Robot-Aided Systems for Improving the Assessment of Upper Limb Spasticity: A Systematic Review
Abstract
1. Introduction
2. Spasticity Management: Assessment and Treatments
2.1. Assessment of Spasticity Degree
2.2. Treatments to Reduce the Spasticity Effects
3. Literature Review Summary
3.1. Search Methods
3.2. Robot-Based Assessment of Spasticity
3.2.1. User’S Arm Behavior Modeling
3.2.2. Control Strategy
| ID | Source | User’s Arm Behavior Modeling * | User’s Arm Motion & Response Capturing | Control Strategy | Morphology of Robotic Device | Characteristics of Robotic Device | User Interfaces (Patient; Therapist) | Approach of Evaluation | Outcome Provided | Correlation Study with | Sample Size | Rehab Mode | Target Human Joint | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) | Norton, B. (1972) [43] | ✘ | 3 EMG electrodes | Passive-assisted | Exoskeleton (1 DOF) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪ ✪✪ | P: None T: None | Hysteresis loops (40 s) | Position, force and EMG recordings | None | 40 subjects + 3 patients | ✘ | Elbow (Hemiplegic) |
| (2) | Reinkensmeyer, D. (1999) [44] | ✔ | None | Active-assistive + passive | Exoskeleton (ARM Guide) (2 DOF) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪ ✪✪ | P: None T: A computer | FUGL motor performance exam (Post-processed) | Force patterns | None | 4 patients | ✔ | Shoulder + Elbow (Hemiplegic) |
| (3) | Pandyan, A. (2001) [45] | ✘ | None | Active | Exoskeleton | Accuracy: Portability: Adaptability: | ✪✪ ✪✪✪ ✪✪✪ | P: None T: None | Correlation between observed and measured MAS and RTPM (7 min) | RTPM | MAS | 16 subjects | ✘ | Elbow (Poststroke) |
| (4) | Lee, H.M. (2004) [46] | ✔ | 1 differential pressure sensor 1 angular rate sensor 2 sensing air bags 1 gyroscope | Active | End-effector | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪✪ ✪ | P: None T: None | Real-time | (Velocity-profile graphics) | MAS, UPDRS | 15 subjects + 15 patients | ✘ | Elbow (Poststroke) |
| (5) | Wu, Y.N. (2004) [47] | ✘ | 2 EMG electrodes | Active | Exoskeleton | Accuracy: Portability: Adaptability: | ✪✪ ✪✪✪ ✪✪ | P: None T: None | Estimation of velocity-dependent viscous component | Biomechanical and neurophysiological data | MAS | 13 patients | ✘ | Elbow (Poststroke) |
| (6) | Chen, J.J.J (2005) [48] | ✔ | 1 differential pressure sensor 1 angular rate sensor 2 sensing air bags 1 gyroscope 2 EMG electrodes | Active | Endeffector | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪✪ ✪ | P: None T: A computer | Estimation of velocity-dependent viscous component | Biomechanic parameters | MAS | 10 patients | ✘ | Elbow (Chronic stroke) |
| (7) | Kumar Raj.T.S (2006) [49] | ✘ | None | Active | Exoskeleton | Accuracy: Portability: Adaptability: | ✪✪ ✪✪✪ ✪✪ | P: None T: None | Linear regression technique (10 min) | RTPM | MAS | 111 patients | ✘ | Elbow (Poststroke) |
| (8) | Fazekas, Gabor (2006) [50] | ✘ | None | Passive | Endeffector (REHAROB) (6 DOF + 6 DOF) | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪ ✪✪ | P: Outer shell with handle T: Hardware Control Panel with predefine programming | Training sessions (30 min) | MAS and FIM score | MAS | 4 subjets + 8 patients | ✔ | Shoulder + Elbow (Hemiparetic) |
| (9) | Pandyan, A (2006) [51] | ✘ | 2 EMG electrodes | Active-assistive | Exoskeleton | Accuracy: Portability: Adaptability: | ✪✪ ✪✪ ✪✪ | P: None T: None | Flexo-extensions (16.7 s) | MAS, RPE, FEMG | MAS, RPE, FEMG | 14 patients | ✘ | Elbow (Poststroke) |
| (10) | Nef and Riener (2007) [52] | ✔ | None | Passive-assistive | Exoskeleton (ARMin)(4 DOF) | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪ ✪✪ | P: 1 graphic display for patient T: 1 graphic display for therapist | Mobilisation therapy and ball game therapy (60 min) | Recorded trajectories and 3D disturbance simulations | None | 11 patients | ✔ | Shoulder + Elbow (Hemiplegic and chronic stroke) |
| (11) | Takahashi Craig.D. (2008) [53] | ✘ | None | Active-assistive | Endeffector (HWARD) (3 DOF) | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪ ✪✪ | P: Computer monitor and + 3 soft straps in hand T: Computer monitor with game difficulty adjusting | Nine different computer games (1.5 h) | MAS, FUGL, ROM, Stroke impact, grasp and pinch force | MAS, FUGL, ROM | 13 patients | ✔ | Hand-wrist (Poststroke) |
| (12) | Calota and Levin (2008) [54] | ✘ | 2 EMG electrodes | Active | Exoskeleton (Montreal Spasticity Measure) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪ ✪✪ | P: Not specified T: A Computer | Flexo extensions (5 min) | TSRT | MAS, Tardieu | 20 patients | ✘ | Elbow (Poststroke) |
| (13) | Bovolenta, F (2009) [55] | ✔ | None | Active and passive | Endeffector (ReoGo) | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪ ✪✪ | P: Computer monitor T: Computer monitor | Nine different computer games (1.5 h) | MAS, FUGL, ROM, Stroke impact, grasp and pinch force | MAS, FUGL, Tardieu | 13 patients | ✘ | Shoulder + Elbow (Poststroke) |
| (14) | Posteraro, F (2009) [56] | ✔ | None | Active-assistive | Endeffector (MIT-MANUS) (2 DOF) | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪ ✪✪ | P: A display T: Not specified | Robot-assisted therapy (60 min) | CM, MSS, MAS, FUGL, ROM | CM, MSS, MAS, FUGL, ROM | 14 patients | ✔ | Shoulder + Elbow (Hemiparetic) |
| (15) | Posteraro, F (2010) [57] | ✘ | None | Active-assistive | Endeffector (MIT-MANUS) (2 DOF) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪ ✪✪ | P: A display T: Not specified | Robot mediated therapies (45 min) | Motor status core, MAS, ROM | MAS, ROM | 34 patients | ✔ | Shoulder + Elbow (Chronic-hemiparetic) |
| (16) | Ferreira, J (2011) [58] | ✘ | 1 goniometer Unknown EMG electrodes | Active | Exoskeleton | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪ ✪✪ | P: None T: A computer | Linear regression technique (Detection algorithm) | TSRT | None | 25 patients | ✘ | Elbow (Post-stroke + cerebral palsy) |
| (17) | Fazekas, Gabor (2011) [59] | ✘ | None | Passive | Endeffector (REHAROB) (6 DOF + 6 DOF) | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪ ✪✪ | P: Outer shell with handle T: Hardware Control Panel with predefine programming | Training sessions (30 min) | RMA, MAS, ROM, FUGL and FIM score | RMA, MAS, ROM, FUGL and FIM score | 30 patients | ✔ | Shoulder + Elbow (Hemiparetic) |
| (18) | Kim, E.H (2011) [60] | ✘ | None | Active | Endeffector (Hand-stretching device) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪✪ ✪✪ | P: None T: Not specified | Finger Stretching (10 min) | Mean MAS | MAS | 15 patients | ✘ | Hand (Hemiparetic) |
| (19) | Hu, X (2013) [61] | ✘ | 4 EMG electrodes | Active | Exoskeleton (2 DOF) | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪✪ ✪✪ | P: A table and a sponge T: Only technician Not developed yet | Training sessions (EMG-triggered algorithm) (30 min) | EMG samples and FUGL, MAS, ARAT and WMFT | FUGL, MAS, ARAT and WMFT | 10 patients | ✔ | Hand-wrist (Chronic-stroke) |
| (20) | Ferreira, J (2013) [62] | ✘ | 1 electrogoniometer Unknown EMG electrodes | Active | Exoskeleton | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪ ✪✪ | P: None T: A computer | Passive muscle stretch at different velocities (Detection algorithm) | TSRT | None | 11 patients | ✘ | Elbow (Post-stroke + cerebral palsy) |
| (21) | Sale and Posteraro (2014) [63] | ✘ | None | Active-assistive | Endeffector (MIT-MANUS) (2 DOF) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪ ✪✪ | P: A display T: Not specified | Robot-assisted therapy (45 min) | MAS-S, MAS-E, pROM | MAS-S, MAS-E, pROM | 53 patients | ✔ | Shoulder + Elbow (Subacute stroke) |
| (22) | Taveggia, G (2016) [64] | ✘ | None | Active-assitive and passive | Exoskeleton (ARMEO spring) | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪ ✪✪ | P: A display T: A computer | Training sessions (SPSS software) (60 min) | Motricity index, MAS and NPRS | MAS | 54 patients | ✘ | Shoulder + Elbow + Wrist (Poststroke) |
| (23) | Pennati, G.V (2016) [65] | ✘ | None | Passive | Exoskeleton (NeuroFlexor) (1 DOF) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪✪ ✪✪ | P: A display T: A digital | Estimation of Neural and Viscous component | Cut-off values | MAS, FUGL | 107 patients | ✘ | Wrist (Poststroke) |
| (24) | Dehem, S (2017) [66] | ✘ | None | Passive | Endeffector (REAplan) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪✪ ✪✪ | P: A display T: Not specified | Correlation between velocity and RF | Velocity-force graphics | MAS | 12 patients | ✘ | Elbow (Chronic stroke) |
| (25) | Lee, D.J. (2017) [67] | ✘ | 1 dynamometer | Active | Exoskeleton (1 DOF) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪ ✪✪ | P: Not developed yet T: Computer monitor and a emergency switches to stop | Stretching sessions (90 s) | Force patterns | MAS | 9 patients | ✔ | Elbow + Hand-wrist (Poststroke) |
| (26) | Calabro, R.S (2017) [68] | ✘ | 3 EMG electrodes | Active-assistive | Exoskeleton (Armeo power) (6 DOF) | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪✪ ✪✪ | P: A display T: Not specified | Training sessions (EMG Algorithm Shapiro-Wilk statistic) (60 min) | MAS, FUGL | MAS, FUGL | 20 patients | ✔ | Shoulder + Elbow (Ischemic stroke) |
| (27) | Posteraro, F (2018) [69] | ✘ | None | Active and passive | Exoskeleton (NEUROExos Elbow Module) (4 DOF) | Accuracy: Portability: Adaptability: | ✪✪✪ ✪✪ ✪✪ | P: Not specified T: Not specified | Isokinetic passive mobilization (45 min) | MAS score | MAS | 5 patients | ✔ | Elbow (Poststroke) |
| (28) | Wang, H. (2019) [12] | ✘ | None | Active and Passive | End-effector (Humac Norm) (1 DOF) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪ ✪✪ | P: A display T: PC interface | Online | Peak torque; Keep time; Rise time | MAS | 14 patients (stroke) | ✘ | Elbow |
| (29) | Sin, M. (2019) [70] | ✘ | 1 EMG | Active and Passive | End-effector (1 DOF) | Accuracy: Portability: Adaptability: | ✪✪ ✪✪✪ ✪✪ | P: Not specified T: Not specified | Manual and Isokinetic mobilization (37 min) | Intraclass correlation coefficient | MAS, MTS | 17 patients (stroke) | ✘ | Elbow |
3.2.3. Morphology of Robotic Devices
3.2.4. User Interfaces
3.2.5. Approach of Evaluation
3.2.6. Outcome Provided
3.2.7. Dual-Operation: Rehab- and Eval-Modes
3.2.8. Target Human Joints
4. Current Status of Robot-Aided Spasticity Assessment Systems
5. Prospects for Improvement in Robot-Aided Upper Limb Spasticity Assessment
5.1. Safety in Human-Robot Interactions
5.2. Intelligent Data Analysis Capabilities
5.3. Standardization of Robot-Aided Procedures
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MAS | Modified Ashworth Scale |
| RTPM | Resistance to passive movement |
| TSRT | Tonic stretch reflex threshold |
| FMA | Fugl-Meyer Assessment |
| MTS | Modified Tardieu Scale |
| ROM | Range of Motion |
| FIM | Functional independent Measure |
| EMG | Electromyography |
| RMA | Rivermead motor assessment scale |
| UPDRS | Unified Parkinson’s Disease Rating Scale |
| FEMG | Free-running electromyography |
| MSS | Multiple sclerosis-related spasticity |
| CM | Chedoke–McMaster Stroke Assessment |
| ARAT | Action Research Arm Test |
| WMFT | Wolf Motor Function Test |
| MAS-S | Modified Ashworth Scale-Shoulder |
| MAS-E | Modified Ashworth Scale-Elbow |
| pROM | Total Passive Range of Motion-Shoulder/Elbow |
| NPRS | Numeric pain rating scale |
| VR | Virtual reality |
| DOF | Degrees of freedom |
References
- Biering-Sørensen, F.; Nielsen, J.B.; Klinge, K. Spasticity-assessment: A review. Spinal Cord 2006, 44, 708–722. [Google Scholar] [CrossRef] [PubMed]
- Lance, J.W. The control of muscle tone, reflexes, and movement: Robert Wartenbeg Lecture. Neurology 1980, 30, 1303. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.L.; Hu, G.C. Post-stroke Spasticity: A Review of Epidemiology, Pathophysiology, and Treatments. Int. J. Gerontol. 2018, 12, 280–284. [Google Scholar] [CrossRef]
- Rekand, T. Clinical assessment and management of spasticity: A review: Clinical assessment and management of spasticity. Acta Neurol. Scand. 2010, 122, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Kwakkel, G.; Meskers, C.G.M. Botulinum toxin A for upper limb spasticity. Lancet Neurol. 2015, 14, 969–971. [Google Scholar] [CrossRef]
- Thibaut, A.; Chatelle, C.; Ziegler, E.; Bruno, M.A.; Laureys, S.; Gosseries, O. Spasticity after stroke: Physiology, assessment and treatment. Brain Inj. 2013, 27, 1093–1105. [Google Scholar] [CrossRef]
- Kinnear, B.Z.; Lannin, N.A.; Cusick, A.; Harvey, L.A.; Rawicki, B. Rehabilitation Therapies After Botulinum Toxin-A Injection to Manage Limb Spasticity: A Systematic Review. Phys. Ther. 2014, 94, 1569–1581. [Google Scholar] [CrossRef]
- Hesse, S.; Reiter, F.; Konrad, M.; Jahnke, M.T. Botulinum toxin type A and short-term electrical stimulation in the treatment of upper limb flexor spasticity after stroke: A randomized, double-blind, placebo-controlled trial. Clin. Rehabil. 1998, 12, 381–388. [Google Scholar] [CrossRef]
- Hoare, B.J.; Imms, C. Upper-Limb Injections of Botulinum Toxin-A in Children With Cerebral Palsy: A Critical Review of the Literature and Clinical Implications for Occupational Therapists. Am. J. Occup. Ther. 2004, 58, 389–397. [Google Scholar] [CrossRef]
- Petek Balci, B. Spasticty measurement. Arch. Neuropsychiatry 2018, 55, s49–s53. [Google Scholar] [CrossRef]
- Sherwood, A.; McKay, W.B. Spasticity and Upper Motor Neuron Dysfunction. In Wiley Encyclopedia of Biomedical Engineering; Akay, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006; p. ebs1109. [Google Scholar] [CrossRef]
- Wang, H.; Huang, P.; Li, X.; Samuel, O.W.; Xiang, Y.; Li, G. Spasticity Assessment Based on the Maximum Isometrics Voluntary Contraction of Upper Limb Muscles in Post-stroke Hemiplegia. Front. Neurol. 2019, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.R. Outcome measures of spasticity. Eur. J. Neurol. 2002, 9, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, N.; Satkunam, L.; Deforge, D. Treatment for spasticity in amyotrophic lateral sclerosis/motor neuron disease. In The Cochrane Database of Systematic Reviews; The Cochrane Collaboration, Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2004; p. CD004156.pub2. [Google Scholar] [CrossRef]
- Walton, K. Management of Patients With Spasticity-A Practical Approach. Pract. Neurol. 2003, 3, 342–353. [Google Scholar]
- Li, S.; Francisco, G.E. New insights into the pathophysiology of post-stroke spasticity. Front. Hum. Neurosci. 2015, 9, 192. [Google Scholar] [CrossRef] [PubMed]
- Trompetto, C.; Marinelli, L.; Mori, L.; Pelosin, E.; Currà, A.; Molfetta, L.; Abbruzzese, G. Pathophysiology of spasticity: Implications for neurorehabilitation. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Priori, A.; Cogiamanian, F.; Mrakic-Sposta, S. Pathophysiology of spasticity. Neurol. Sci. 2006, 27, s307–s309. [Google Scholar] [CrossRef]
- Ward, A.B. A literature review of the pathophysiology and onset of post-stroke spasticity. Eur. J. Neurol. 2012, 19, 21–27. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chakravarty, A. Spasticity mechanisms–for the clinician. Front. Neurol. 2010, 1, 149. [Google Scholar] [CrossRef]
- Malhotra, S.; Pandyan, A.; Day, C.; Jones, P.; Hermens, H. Spasticity, an impairment that is poorly defined and poorly measured. Clin. Rehabil. 2009, 23, 651–658. [Google Scholar] [CrossRef]
- Denny-Brown, D. The Cerebral Control of Movement; Liverpool University Press: Liverpool, UK, 1966. [Google Scholar]
- Burridge, J.; Wood, D.; Hermens, H.J.; Voerman, G.; Johnson, G.; Wijck, F.V.; Platz, T.; Gregoric, M.; Hitchcock, R.; Pandyan, A. Theoretical and methodological considerations in the measurement of spasticity. Disabil. Rehabil. 2005, 27, 69–80. [Google Scholar] [CrossRef]
- Petropoulou, K. Spasticity - Management with a Focus on Rehabilitation. International Neuromodulation Society. 2017. Available online: https://www.neuromodulation.com/fact_sheet_spasticity (accessed on 25 June 2020).
- Aloraini, S.M.; Gäverth, J.; Yeung, E.; MacKay-Lyons, M. Assessment of spasticity after stroke using clinical measures: A systematic review. Disabil. Rehabil. 2015, 37, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Hugos, C.L.; Cameron, M.H. Assessment and Measurement of Spasticity in MS: State of the Evidence. Curr. Neurol. Neurosci. Rep. 2019, 19, 79. [Google Scholar] [CrossRef]
- Platz, T.; Eickhof, C.; Nuyens, G.; Vuadens, P. Clinical scales for the assessment of spasticity, associated phenomena, and function: A systematic review of the literature. Disabil. Rehabil. 2005, 27, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Carson, L.; Kinnin, E.; Patterson, V. The Ashworth Scale: A Reliable and Reproducible Method of Measuring Spasticity. Neurorehabilit. Neural Repair 1989, 3, 205–209. [Google Scholar] [CrossRef]
- Pandyan, A.D.; Johnson, G.R.; Price, C.I.M.; Curless, R.H.; Barnes, M.P.; Rodgers, H. A review of the properties and limitations of the Ashworth and modified Ashworth Scales as measures of spasticity. Clin. Rehabil. 1999, 13, 373–383. [Google Scholar] [CrossRef]
- Oña Simbaña, E.D.; Sánchez-Herrera Baeza, P.; Jardón Huete, A.; Balaguer, C. Review of Automated Systems for Upper Limbs Functional Assessment in Neurorehabilitation. IEEE Access 2019, 7, 32352–32367. [Google Scholar] [CrossRef]
- Lum, P.S.; Burgar, C.G.; Loos, M.V.d.; Shor, P.C.; Majmundar, M.; Yap, R. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: A follow-up study. J. Rehabil. Res. Dev. 2006, 43, 631. [Google Scholar] [CrossRef]
- Volpe, B.T.; Lynch, D.; Rykman-Berland, A.; Ferraro, M.; Galgano, M.; Hogan, N.; Krebs, H.I. Intensive Sensorimotor Arm Training Mediated by Therapist or Robot Improves Hemiparesis in Patients With Chronic Stroke. Neurorehabilit. Neural Repair 2008, 22, 305–310. [Google Scholar] [CrossRef]
- Rosati, G.; Gallina, P.; Masiero, S. Design, Implementation and Clinical Tests of a Wire-Based Robot for Neurorehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2007, 15, 560–569. [Google Scholar] [CrossRef]
- Kung, P.C.; Ju, M.S.; Lin, C.C.K. Design of a forearm rehabilitation robot. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, Netherlands, 13–15 June 2007; pp. 228–233. [Google Scholar] [CrossRef]
- Fonseca, L.A.; Grecco, L.A.C.; Politti, F.; Frigo, C.; Pavan, E.; Corrêa, J.C.F.; Oliveira, C.S. Use a Portable Device for Measuring Spasticity in Individuals with Cerebral Palsy. J. Phys. Ther. Sci. 2013, 25, 271–275. [Google Scholar] [CrossRef][Green Version]
- Lunenburger, L.; Colombo, G.; Riener, R.; Dietz, V. Clinical Assessments Performed During Robotic Rehabilitation by the Gait Training Robot Lokomat. In Proceedings of the 9th International Conference on Rehabilitation Robotics, ICORR 2005, Chicago, IL, USA, 28 June–1 July 2005; pp. 345–348. [Google Scholar] [CrossRef]
- Oña, E.D.; Cano-de-la Cuerda, R.; Sánchez-Herrera, P.; Balaguer, C.; Jardón, A. A review of robotics in neurorehabilitation: Towards an automated process for upper limb. J. Healthc. Eng. 2018, 2018, 9758939. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Sun, Y.; Wang, N.; Gao, F.; Wei, K.; Wang, Q. Robot-Assisted Rehabilitation of Ankle Plantar Flexors Spasticity: A 3-Month Study with Proprioceptive Neuromuscular Facilitation. Front. Neurorobot. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Abu-Dakk, F.J.; Valera, A.; Escalera, J.; Vallés, M.; Mata, V.; Abderrahim, M. Trajectory Adaptation and Learning for Ankle Rehabilitation Using a 3-PRS Parallel Robot. In Intelligent Robotics and Applications; Liu, H., Kubota, N., Zhu, X., Dillmann, R., Zhou, D., Eds.; Springer International Publishing: Cham, Switzerland; Berlin/Heidelberg, Germany, 2015; Volume 9245, pp. 483–494. [Google Scholar] [CrossRef]
- Bucca, G.; Bezzolato, A.; Bruni, S.; Molteni, F. A Mechatronic Device for the Rehabilitation of Ankle Motor Function. J. Biomech. Eng. 2009, 131, 125001. [Google Scholar] [CrossRef] [PubMed]
- Adewusi, S.; Rakheja, S.; Marcotte, P. Biomechanical models of the human hand-arm to simulate distributed biodynamic responses for different postures. Int. J. Ind. Ergon. 2012, 42, 249–260. [Google Scholar] [CrossRef]
- Basteris, A.; Nijenhuis, S.M.; Stienen, A.H.; Buurke, J.H.; Prange, G.B.; Amirabdollahian, F. Training modalities in robot-mediated upper limb rehabilitation in stroke: A framework for classification based on a systematic review. J. Neuroeng. Rehabil. 2014, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Norton, B.J.; Bomze, H.A.; Chaplin, H. An Approach to the Objective Measurement of Spasticity. Phys. Ther. 1972, 52, 15–24. [Google Scholar] [CrossRef][Green Version]
- Reinkensmeyer, D.; Dewald, J.; Rymer, W. Guidance-based quantification of arm impairment following brain injury: A pilot study. IEEE Trans. Rehabil. Eng. 1999, 7, 1–11. [Google Scholar] [CrossRef]
- Pandyan, A.; Price, C.; Rodgers, H.; Barnes, M.; Johnson, G. Biomechanical examination of a commonly used measure of spasticity. Clin. Biomech. 2001, 16, 859–865. [Google Scholar] [CrossRef]
- Lee, H.M.; Chen, J.J.J.; Ju, M.S.; Lin, C.C.K.; Poon, P.P. Validation of portable muscle tone measurement device for quantifying velocity-dependent properties in elbow spasticity. J. Electromyogr. Kinesiol. 2004, 14, 577–589. [Google Scholar] [CrossRef]
- Wu, Y.N.; Huang, S.C.; Chen, J.J.J.; Wang, Y.L.; Piotrkiewicz, M. Spasticity Evaluation of Hemiparetic Limbs in Stroke Patients before Intervention by Using Portable Stretching Device and EMG. J. Med. Biol. Eng. 2004, 24, 6. [Google Scholar]
- Chen, J.J.J.; Wu, Y.N.; Huang, S.C.; Lee, H.M.; Wang, Y.L. The Use of a Portable Muscle Tone Measurement Device to Measure the Effects of Botulinum Toxin Type A on Elbow Flexor Spasticity. Arch. Phys. Med. Rehabil. 2005, 86, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.T.S.; Pandyan, A.D.; Sharma, A.K. Biomechanical measurement of post-stroke spasticity. Age Ageing 2006, 35, 371–375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fazekas, G.; Horvath, M.; Toth, A. A novel robot training system designed to supplement upper limb physiotherapy of patients with spastic hemiparesis. Int. J. Rehabil. Res. 2006, 29, 251–254. [Google Scholar] [CrossRef] [PubMed]
- Pandyan, A.D.; Van Wijck, F.M.J.; Stark, S.; Vuadens, P.; Johnson, G.R.; Barnes, M.P. The construct validity of a spasticity measurement device for clinical practice: An alternative to the Ashworth scales. Disabil. Rehabil. 2006, 28, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Nef, T.; Mihelj, M.; Riener, R. ARMin: A robot for patient-cooperative arm therapy. Med. Biol. Eng. Comput. 2007, 45, 887–900. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, C.D.; Der-Yeghiaian, L.; Le, V.; Motiwala, R.R.; Cramer, S.C. Robot-based hand motor therapy after stroke. Brain 2008, 131, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Calota, A.; Feldman, A.G.; Levin, M.F. Spasticity measurement based on tonic stretch reflex threshold in stroke using a portable device. Clin. Neurophysiol. 2008, 119, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Bovolenta, F.; Goldoni, M.; Clerici, P.; Agosti, M.; Franceschini, M. Robot therapy for functional recovery of the upper limbs: A pilot study on patients after stroke. J. Rehabil. Med. 2009, 41, 971–975. [Google Scholar] [CrossRef]
- Posteraro, F.; Mazzoleni, S.; Aliboni, S.; Cesqui, B.; Battaglia, A.; Dario, P.; Micera, S. Robot-mediated therapy for paretic upper limb of chronic patients following neurological injury. J. Rehabil. Med. 2009, 41, 976–980. [Google Scholar] [CrossRef]
- Posteraro, F.; Mazzoleni, S.; Aliboni, S.; Cesqui, B.; Battaglia, A.; Carrozza, M.; Dario, P.; Micera, S. Upper limb spasticity reduction following active training: A robot-mediated study in patients with chronic hemiparesis. J. Rehabil. Med. 2010, 42, 279–281. [Google Scholar] [CrossRef]
- Ferreira, J.; Moreira, V.; Machado, J.; Soares, F. Biomedical device for spasticity quantification based on the velocity dependence of the Stretch Reflex threshold. In Proceedings of the ETFA2011, Toulouse, France, 5–9 September 2011; pp. 1–4. [Google Scholar] [CrossRef]
- Fazekas, G.; Zsiga, K.; Dénes, Z. Robot-mediated upper limb physiotherapy: Review and recommendations for future clinical trials. Int. J. Rehabil. Res. 2011, 34, 196–202. [Google Scholar] [CrossRef]
- Kim, E.H.; Jang, M.C.; Seo, J.P.; Jang, S.H.; Song, J.C.; Jo, H.M. The Effect of a Hand-Stretching Device During the Management of Spasticity in Chronic Hemiparetic Stroke Patients. Ann. Rehabil. Med. 2013, 37, 235. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Tong, K.; Wei, X.; Rong, W.; Susanto, E.; Ho, S. The effects of post-stroke upper-limb training with an electromyography (EMG)-driven hand robot. J. Electromyogr. Kinesiol. 2013, 23, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Moreira, V.; Machado, J.; Soares, F. Improved biomedical device for spasticity quantification. In Proceedings of the 2013 IEEE 3rd Portuguese Meeting in Bioengineering (ENBENG), Braga, Portugal, 20–23 February 2013; pp. 1–4. [Google Scholar] [CrossRef]
- Sale, P.; Franceschini, M.; Mazzoleni, S.; Palma, E.; Agosti, M.; Posteraro, F. Effects of upper limb robot-assisted therapy on motor recovery in subacute stroke patients. J. Neuroeng. Rehabil. 2014, 11, 104. [Google Scholar] [CrossRef] [PubMed]
- Taveggia, G.; Borboni, A.; Salvi, L.; Mulé, C.; Fogliaresi, S.; Villafañe, J.H.; Casale, R. Efficacy of robot-assisted rehabilitation for the functional recovery of the upper limb in post-stroke patients: A randomized controlled study. Eur. J. Phys. Rehabil. Med. 2016, 52, 767–773. [Google Scholar]
- Pennati, G.V.; Plantin, J.; Borg, J.; Lindberg, P.G. Normative NeuroFlexor data for detection of spasticity after stroke: A cross-sectional study. J. Neuroeng. Rehabil. 2016, 13, 30. [Google Scholar] [CrossRef]
- Dehem, S.; Gilliaux, M.; Lejeune, T.; Detrembleur, C.; Galinski, D.; Sapin, J.; Vanderwegen, M.; Stoquart, G. Assessment of upper limb spasticity in stroke patients using the robotic device REAplan. J. Rehabil. Med. 2017, 49, 565–571. [Google Scholar] [CrossRef]
- Lee, D.J.; Bae, S.J.; Jang, S.H.; Chang, P.H. Design of a clinically relevant upper-limb exoskeleton robot for stroke patients with spasticity. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 622–627. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Naro, A.; Russo, M.; Milardi, D.; Leo, A.; Filoni, S.; Trinchera, A.; Bramanti, P. Is two better than one? Muscle vibration plus robotic rehabilitation to improve upper limb spasticity and function: A pilot randomized controlled trial. PLoS ONE 2017, 12, e0185936. [Google Scholar] [CrossRef]
- Posteraro, F.; Crea, S.; Mazzoleni, S.; Berteanu, M.; Ciobanu, I.; Vitiello, N.; Cempini, M.; Gervasio, S.; Mrachacz-Kersting, N. Technologically-advanced assessment of upper-limb spasticity: A pilot study. Eur. J. Phys. Rehabil. Med. 2018, 54, 9. [Google Scholar]
- Sin, M.; Kim, W.S.; Cho, K.; Paik, N.J. Isokinetic Robotic Device to Improve Test-Retest and Inter-Rater Reliability for Stretch Reflex Measurements in Stroke Patients with Spasticity. J. Vis. Exp. 2019, 59814. [Google Scholar] [CrossRef]
- Htoon, Z.L.; Sidek, S.N.; Fatai, S.; Rashid, M.M. Estimation of Upper Limb Impedance Parameters Using Recursive Least Square Estimator. In Proceedings of the 2016 International Conference on Computer and Communication Engineering (ICCCE), Kuala Lumpur, Malaysia, 26–27 July 2016; pp. 144–148. [Google Scholar] [CrossRef]
- Sidiropoulos, A.; Karayiannidis, Y.; Doulgeri, Z. Human-robot collaborative object transfer using human motion prediction based on dynamic movement primitives. In Proceedings of the 2019 18th European Control Conference (ECC), Naples, Italy, 25–28 June 2019; pp. 2583–2588. [Google Scholar]
- Oña, E.D.; Jardón, A.; Cuesta-Gómez, A.; Sánchez-Herrera-Baeza, P.; Cano-de-la Cuerda, R.; Balaguer, C. Validity of a Fully-Immersive VR-Based Version of the Box and Blocks Test for Upper Limb Function Assessment in Parkinson’s Disease. Sensors 2020, 20, 2773. [Google Scholar] [CrossRef] [PubMed]
- Scano, A.; Molteni, F.; Molinari Tosatti, L. Low-Cost Tracking Systems Allow Fine Biomechanical Evaluation of Upper-Limb Daily-Life Gestures in Healthy People and Post-Stroke Patients. Sensors 2019, 19, 1224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Q.; Chung, S.; Lin, A.; van Rey, E.; Bai, Z.; Grant, T.; Roth, E. A portable intelligent stretching device for treating spasticity and contracture with outcome evaluation. In Proceedings of the Second Joint 24th Annual Conference and the Annual Fall Meeting of the Biomedical Engineering Society, Engineering in Medicine and Biology, Houston, TX, USA, 23–26 October 2002; Volume 3, pp. 2453–2454. [Google Scholar] [CrossRef]
- Ang, W.S.; Geyer, H.; Chen, I.M.; Ang, W.T. Objective Assessment of Spasticity With a Method Based on a Human Upper Limb Model. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, P.P.; Forkan, A.R.M.; Morshed, A.; Haghighi, P.D.; Kang, Y.B. Healthcare 4.0: A review of frontiers in digital health. Wiley Interdiscip. Rev. Data Min. Knowl. Discov. 2020, 10, e1350. [Google Scholar] [CrossRef]
- Heung, H.L.; Tang, Z.Q.; Shi, X.Q.; Tong, K.Y.; Li, Z. Soft Rehabilitation Actuator With Integrated Post-stroke Finger Spasticity Evaluation. Front. Bioeng. Biotechnol. 2020, 8, 111. [Google Scholar] [CrossRef] [PubMed]
- Oña, E.D.; Garcia-Haro, J.M.; Jardón, A.; Balaguer, C. Robotics in health care: Perspectives of robot-aided interventions in clinical practice for rehabilitation of upper limbs. Appl. Sci. 2019, 9, 2586. [Google Scholar] [CrossRef]
- Yee, J.; Low, C.Y.; Ong, P.; Soh, W.S.; Hanapiah, F.A.; Zakaria, N.A.C.; Enzberg, S.v.; Asmar, L.; Dumitrescu, R. Verification of Mathematical Model for Upper Limb Spasticity with Clinical Data. Iop Conf. Ser. Mater. Sci. Eng. 2020, 824, 012013. [Google Scholar] [CrossRef]
- Fleuren, J.F.; Voerman, G.E.; Erren-Wolters, C.V.; Snoek, G.J.; Rietman, J.S.; Hermens, H.J.; Nene, A.V. Stop using the Ashworth Scale for the assessment of spasticity. J. Neurol. Neurosurg. Psychiatry 2010, 81, 46–52. [Google Scholar] [CrossRef]
- Stein, J.; Krebs, H.I.; Frontera, W.R.; Fasoli, S.E.; Hughes, R.; Hogan, N. Comparison of Two Techniques of Robot-Aided Upper Limb Exercise Training After Stroke. Am. J. Phys. Med. Rehabil. 2004, 83, 720–728. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Riener, R.; Nef, T.; Colombo, G. Robot-aided neurorehabilitation of the upper extremities. Med. Biol. Eng. Comput. 2005, 43, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Toigo, M.; Flück, M.; Riener, R.; Klamroth-Marganska, V. Robot-assisted assessment of muscle strength. J. Neuroeng. Rehabil. 2017, 14, 103. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.L.; Tong, K.Y.; Song, R.; Zheng, X.J.; Lui, K.H. Robot-assisted wrist training for chronic stroke: A comparison between electromyography (EMG) driven robot and passive motion. In Proceedings of the 2008 2nd IEEE RAS & EMBS International Conference on Biomedical Robotics and Biomechatronics, Scottsdale, AZ, USA, 19–22 October 2008; pp. 637–641. [Google Scholar] [CrossRef]
- Malhotra, S.; Cousins, E.; Ward, A.; Day, C.; Jones, P.; Roffe, C.; Pandyan, A. An investigation into the agreement between clinical, biomechanical and neurophysiological measures of spasticity. Clin. Rehabil. 2008, 22, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Jobin, A.; Levin, M.F. Regulation of stretch reflex threshold in elbow flexors in children with cerebral palsy: A new measure of spasticity. Dev. Med. Child Neurol. 2000, 42, 531–540. [Google Scholar] [CrossRef] [PubMed]
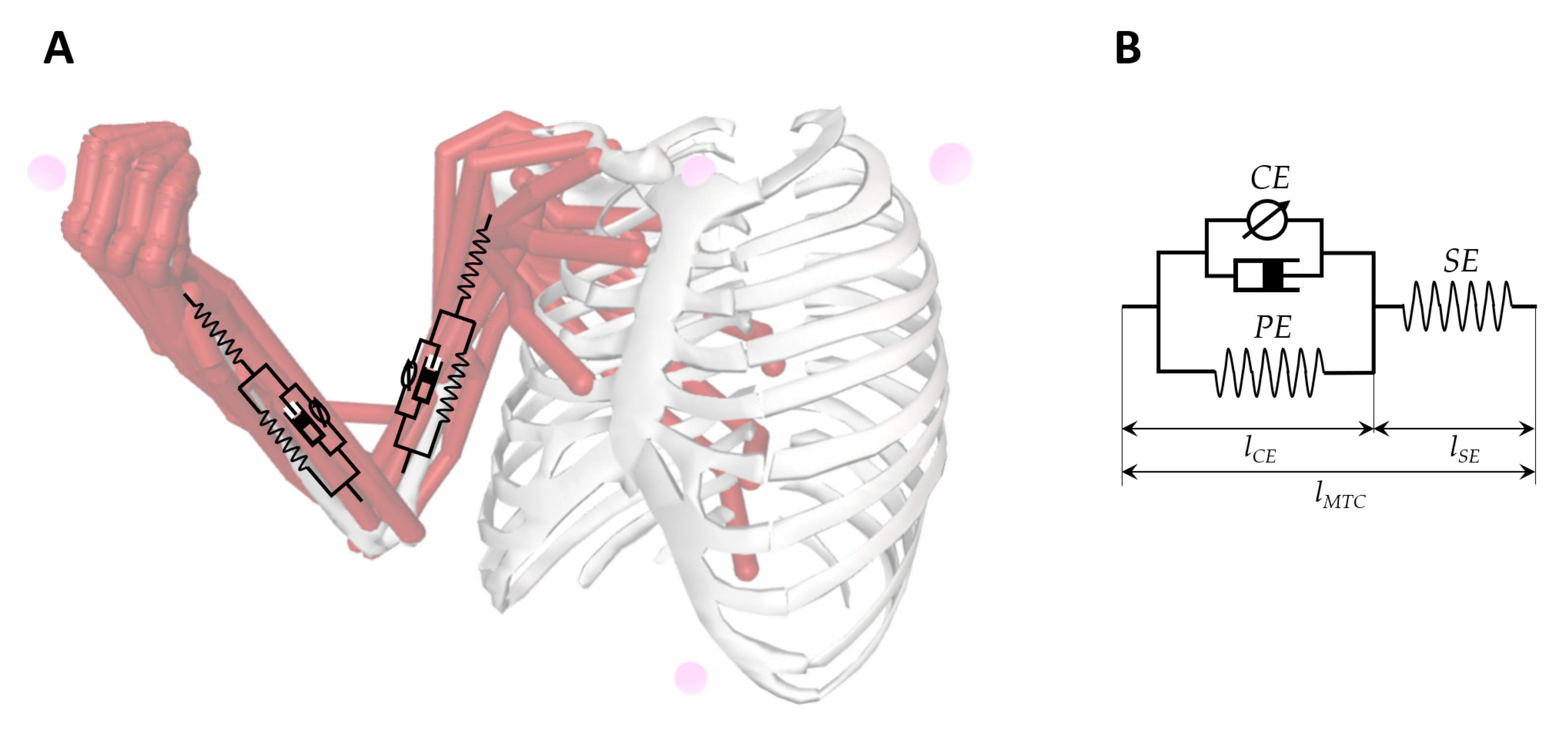
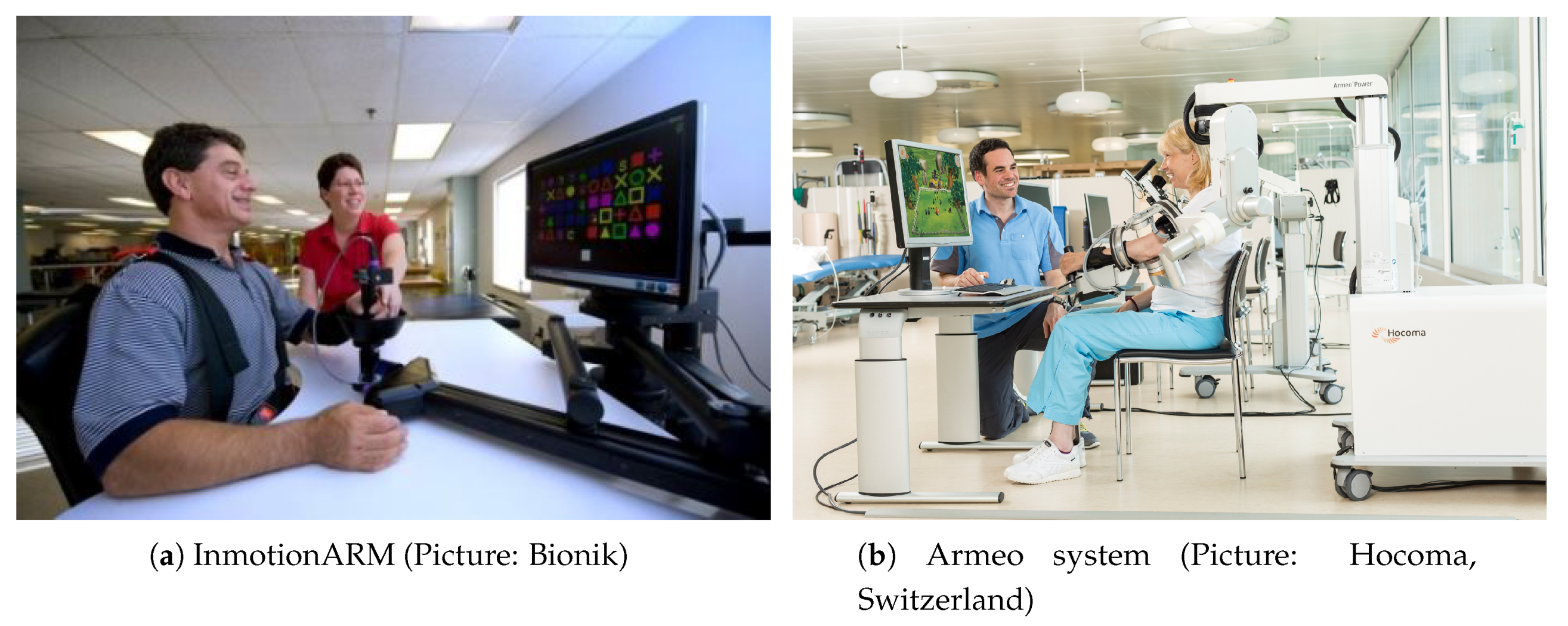

| Category | Scale | Principle | Outcome |
|---|---|---|---|
| Observational | Ashworth Scale | Rating the resistance to manually limb mobilization | 0–4 scale (1-point extra in modified version) |
| Tardieu Scale | Rating the resistance to manually limb mobilization and the angle where this resistance occurs | 0–4 scale (1-point extra in modified version) + Two angles (R1, R2) | |
| Pendulum test | Observing a muscle’s response and oscillations to sudden stretch imposed by gravity | There is no accepted scale (observation-based rating) | |
| Tone Assessment Scale (TAS) | Evaluating the resting posture, the response to passive movement and the response to active efforts (multi-item) | 0–4 scale | |
| Self-reported | Penn Spasm Frequency Scale | Counting the number of spasms experienced in a specified time frame | 0–4 scale |
| Numeric Rating Scale (NRS) | Self-appreciation of severity of their symptoms | 0–10 scale | |
| Instrumented | Ultrasound muscle elastography | Examining the mechanical elastic properties of tissues | 5-point scale |
| Instrumented Hofmann’s reflex | Measuring the threshold spinal reflex reaction by electromyography (EMG) | H-reflex | |
| Instrumented Pendulum Scale | Markers are adhered to limb and the trial is videotaped to allow computerized motion analysis | Angular displacement, velocity, and acceleration response | |
| Instrumented Tardieu Scale | Integrating biomechanical and electrophysiological signals during limb mobilization | Joint angle and torque + surface electromyography |
| Category | Procedure | Aim |
|---|---|---|
| Non-interventional | Physical therapy | Improving movement |
| Occupational therapy | Improving autonomy in ADL | |
| Casting or bracing | Reducing secondary damage | |
| Pharmacological (oral medication and injections) | Improving movement | |
| Interventional | Selective dorsal rhizotomy (SDR) | Balancing electrical stimulus |
| Intrathecal baclofen (ITB) pump | Supplying medication at spinal fluid | |
| Neurectomies | Removal damaged nerves |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de-la-Torre, R.; Oña, E.D.; Balaguer, C.; Jardón, A. Robot-Aided Systems for Improving the Assessment of Upper Limb Spasticity: A Systematic Review. Sensors 2020, 20, 5251. https://doi.org/10.3390/s20185251
de-la-Torre R, Oña ED, Balaguer C, Jardón A. Robot-Aided Systems for Improving the Assessment of Upper Limb Spasticity: A Systematic Review. Sensors. 2020; 20(18):5251. https://doi.org/10.3390/s20185251
Chicago/Turabian Stylede-la-Torre, Rubén, Edwin Daniel Oña, Carlos Balaguer, and Alberto Jardón. 2020. "Robot-Aided Systems for Improving the Assessment of Upper Limb Spasticity: A Systematic Review" Sensors 20, no. 18: 5251. https://doi.org/10.3390/s20185251
APA Stylede-la-Torre, R., Oña, E. D., Balaguer, C., & Jardón, A. (2020). Robot-Aided Systems for Improving the Assessment of Upper Limb Spasticity: A Systematic Review. Sensors, 20(18), 5251. https://doi.org/10.3390/s20185251







