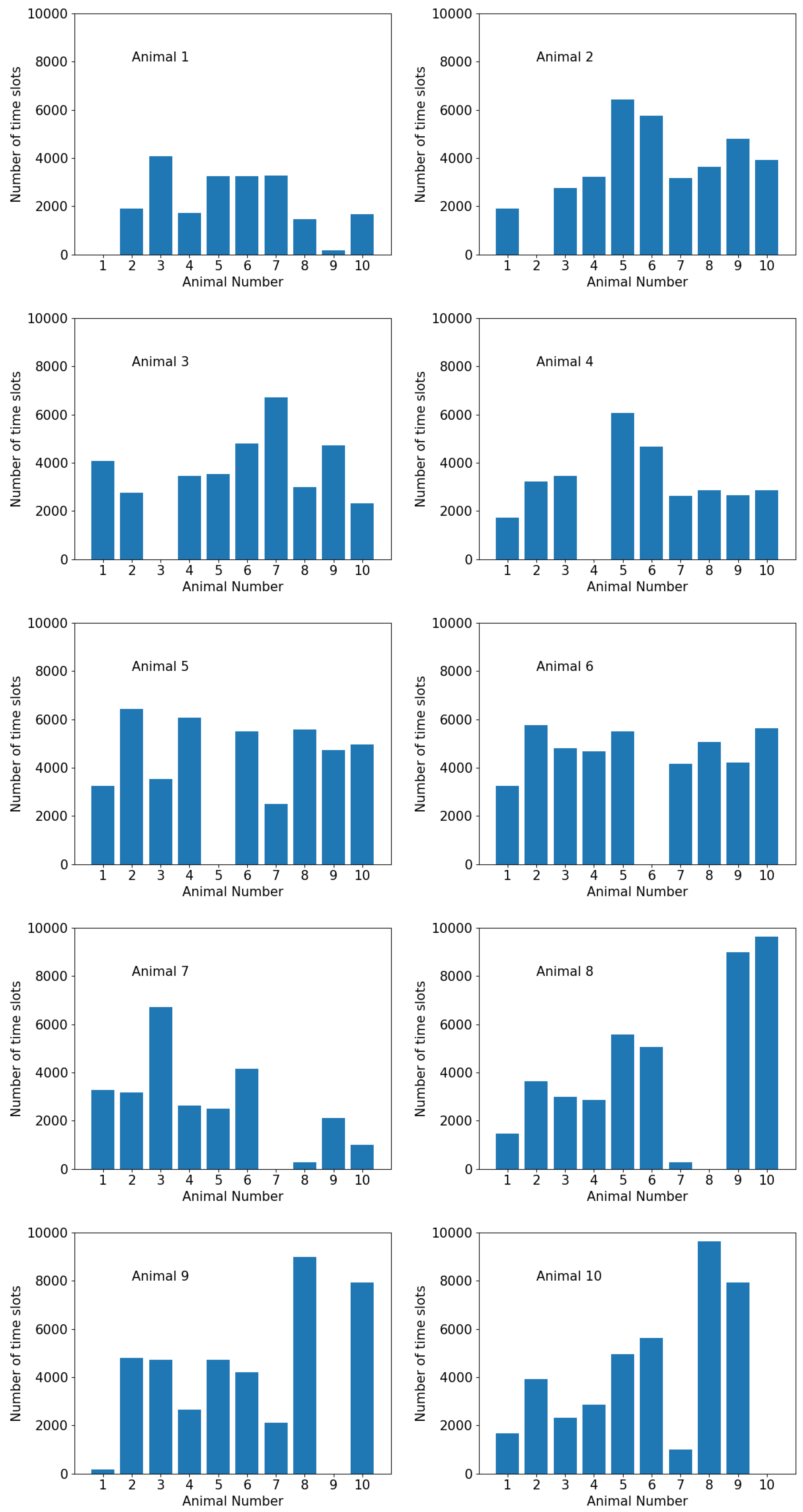
3.1. Graph-Theoretic Interpretation of Intra-Group Closeness
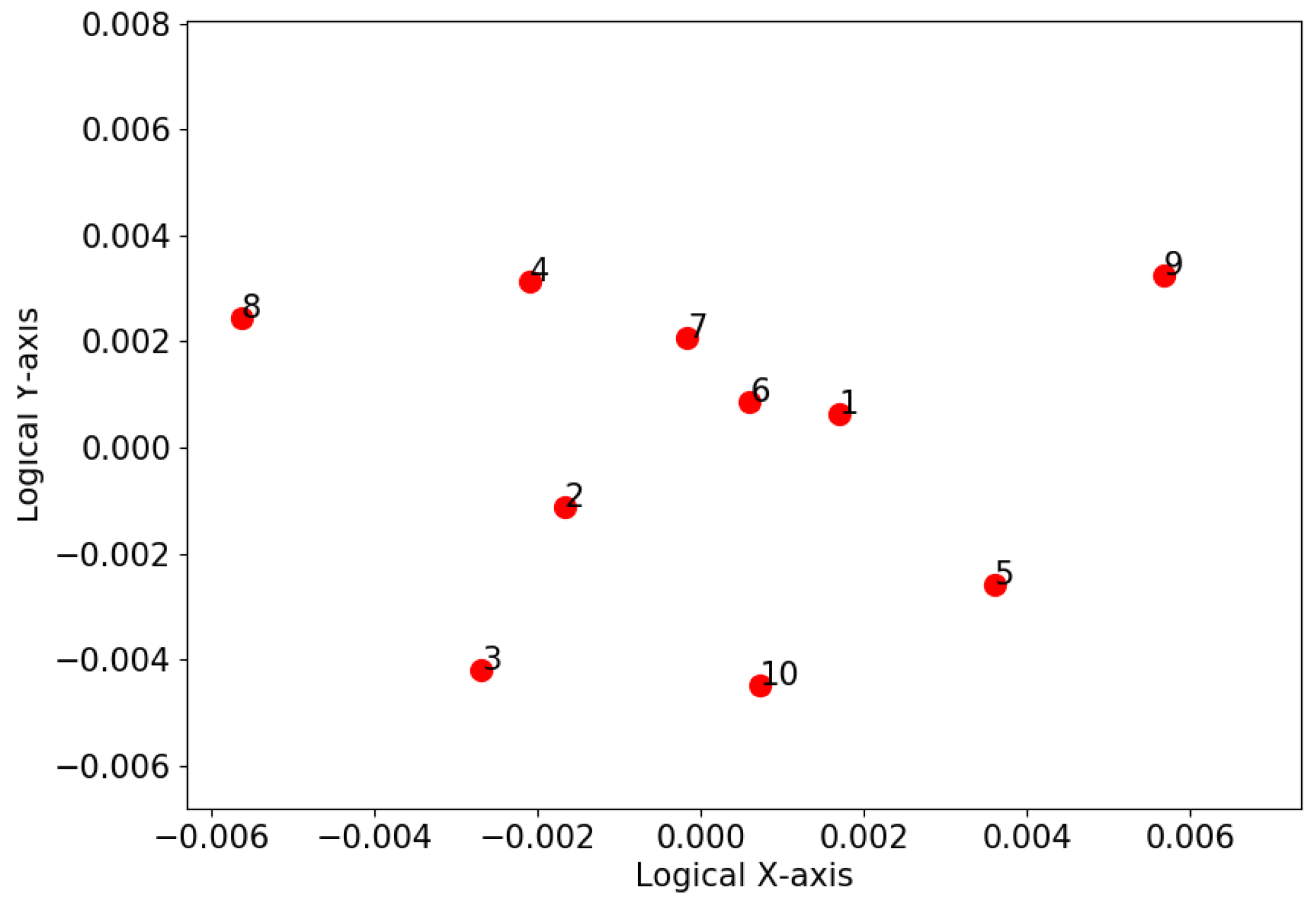
3.2. K-Means Clustering Based on Dimension Reduction
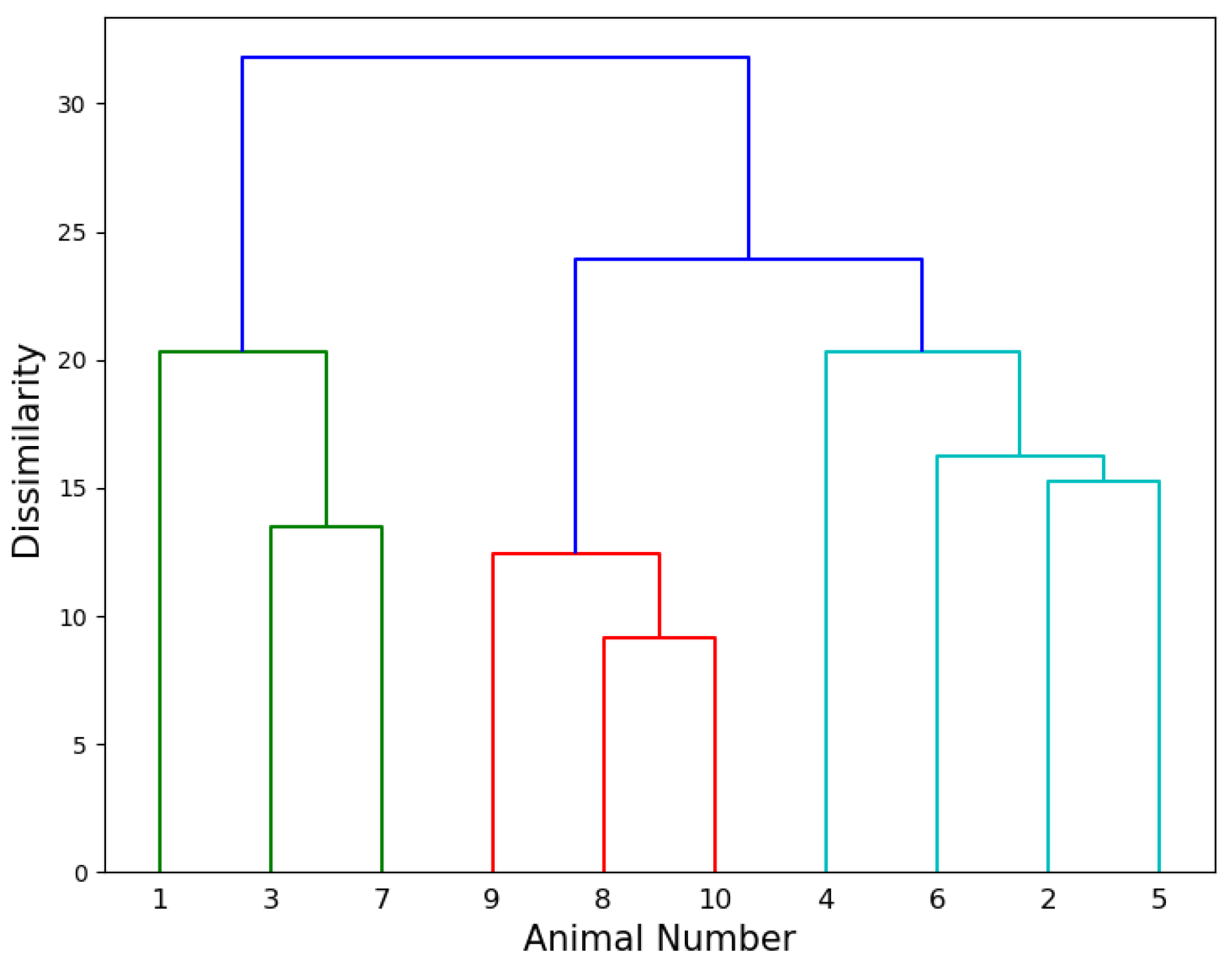
3.3. Agglomerative Hierarchical Clustering
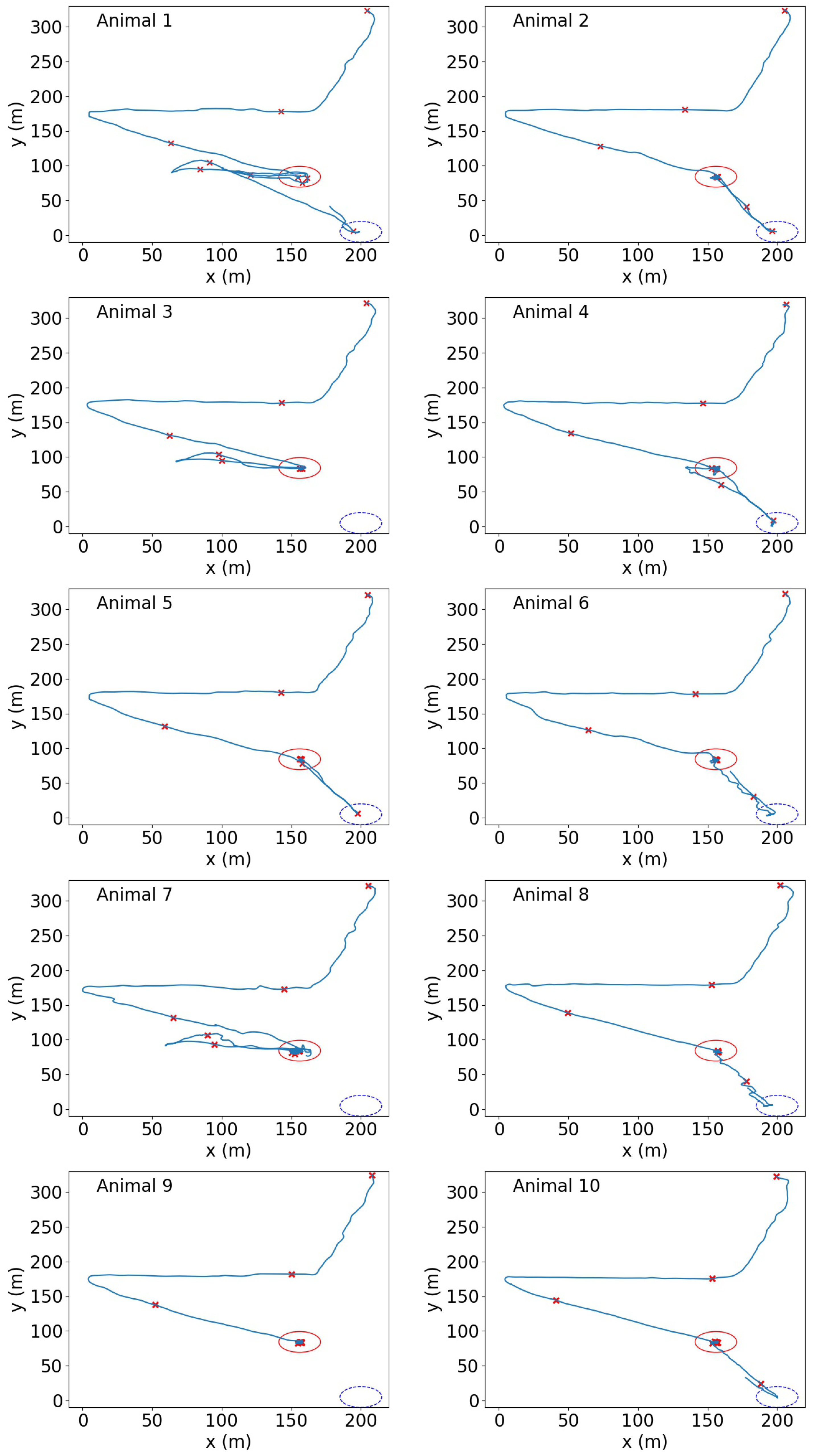
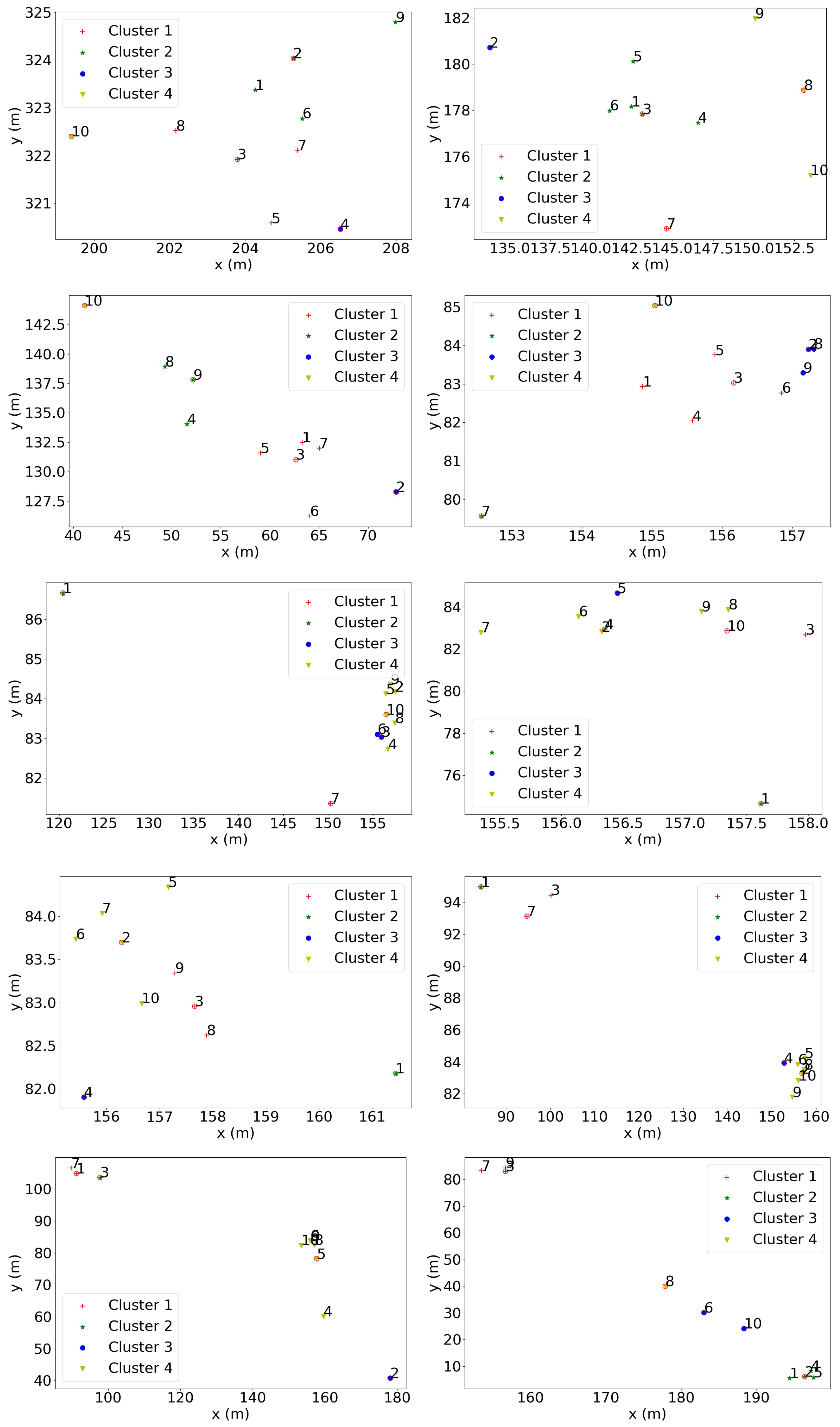
3.4. Herd Behaviour Based on Geo-Spatial Locations
4. Results
4.1. Captured Positional Data
4.2. Graph-Theoretic Interpretation of Intra-Group Closeness
4.3. K-Means Clustering Based on Dimension Reduction
4.4. Agglomerative Hierarchical Clustering
4.5. Herd Behaviour Based on Geo-Spatial Locations
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Koene, P.; Ipema, B. Social Networks and Welfare in Future Animal Management. Animals 2014, 4, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Foris, B.; Zebunke, M.; Langbein, J.; Melzer, N. Comprehensive analysis of affiliative and agonistic social networks in lactating dairy cattle groups. Appl. Anim. Behav. Sci. 2019, 210, 60–67. [Google Scholar] [CrossRef]
- Gygax, L.; Neisen, G.; Wechsler, B. Socio-Spatial Relationships in Dairy Cows. Ethology 2010, 116, 10–23. [Google Scholar] [CrossRef]
- Stephenson, M.B.; Bailey, D.W.; Jensen, D. Association patterns of visually-observed cattle on Montana, USA foothill rangelands. Appl. Anim. Behav. Sci. 2016, 178, 7–15. [Google Scholar] [CrossRef]
- Harris, N.R.; Johnson, D.E.; McDougald, N.K.; George, M.R. Social associations and dominance of individuals in small herds of cattle. Rangel. Ecol. Manag. 2007, 60, 339–349. [Google Scholar] [CrossRef]
- Sueur, C.; Kuntz, C.; Debergue, E.; Keller, B.; Robic, F.; Siegwalt-Baudin, F.; Richer, C.; Ramos, A.; Pelé, M. Leadership linked to group composition in Highland cattle (Bos taurus): Implications for livestock management. Appl. Anim. Behav. Sci. 2018, 198, 9–18. [Google Scholar] [CrossRef]
- Šárová, R.; Špinka, M.; Panamá, J.L.A.; Šimeček, P. Graded leadership by dominant animals in a herd of female beef cattle on pasture. Anim. Behav. 2010, 79, 1037–1045. [Google Scholar] [CrossRef]
- Llonch, P.; Mainau, E.; Ipharraguerre, I.R.; Bargo, F.; Tedó, G.; Blanch, M.; Manteca, X. Chicken or the Egg: The reciprocal association between feeding behavior and animal welfare and their impact on productivity in dairy cows. Front. Vet. Sci. 2018, 5, 305. [Google Scholar] [CrossRef]
- Weary, D.; Huzzey, J.; Von Keyserlingk, M. Board-invited review: Using behavior to predict and identify ill health in animals. J. Anim. Sci. 2009, 87, 770–777. [Google Scholar] [CrossRef]
- Rushen, J.; Chapinal, N.; de Passille, A.M. Automated monitoring of behavioural-based animal welfare indicators. Anim. Welf. 2012, 21, 339–350. [Google Scholar] [CrossRef]
- Borchers, M.; Chang, Y.; Tsai, I.; Wadsworth, B.; Bewley, J. A validation of technologies monitoring dairy cow feeding, ruminating, and lying behaviors. J. Dairy Sci. 2016, 99, 7458–7466. [Google Scholar] [CrossRef] [PubMed]
- Grinter, L.; Campler, M.; Costa, J. Technical note: Validation of a behavior-monitoring collar’s precision and accuracy to measure rumination, feeding, and resting time of lactating dairy cows. J. Dairy Sci. 2019, 102, 3487–3494. [Google Scholar] [CrossRef] [PubMed]
- Theurer, M.E.; Amrine, D.E.; White, B.J. Remote Noninvasive Assessment of Pain and Health Status in Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Dumont, B.; Boissy, A.; Achard, C.; Sibbald, A.; Erhard, H. Consistency of animal order in spontaneous group movements allows the measurement of leadership in a group of grazing heifers. Appl. Anim. Behav. Sci. 2005, 95, 55–66. [Google Scholar] [CrossRef]
- Guo, Y.; Poulton, G.; Corke, P.; Bishop-Hurley, G.; Wark, T.; Swain, D.L. Using accelerometer, high sample rate GPS and magnetometer data to develop a cattle movement and behaviour model. Ecol. Model. 2009, 220, 2068–2075. [Google Scholar] [CrossRef]
- Mufford, J.; Hill, D.; Flood, N.; Church, J. Use of unmanned aerial vehicles (UAVs) and photogrammetric image analysis to quantify spatial proximity in beef cattle. J. Unmanned Veh. Syst. 2019, 7. [Google Scholar] [CrossRef]
- Rocha, L.E.; Terenius, O.; Veissier, I.; Meunier, B.; Nielsen, P.P. Persistence of sociality in group dynamics of dairy cattle. Appl. Anim. Behav. Sci. 2020, 223, 104921. [Google Scholar] [CrossRef]
- Boyland, N.K.; Mlynski, D.T.; James, R.; Brent, L.J.; Croft, D.P. The social network structure of a dynamic group of dairy cows: From individual to group level patterns. Appl. Anim. Behav. Sci. 2016, 174, 1–10. [Google Scholar] [CrossRef]
- O’Neill, C.; Bishop-Hurley, G.; Williams, P.; Reid, D.; Swain, D. Using UHF proximity loggers to quantify male–female interactions: A scoping study of estrous activity in cattle. Anim. Reprod. Sci. 2014, 151, 1–8. [Google Scholar] [CrossRef]
- Swain, D.; Patison, K.; Heath, B.; Bishop-Hurley, G.; Finger, A. Pregnant cattle associations and links to maternal reciprocity. Appl. Anim. Behav. Sci. 2015, 168, 10–17. [Google Scholar] [CrossRef]
- Swain, D.L.; Bishop-Hurley, G.J. Using contact logging devices to explore animal affiliations: Quantifying cow–calf interactions. Appl. Anim. Behav. Sci. 2007, 102, 1–11. [Google Scholar] [CrossRef]
- Watson-Haigh, N.S.; O’Neill, C.J.; Kadarmideen, H.N. Proximity Loggers: Data Handling and Classification for Quality Control. IEEE Sens. J. 2012, 12, 1611–1617. [Google Scholar] [CrossRef]
- Porto, S.; Arcidiacono, C.; Giummarra, A.; Anguzza, U.; Cascone, G. Localisation and identification performances of a real-time location system based on ultra wide band technology for monitoring and tracking dairy cow behaviour in a semi-open free-stall barn. Comput. Electron. Agric. 2014, 108, 221–229. [Google Scholar] [CrossRef]
- Handcock, R.N.; Swain, D.L.; Bishop-Hurley, G.J.; Patison, K.P.; Wark, T.; Valencia, P.; Corke, P.; O’Neill, C.J. Monitoring animal behaviour and environmental interactions using wireless sensor networks, GPS collars and satellite remote sensing. Sensors 2009, 9, 3586–3603. [Google Scholar] [CrossRef] [PubMed]
- Valletta, J.J.; Torney, C.; Kings, M.; Thornton, A.; Madden, J. Applications of machine learning in animal behaviour studies. Anim. Behav. 2017, 124, 203–220. [Google Scholar] [CrossRef]
- McVey, C.; Hsieh, F.; Manriquez, D.; Pinedo, P.; Horback, K. Mind the Queue: A Case Study in Visualizing Heterogeneous Behavioral Patterns in Livestock Sensor Data Using Unsupervised Machine Learning Techniques. Front. Vet. Sci. 2020, 7, 523. [Google Scholar] [CrossRef]
- Morota, G.; Ventura, R.V.; Silva, F.F.; Koyama, M.; Fernando, S.C. Big data analytics and precision animal agriculture symposium: Machine learning and data mining advance predictive big data analysis in precision animal agriculture. J. Anim. Sci. 2018, 96, 1540–1550. [Google Scholar] [CrossRef]
- Neethirajan, S. The role of sensors, big data and machine learning in modern animal farming. Sens. Bio-Sens. Res. 2020, 29, 100367. [Google Scholar] [CrossRef]
- Liakos, K.; Busato, P.; Moshou, D.; Pearson, S.; Bochtis, D. Machine Learning in Agriculture: A Review. Sensors 2018, 18, 2674. [Google Scholar] [CrossRef]
- Rahman, A.; Smith, D.; Little, B.; Ingham, A.; Greenwood, P.; Bishop-Hurley, G. Cattle behaviour classification from collar, halter, and ear tag sensors. Inf. Process. Agric. 2018, 5, 124–133. [Google Scholar] [CrossRef]
- Dutta, R.; Smith, D.; Rawnsley, R.; Bishop-Hurley, G.; Hills, J.; Timms, G.; Henry, D. Dynamic cattle behavioural classification using supervised ensemble classifiers. Comput. Electron. Agric. 2015, 111, 18–28. [Google Scholar] [CrossRef]
- Pegorini, V.; Zen Karam, L.; Pitta, C.S.R.; Cardoso, R.; Da Silva, J.C.C.; Kalinowski, H.J.; Ribeiro, R.; Bertotti, F.L.; Assmann, T.S. In vivo pattern classification of ingestive behavior in ruminants using FBG sensors and machine learning. Sensors 2015, 15, 28456–28471. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Mac Parthaláin, N.; Brewer, P.; James, W.; Rose, M. A novel behavioral model of the pasture-based dairy cow from GPS data using data mining and machine learning techniques. J. Dairy Sci. 2016, 99, 2063–2075. [Google Scholar] [CrossRef] [PubMed]
- Borchers, M.; Chang, Y.; Proudfoot, K.; Wadsworth, B.; Stone, A.; Bewley, J. Machine-learning-based calving prediction from activity, lying, and ruminating behaviors in dairy cattle. J. Dairy Sci. 2017, 100, 5664–5674. [Google Scholar] [CrossRef] [PubMed]
- Taneja, M.; Byabazaire, J.; Jalodia, N.; Davy, A.; Olariu, C.; Malone, P. Machine learning based fog computing assisted data-driven approach for early lameness detection in dairy cattle. Comput. Electron. Agric. 2020, 171, 105286. [Google Scholar] [CrossRef]
- Higaki, S.; Miura, R.; Suda, T.; Andersson, L.M.; Okada, H.; Zhang, Y.; Itoh, T.; Miwakeichi, F.; Yoshioka, K. Estrous detection by continuous measurements of vaginal temperature and conductivity with supervised machine learning in cattle. Theriogenology 2019, 123, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Shahinfar, S.; Page, D.; Guenther, J.; Cabrera, V.; Fricke, P.; Weigel, K. Prediction of insemination outcomes in Holstein dairy cattle using alternative machine learning algorithms. J. Dairy Sci. 2014, 97, 731–742. [Google Scholar] [CrossRef]
- Schwager, M.; Anderson, D.M.; Butler, Z.; Rus, D. Robust classification of animal tracking data. Comput. Electron. Agric. 2007, 56, 46–59. [Google Scholar] [CrossRef]
- Rufener, C.; Berezowski, J.; Sousa, F.M.; Abreu, Y.; Asher, L.; Toscano, M.J. Finding hens in a haystack: Consistency of movement patterns within and across individual laying hens maintained in large groups. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Sibanda, T.Z.; Welch, M.; Schneider, D.; Kolakshyapati, M.; Ruhnke, I. Characterising Free-Range Layer Flocks Using Unsupervised Cluster Analysis. Animals 2020, 10, 855. [Google Scholar] [CrossRef]
- Sathyan, T.; Humphrey, D.; Hedley, M. WASP: A system and algorithms for accurate radio localization using low-cost hardware. IEEE Trans. Syst. Man Cybern. Part C (Appl. Rev.) 2010, 41, 211–222. [Google Scholar] [CrossRef]
- Lefebvre, T.; Bruyninckx, H.; Schutter, J.D. Kalman filters for non-linear systems: A comparison of performance. Int. J. Control 2004, 77, 639–653. [Google Scholar] [CrossRef]
- Wish, M.; Carroll, J.D. 14 Multidimensional scaling and its applications. Handb. Stat. 1982, 2, 317–345. [Google Scholar]
- Hughes, A.; Ruan, Y.; Ekanayake, S.; Bae, S.H.; Dong, Q.; Rho, M.; Qiu, J.; Fox, G. Interpolative multidimensional scaling techniques for the identification of clusters in very large sequence sets. BMC Bioinform. 2012, 13, S9. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Varshney, S. Analysis of k-means and k-medoids algorithm for big data. Procedia Comput. Sci. 2016, 78, 507–512. [Google Scholar] [CrossRef]
- Kodinariya, T.M.; Makwana, P.R. Review on determining number of Cluster in K-Means Clustering. Int. J. 2013, 1, 90–95. [Google Scholar]
- Kanungo, T.; Mount, D.M.; Netanyahu, N.S.; Piatko, C.D.; Silverman, R.; Wu, A.Y. An efficient k-means clustering algorithm: Analysis and implementation. IEEE Trans. Pattern Anal. Mach. Intell. 2002, 24, 881–892. [Google Scholar] [CrossRef]
- Sasirekha, K.; Baby, P. Agglomerative hierarchical clustering algorithm-a. Int. J. Sci. Res. Publ. 2013, 83, 83. [Google Scholar]
- Rani, Y.; Rohil, H. A study of hierarchical clustering algorithm. Int. J. Inf. Comput. Technol. 2013, 3, 1115–1122. [Google Scholar]
- Zhang, W.; Wang, X.; Zhao, D.; Tang, X. Graph degree linkage: Agglomerative clustering on a directed graph. In European Conference on Computer Vision; Springer: Florence, Italy, 2012; pp. 428–441. [Google Scholar]
- Murtagh, F. A survey of recent advances in hierarchical clustering algorithms. Comput. J. 1983, 26, 354–359. [Google Scholar] [CrossRef]
- Šárová, R.; Špinka, M.; Stěhulová, I.; Ceacero, F.; Šimečková, M.; Kotrba, R. Pay respect to the elders: Age, more than body mass, determines dominance in female beef cattle. Anim. Behav. 2013, 86, 1315–1323. [Google Scholar] [CrossRef]
- Finkemeier, M.A.; Langbein, J.; Puppe, B. Personality Research in Mammalian Farm Animals: Concepts, Measures, and Relationship to Welfare. Front. Vet. Sci. 2018, 5, 131. [Google Scholar] [CrossRef] [PubMed]
- Kwong, K.H.; Wu, T.T.; Goh, H.G.; Sasloglou, K.; Stephen, B.; Glover, I.; Shen, C.; Du, W.; Michie, C.; Andonovic, I. Practical considerations for wireless sensor networks in cattle monitoring applications. Comput. Electron. Agric. 2012, 81, 33–44. [Google Scholar] [CrossRef]



| Animal | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Animal | |||||||||||
| 1 | 0 | 6.06 | 3.61 | 5.28 | 4.45 | 4.11 | 4.32 | 5.79 | 6.19 | 5.19 | |
| 2 | 6.43 | 0 | 5.29 | 4.92 | 3.52 | 3.65 | 5.81 | 5.34 | 4.90 | 5.15 | |
| 3 | 5.46 | 5.70 | 0 | 4.69 | 5.19 | 4.24 | 4.12 | 5.81 | 4.43 | 5.36 | |
| 4 | 6.68 | 5.27 | 4.94 | 0 | 3.62 | 4.10 | 6.48 | 5.23 | 4.58 | 4.10 | |
| 5 | 6.17 | 4.34 | 5.26 | 4.35 | 0 | 3.99 | 6.69 | 4.36 | 4.79 | 5.05 | |
| 6 | 6.10 | 4.55 | 4.71 | 4.72 | 3.98 | 0 | 5.59 | 5.27 | 5.28 | 4.79 | |
| 7 | 4.86 | 5.59 | 3.28 | 4.62 | 5.13 | 3.55 | 0 | 6.75 | 5.40 | 5.83 | |
| 8 | 6.62 | 5.78 | 5.29 | 4.99 | 3.63 | 4.79 | 7.31 | 0 | 3.52 | 3.06 | |
| 9 | 7.39 | 5.29 | 4.65 | 4.94 | 4.42 | 4.81 | 6.39 | 3.40 | 0 | 3.69 | |
| 10 | 6.51 | 5.79 | 5.61 | 4.48 | 4.55 | 4.65 | 6.91 | 2.99 | 3.49 | 0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Li, S.; Lee, C.; Ni, W.; Abbott, D.; Johnson, M.; Lea, J.M.; Yuan, J.; Campbell, D.L.M. Analysis of Cattle Social Transitional Behaviour: Attraction and Repulsion. Sensors 2020, 20, 5340. https://doi.org/10.3390/s20185340
Xu H, Li S, Lee C, Ni W, Abbott D, Johnson M, Lea JM, Yuan J, Campbell DLM. Analysis of Cattle Social Transitional Behaviour: Attraction and Repulsion. Sensors. 2020; 20(18):5340. https://doi.org/10.3390/s20185340
Chicago/Turabian StyleXu, Haocheng, Shenghong Li, Caroline Lee, Wei Ni, David Abbott, Mark Johnson, Jim M. Lea, Jinhong Yuan, and Dana L. M. Campbell. 2020. "Analysis of Cattle Social Transitional Behaviour: Attraction and Repulsion" Sensors 20, no. 18: 5340. https://doi.org/10.3390/s20185340
APA StyleXu, H., Li, S., Lee, C., Ni, W., Abbott, D., Johnson, M., Lea, J. M., Yuan, J., & Campbell, D. L. M. (2020). Analysis of Cattle Social Transitional Behaviour: Attraction and Repulsion. Sensors, 20(18), 5340. https://doi.org/10.3390/s20185340





