ZnO Metal Oxide Semiconductor in Surface Acoustic Wave Sensors: A Review
Abstract
1. Introduction
2. SAW Sensors for Gas Detection
3. Sensitive Materials Used in Gas Detection
4. ZnO in SAW Sensors
5. Summary and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lang, L.; Zhu, W.; Zhu, G.; Bao, C.; Xu, H.; Li, X.; Shen, X. Folic acid mediated synthesis of hierarchical ZnO micro-flower with improved gas sensing properties. Adv. Powder Technol. 2020, 31, 2227–2234. [Google Scholar] [CrossRef]
- Johny, J.; Prabhu, R.; Fung, W.K.; Watson, J. Investigation of positioning of FBG sensors for smart monitoring of oil and gas subsea structures. In Proceedings of the Oceans 2016, Shanghai, China, 10–13 April 2016; pp. 1–4. [Google Scholar]
- Harrou, F.; Dairi, A.; Sun, Y.; Kadri, F. Detecting Abnormal Ozone Measurements with a Deep Learning-Based Strategy. IEEE Sens. J. 2018, 18, 7222–7232. [Google Scholar] [CrossRef]
- Feng, S.; Farha, F.; Li, Q.; Wan, Y.; Xu, Y.; Zhang, T.; Ning, H. Review on Smart Gas Sensing Technology. Sensors 2019, 19, 3760. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Lin, W.; Liu, Z.; Wu, S.; Qiu, X. Pipeline Leak Detection by Using Time-Domain Statistical Features. IEEE Sens. J. 2017, 17, 6431–6442. [Google Scholar] [CrossRef]
- Ren, Q.; Cao, Y.-Q.; Arulraj, D.; Liu, C.; Wu, D.; Li, W.-M.; Li, A.-D. Review—Resistive-Type Hydrogen Sensors Based on Zinc Oxide Nanostructures. J. Electrochem. Soc. 2020, 167, 067528. [Google Scholar] [CrossRef]
- Hjiri, M.; Bahanan, F.; Aida, M.S.; El Mir, L.; Neri, G. High Performance CO Gas Sensor Based on ZnO Nanoparticles. J. Inorg. Organomet. Polym. Mater. 2020. [Google Scholar] [CrossRef]
- Li, D.; Le, X.; Pang, J.; Peng, L.; Xu, Z.; Gao, C.; Xie, J. A SAW hydrogen sensor based on decoration of graphene oxide by palladium nanoparticles on AIN/Si layered structure. J. Micromech. Microeng. 2019, 29, 045007. [Google Scholar] [CrossRef]
- Yang, L.; Yin, C.; Zhang, Z.; Zhou, J.; Xu, H. The investigation of hydrogen gas sensing properties of SAW gas sensor based on palladium surface modified SnO2 thin film. Mat. Sci. Semicond. Process. 2017, 60, 16–28. [Google Scholar] [CrossRef]
- Lin, T.; Lv, X.; Hu, Z.; Xu, A.; Feng, C. Semiconductor Metal Oxides as Chemoresistive Sensors for Detecting Volatile Organic Compounds. Sensors 2019, 19, 233. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wang, Y.; Li, X.; Guo, Y. The impact of carrier gas on room-temperature trace nitrogen dioxide sensing of ZnO nanowire-integrated film under UV illumination. Ceram. Int. 2020, 46, 16056–16061. [Google Scholar] [CrossRef]
- Yagati, A.K.; Lee, T.; Min, J.; Choi, J. Electrochemical performance of gold nanoparticle–cytochrome c hybrid interface for H2O2 detection. Colloids Surf. B 2012, 92, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K.; Kurita, R.; Niwa, O. Electrochemical surface plasmon resonance measurement based on gold nanohole array fabricated by nanoimprinting technique. Anal. Chem. 2012, 84, 3187–3191. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B-Adv. 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Illyaskutty, N.; Kansizoglu, O.; Akdag, O.; Ojha, B.; Knoblauch, J.; Kohler, H. Miniaturized Single Chip Arrangement of a Wheatstone Bridge Based Calorimetric Gas Sensor. Chemosensors 2018, 6, 22. [Google Scholar] [CrossRef]
- Sarf, F. Metal Oxide Gas Sensors by Nanostructures. Gas Sensors 2020. [Google Scholar] [CrossRef]
- Ortiz Cebolla, R.; Weidner, E.; Buttner, W.; Bonato, C.; Hartmann, K.; Schmidt, K. Test methodologies for hydrogen sensor performance assessment: Chamber vs. flow-through test apparatus. Int. J. Hydrogen Energy 2018, 43, 21149–21160. [Google Scholar] [CrossRef]
- Westerwaal, R.J.; Duim, N.; Nieuwenhuijse, I.; Perrotton, C.; Dabirian, A.; Van Leeuwen, J.M.; Palmisano, V.; Dam, B. Thin film based sensors for a continuous monitoring of hydrogen concentrations. Sens. Actuator B Chem. 2012, 165, 88–96. [Google Scholar] [CrossRef]
- Wohltjen, H.; Dessy, R. Surface Acoustic-Wave Probe for Chemical-Analysis. 1. Introduction and Instrument Description. Anal. Chem. 1979, 51, 1458–1464. [Google Scholar] [CrossRef]
- Wohltjen, H.; Dessy, R. Surface Acoustic-Wave Probes for Chemical-Analysis. 2. Gas-Chromatography Detector. Anal. Chem. 1979, 51, 1465–1470. [Google Scholar] [CrossRef]
- Lange, K. Bulk and Surface AcousticWave Sensor Arrays for Multi-Analyte Detection: A Review. Sensors 2019, 19, 5382. [Google Scholar] [CrossRef] [PubMed]
- Constantinoiu, I.; Miu, D.; Viespe, C. Surface Acoustic Wave Sensors for Ammonia Detection at Room Temperature Based on SnO2/Co3O4 Bilayers. J. Sens. 2019. [Google Scholar] [CrossRef]
- Han, Z.; Ren, J.; Zhou, J.; Zhang, S.; Zhang, Z.; Yang, L.; Yin, C. Multilayer porous Pd-WO3 composite thin films prepared by sol-gel process for hydrogen sensing. Int. J. Hydrogen Energy 2020, 45, 7223–7233. [Google Scholar] [CrossRef]
- Mao, S.; Zhou, H.; Wu, S.; Yang, J.; Li, Z.; Wei, X.; Wang, X.; Wang, Z.; Li, J. High performance hydrogen sensor based on Pd/TiO2 composite film. Int. J. Hydrogen Energy 2018, 43, 22727–22732. [Google Scholar] [CrossRef]
- Hamidon, M.N.; Yunusa, Z. Sensing materials for acoustic wave chemical sensors. In Progresses in Chemical Sensor; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Dwiputra, M.A.; Fadhila, F.; Imawan, C.; Fauzia, V. The enhanced performance of capacitive-type humidity sensors based on ZnO nanorods/WS2 nanosheets heterostructure. Sens. Actuator B Chem. 2020, 310, 127810. [Google Scholar] [CrossRef]
- Karapetyana, G.Y.; Kaydashevb, V.E.; Zhilina, D.A.; Kutepova, M.E.; Minasyana, T.A.; Kaidashev, E.M. A surface acoustic wave impedance-loaded high sensitivity sensor with wide dynamic range for ultraviolet light detection. Sens. Actuator A-Phys. 2019, 296, 70–78. [Google Scholar] [CrossRef]
- Dinca, V.; Viespe, C.; Brajnicov, S.; Constantinoiu, I.; Moldovan, A.; Bonciu, A.; Toader, C.N.; Ginghina, R.E.; Grigoriu, N.; Dinescu, M.; et al. MAPLE Assembled Acetylcholinesterase–Polyethylenimine Hybrid and Multilayered Interfaces for Toxic Gases Detection. Sensors 2018, 18, 4265. [Google Scholar] [CrossRef]
- Puiu, M.; Gurban, A.M.; Rotariu, L.; Brajnicov, S.; Viespe, C.; Bala, C. Enhanced Sensitive Love Wave Surface Acoustic Wave Sensor Designed for Immunoassay Formats. Sensors 2015, 15, 10511–10525. [Google Scholar] [CrossRef]
- Ballentine, D.S.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.T.; Frye, G.C.; Wohtjen, H. Acoustic Wave Sensors, Theory, Design and Physico-Chemical Applications; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Devkota, J.; Ohodnicki, R.P.; Greve, W.D. SAW Sensors for Chemical Vapors and Gases. Sensors 2017, 17, 801. [Google Scholar] [CrossRef]
- Luo, W.; Fu, Q.; Zhou, D.; Deng, J.; Liu, H.; Yan, G. A surface acoustic wave H2S gas sensor employing nanocrystalline SnO2 thin film. Sens. Actuators B Chem. 2013, 176, 746–752. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, F.L. SAW and Functional Polymers. In Gas Sensing Fundamentals; Kohl, C.-D., Wagner, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 213–245. [Google Scholar]
- Li, Z.; Li, H.; Wu, Z.; Wang, M.; Luo, J.; Torun, H.; Hu, P.; Yang, C.; Grundmann, M.; Liu, X.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef]
- Mujahid, A.; Dickert, L.F. Surface Acoustic Wave (SAW) for Chemical Sensing Applications of Recognition Layers. Sensors 2017, 17, 2716. [Google Scholar] [CrossRef] [PubMed]
- Drafts, B. Acoustic wave technology sensors. IEEE Trans. Microw. Theory Tech. 2001, 49, 795–802. [Google Scholar] [CrossRef]
- Chen, C.; Jin, J. Surface Acoustic Wave Vapor Sensor with Graphene Interdigital Transducer for TNT Detection. Sens. Imaging 2020, 21, 24. [Google Scholar] [CrossRef]
- Manbachi, A.; Cobbold, R.S.C. Development and Application of Piezoelectric Materials for Ultrasound Generation and Detection. Ultrasound 2011, 19, 187–196. [Google Scholar] [CrossRef]
- Curie, P.; Curie, J. Développement par pression de l’électricité polaire dans les hémièdres à faces inclinées. C. R. Acad. Sci. 1880, 91, 294–295. [Google Scholar]
- Wohltjen, H. Mechanism of Operation and Design Considerations for Surface Acoustic-Wave Device Vapor Sensors. Sens. Actuator 1984, 5, 307–325. [Google Scholar] [CrossRef]
- Campbell, C.K. Applications of surface acoustic and shallow bulk acoustic wave devices. Proc. IEEE 1989, 77, 1453–1484. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Takeuchi, M. Applications for Piezoelectric Leaky Surface Waves. In Proceedings of the IEEE Symposium on Ultrasonics, Honolulu, HI, USA, 4–7 December 1990; Volume 1, pp. 11–18. [Google Scholar]
- Dorozhkin, L.M.; Rozanov, I.A. Acoustic wave chemical sensors for gases. J. Anal. Chem. 2001, 56, 399–416. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Detection of Volatile Organic Compounds Using Surface Acoustic Wave Sensor Based on Nanoparticles Incorporated in Polymer. Coatings 2019, 9, 373. [Google Scholar] [CrossRef]
- Strutt, J.W. On waves propagated along the plane surface of an elastic solid. Proc. Lond. Math. Soc. 1885, 17, 4–11. [Google Scholar]
- Caliendo, C.; Verardi, P.; Verona, E.; Amico, A.D.; Natale, C.D.; Saggio, G.; Serafini, M.; Paolesse, R.; Huq, S.E. Advances in SAW-based gas sensors. Smart Mater. Struct. 1997, 6, 689. [Google Scholar] [CrossRef]
- Jakubik, W.P. Surface acoustic wave-based gas sensors. Thin Solid Films 2011, 520, 986–993. [Google Scholar] [CrossRef]
- Liu, B.; Chen, X.; Cai, H.; Mohammad Ali, M.; Tian, X.; Tao, L.; Yang, Y.; Ren, T. Surface acoustic wave devices for sensor applications. J. Semicond. 2016, 37, 021001. [Google Scholar] [CrossRef]
- Marcu, A.; Nicolae, I.; Viespe, C. Active surface geometrical control of noise in nanowire-SAW sensors. Sens. Actuator B-Chem. 2016, 231, 469–473. [Google Scholar] [CrossRef]
- Arn, D.; Amati, D.; Blom, N.; Ehrat, M.; Widmer, H.M. Surface acoustic wave gas sensors: Developments in the chemical industry. Sens. Actuator B Chem. 1992, 8, 27–31. [Google Scholar] [CrossRef]
- Cheeke, J.D.N.; Wang, Z. Acoustic wave gas sensors. Sens. Actuators B Chem. 1999, 59, 146–153. [Google Scholar] [CrossRef]
- Rana, L.; Gupta, R.; Tomar, M.; Gupta, V. ZnO/ST-Quartz SAW resonator: An efficient NO2gas sensor. Sens Actuator B Chem. 2017, 252, 840–845. [Google Scholar] [CrossRef]
- Fan, S.B.; Pan, G.F.; Liang, J.; Tian, Z.Y. Tailored synthesis of CoOX thin films for catalytic application. RSC Adv. 2015, 5, 97272–97278. [Google Scholar] [CrossRef]
- Goktas, A.; Tumbul, A.; Aba, Z.; Kilic, A.; Aslan, F. Enhancing crystalline/optical quality, and photoluminescence properties of the Na and Sn substituted ZnS thin films for optoelectronic and solar cell applications; a comparative study. Opt. Mater. 2020, 107, 110073. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Ding, S.; Li, Y.; Tian, Y. Preparation of highly transparent conductive aluminum-doped zinc oxide thin films using a low-temperature aqueous solution process for thin-film solar cells applications. Sol. Energy Mater. Sol. Cells 2019, 203, 110161. [Google Scholar] [CrossRef]
- Jinga, S.I.; Skokin, M.; Vasile, B.S.; Constantinoiu, I.; Miu, D.; Bacalum, M.; Busuioc, C. Development of Vitroceramic Coatings and Analysis of Their Suitability for Biomedical Applications. Coatings 2019, 9, 671. [Google Scholar] [CrossRef]
- Daves, W.; Krauss, A.; Behnel, N.; Häublein, V.; Bauer, A.; Frey, L. Amorphous silicon carbide thin films (a-SiC:H) deposited by plasma-enhanced chemical vapor deposition as protective coatings for harsh environment applications. Thin Solid Films 2011, 519, 5892–5898. [Google Scholar] [CrossRef]
- Charlesbabu, J.; Gopalakrishnan, K.; Elango, M.; Vasudevan, K. Preparation and characterization of Cd-doped ZnO thin films by spin coating method. Inorg. Nano-Metal Chem. 2017, 47, 1298–1303. [Google Scholar] [CrossRef]
- Zhang, Z.; Yin, C.; Yang, L.; Jiang, J.; Guo, Y. Optimizing the gas sensing characteristics of Co-doped SnO2 thin film based hydrogen sensor. J. Alloy Compd. 2019, 785, 819–825. [Google Scholar] [CrossRef]
- Negrea, R.; Busuioc, C.; Constantinoiu, I.; Miu, D.; Enache, C.; Iordache, F.; Jinga, S.I. Akermanite-based coatings grown by pulsed laser deposition for metallic implants employed in orthopaedics. Surf. Coat. Technol. 2019, 357, 1015–1026. [Google Scholar] [CrossRef]
- Nagaraju, P.; Vijayakumar, Y.; Ramana Reddy, M.V. Effect of oxygen partial pressure on the microstructural, optical and gas sensing characterization of nanostructured Gd doped ceria thin films deposited by pulsed laser deposition. J. Asian Ceram. Soc. 2017, 5, 402–409. [Google Scholar]
- Urper, O.; Baydogan, N. Effect of Al concentration on optical parameters of ZnO thin film derived by Sol-Gel dip coating technique. Mater. Lett. 2020, 274, 128000. [Google Scholar] [CrossRef]
- Zhao, S.; Shn, Y.; Zhou, P.; Zhang, J.; Zhang, W.; Chen, X.; Wei, D.; Fang, P.; Shen, Y. Highly selective NO2 sensor based on p-type nanocrystalline NiO thin films prepared by sol–gel dip coating. Ceram. Int. 2018, 44, 753–759. [Google Scholar] [CrossRef]
- Sakai, T.; Kato, T.; Katsui, H.; Tanaka, Y.; Goto, T. Preparation of Y-doped BaZrO3 thin film electrolyte by laser chemical vapor deposition. Mater. Today Commun. 2020, 24, 101184. [Google Scholar] [CrossRef]
- Ding, J.; Chen, S.; Han, N.; Shy, Y.; Hu, P.; Li, H.; Wang, J. Aerosol assisted chemical vapour deposition of nanostructured ZnO thin films for NO2 and ethanol monitoring. Ceram. Int. 2020, 46, 15152–15158. [Google Scholar] [CrossRef]
- Wu, Y.J.; Hsu, S.C.; Lin, Y.C.; Xu, Y.; Chuang, T.H.; Chen, S.C. Study on thermoelectric property optimization of mixed-phase bismuth telluride thin films deposited by co-evaporation process. Surf. Coat. Technol. 2020, 394, 125694. [Google Scholar] [CrossRef]
- Dev, S.; Kumar, P.; Rani, A.; Agarwal, A.; Dhar, R. Development of indium doped ZnO thin films for highly sensitive acetylene (C2H2) gas sensing. Superlattices Microstruct. 2020, 145, 106638. [Google Scholar] [CrossRef]
- Chen, X.; Bai, R.; Huang, M. Optical properties of amorphous Ta2O5 thin films deposited by RF magnetron sputtering. Opt. Mater. 2019, 97, 109404. [Google Scholar] [CrossRef]
- Ponmudi, S.; Sivakumar, R.; Sanjeeviraja, C.; Gopalakrishnan, C.; Jeyadheepan, K. Tuning the morphology of Cr2O3:CuO (50:50) thin films by RF magnetron sputtering for room temperature sensing application. Appl. Surf. Sci. 2019, 466, 703–714. [Google Scholar] [CrossRef]
- Li, Y.; Deng, D.; Xing, X.; Chen, N.; Liu, X.; Xiao, X.; Wang, Y. A high-performance methanol gas sensor based on palladium-platinum-In2O3 composited nanocrystalline SnO2. Sens. Actuator. B-Chem. 2016, 237, 133–141. [Google Scholar] [CrossRef]
- Wang, J.; Hu, C.; Xia, Y.; Komarneni, S. Highly sensitive, fast and reversible NO2 sensors at room-temperature utilizing nonplasmonic electrons of ZnO/Pd hybrids. Ceram. Int. 2020, 46, 8462–8468. [Google Scholar] [CrossRef]
- Bhati, V.S.; Nathanl, A.; Nigam, A.; Sharma, C.S.; Kumar, M. PAN/(PAN-b-PMMA) derived nanoporous carbon nanofibers loaded on ZnO nanostructures for hydrogen detection. Sens. Actuator B-Chem. 2019, 299, 126980. [Google Scholar] [CrossRef]
- Saaedi, A.; Shabani, P.; Yousefi, R. High performance of methanol gas sensing of ZnO/PAni nanocomposites synthesized under different magnetic field. J. Alloy. Compd. 2019, 802, 335–344. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Jiang, C.; Zhang, Y. Room temperature hydrogen gas sensor based on palladium decorated tin oxide/molybdenum disulfide ternary hybrid via hydrothermal route. Sens. Actuator B-Chem. 2017, 242, 15–24. [Google Scholar] [CrossRef]
- Paulowicz, I.; Hrkac, V.; Kaps, S.; Cretu, V.; Lupan, O.; Braniste, T.; Duppel, V.; Mishra, Y.K. Nanowire Networks: Three-dimensional SnO2 nanowire networks for multifunctional applications: From high-temperature stretchable ceramics to ultraresponsive sensors. Adv. Electron. Mater. 2015, 1500081. [Google Scholar] [CrossRef]
- Zang, Z.; Tang, X. Enhanced fluorescence imaging performance of hydrophobic colloidal ZnO nanoparticles by a facile method. J. Alloy. Compd. 2015, 619, 98–101. [Google Scholar] [CrossRef]
- Li, C.; Han, C.; Zhang, Y.; Zang, Z.; Wang, M.; Tang, X.; Du, J. Enhanced photoresponse of self-powered perovskite photodetector based on ZnO nanoparticles decorated CsPbBr3 films. Sol. Energy Mater. Sol. Cells 2017, 172, 341–346. [Google Scholar] [CrossRef]
- Khorramshahi, V.; Karamdel, J.; Yousefi, R. Acetic acid sensing of Mg-doped ZnO thin films fabricated by the sol–gel method. J. Mater. Sci. Mater. Electron. 2018, 29, 14679–14688. [Google Scholar] [CrossRef]
- Khorramshahi, V.; Karamdel, J.; Yousefi, R. High acetic acid sensing performance of Mg-doped ZnO/rGO nanocomposites. Ceram. Int. 2019, 45, 7034–7043. [Google Scholar] [CrossRef]
- Wang, S.Y.; Ma, J.Y.; Li, Z.J.; Su, H.Q.; Alkurd, N.R.; Zhou, W.L.; Wang, L.; Du, B.; Tang, Y.L.; Ao, D.Y.; et al. Surface acoustic wave ammonia sensor based on ZnO/SiO2 composite film. J. Hazard. Mater. 2015, 285, 368–374. [Google Scholar] [CrossRef]
- Wang, S.H.; Shen, C.Y.; Su, J.M.; Chang, S.W. A Room Temperature Nitric Oxide Gas Sensor Based on a Copper-Ion-Doped Polyaniline/Tungsten Oxide Nanocomposite. Sensors 2015, 15, 7084–7095. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, X.; Han, S.; Cai, C.; Du, H.; Zhu, H.; Zu, X.; Fu, Y. ZnO-Al2O3 nanocomposite as a sensitive layer for high performance surface acoustic wave H2S gas sensor with enhanced elastic loading effect. Sens. Actuators B Chem. 2020, 304, 127395. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhang, C.; Shi, S.; Zhang, H. High temperature CO2 sensing and its cross-sensitivity towards H2 and CO gas using calcium doped ZnO thin film coated langasite SAW sensor. Sens. Actuator B-Chem. 2019, 301, 126958. [Google Scholar] [CrossRef]
- Agarwal, S.; Rai, P.; Gatell, E.N.; Llobet, E.; Guell, F.; Kumar, M.; Awasthi, K. Gas sensing properties of ZnO nanostructures (flowers/rods) synthesized by hydrothermal method. Sens. Actuator B-Chem. 2019, 292, 24–31. [Google Scholar] [CrossRef]
- Anukunprasert, T.; Saiwan, C.; Traversa, E. The development of gas sensor for carbon monoxide monitoring using nanostructure Nb-TiO2. Sci. Technol. Adv. Mater. 2005, 6, 359–363. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Geng, X.; Wu, K.; Debliquy, M. Metal oxide semiconductors with highly concentrated oxygenvacancies for gas sensing materials: A review. Sens. Actuator B-Chem. 2020, 309, 112026. [Google Scholar] [CrossRef]
- Brattain, W.H.; Bardeen, J. Surface properties of germanium. Bell Syst. Tech. J. 1953, 32, 1–41. [Google Scholar] [CrossRef]
- Seiyama, T.; Fujisishi, K. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- Li, Y.; Li, K.; Luo, Y.; Liu, B.; Wang, H.; Gao, L.; Duan, G. Synthesis of Co3O4/ZnO nano-heterojunctions by one-off processing ZIF-8@ ZIF-67 and their gas-sensing performances for trimethylamine. Sens. Actuator B-Chem. 2020, 308, 127657. [Google Scholar] [CrossRef]
- Jiao, W.; Zhang, L. Fabrication of new C/ZnO/ZnO composite material and their enhanced gas sensing properties. Mater. Sci. Semicond. Process. 2018, 86, 63–68. [Google Scholar] [CrossRef]
- Xie, F.; Centeno, A.; Zou, B.; Ryan, M.P.; Riley, D.J.; Alford, N.M. Tunable synthesis of ordered Zinc Oxide nanoflower-like arrays. J. Colloid Interface Sci. 2013, 395, 85–90. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, X.Y.; Song, J.W.; Yao, J.H. Optical properties of ZnO andMn-doped ZnO nanocrystals by vapor phase transport processes. Nano-Micro Lett. 2009, 1, 45–48. [Google Scholar] [CrossRef]
- Iqbal, J.; Jan, T.; Ronghai, Y.; Naqvi, S.H.; Ahmad, I. Doping induced tailoring inthe morphology, band-gap and ferromagnetic properties of biocompatibleZnO nanowires, nanorods and nanoparticles. Nano-Micro Lett. 2014, 6, 242–251. [Google Scholar] [CrossRef]
- Patil, V.L.; Vanalakar, S.A.; Tarwal, N.L.; Patil, A.P.; Dongale, T.D.; Kim, J.H.; Patil, P.S. Construction of Cu doped ZnO nanorods by chemical method for Low temperature detection of NO2 gas. Sens. Actuator A-Phys. 2019, 299, 111611. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, Y.; Yang, Z.; Wu, J. Nano heterostructure construction and DFT study of Ni-doped In2O3nanocubes/WS2hexagon nanosheets for formaldehyde sensing at room temperature. ACS Appl. Mater. Interfaces 2020, 12, 11979–11989. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, J.; Li, P.; Cao, Y.; Yang, Z. Hierarchical nanoheterostructure of tungsten disulfide nanoflowers doped with zinc oxide hollow spheres: Benzene gas sensing properties and first-principles study. ACS Appl. Mater. Interfaces 2019, 11, 31245–31256. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Q.; Zheng, B.; You, J.; Luo, Y. Synthesis and acetone gas sensingproperties of Ag activated hollow sphere structured ZnFe2O4. Ceram. Int. 2018, 44, 20700–20707. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Yu, S.; Mi, Q.; Pan, Q. Diversiform metal oxide-based hybrid nanostructures for gas sensing with versatile prospects. Coord. Chem. Rev. 2020, 413, 213272. [Google Scholar] [CrossRef]
- Qin, S.; Tang, P.; Feng, Y.; Li, D. Novel ultrathin mesoporous ZnO-SnO2 n-n heterojunction nanosheets with high sensitivity to ethanol. Sens. Actuator B-Chem 2020, 309, 127801. [Google Scholar] [CrossRef]
- Abraham, N.; Krishnakumar, R.R.; Unni, C.; Philip, D. Simulation studies on the responses of ZnO-CuO/CNT nanocomposite-based SAW sensor to various volatile organic chemicals. J. Sci. 2019, 4, 125–131. [Google Scholar] [CrossRef]
- Mateti, S.; Glushenkov, A.M.; Hua Li, L.; Ma, Q.; Zhi, C.; Chen, Y. In situ doping and synthesis of two-dimensional nanomaterials using mechano-chemistry. Nanoscale Horiz. 2019, 4, 642. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced Micro- and Nano-Gas Sensor Technology: A Review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef]
- Langley, D.; Giusti, G.; Mayousse, C.; Celle, C.; Bellet, D.; Simonato, J.P. Flexible transparent conductive materials based on silver nanowire networks: A review. Nanotechnology 2013, 24, 452001. [Google Scholar] [CrossRef]
- Mandal, B.; Bhardwaj, R.; Maiti, S.; Sharma, D.S.; Das, A.K.; Mukherjee, S. Functionalized Oligo(p-Phenylenevinylene) and ZnO-Based Nanohybrid for Selective Ammonia Sensing at Room Temperature. IEEE Sens. J. 2019, 19, 2847–2854. [Google Scholar] [CrossRef]
- Pimentel, A.; Henriques Ferreira, S.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study. Materials 2016, 9, 299. [Google Scholar] [CrossRef]
- Bochenkov, V.E.; Sergeev, G.B. Sensitivity, Selectivity, and Stability of Gas-Sensitive Metal-Oxide Nanostructures. In Metal Oxide Nanostructures and Their Applications; American Scientific Publishers: Valencia, CA, USA, 2010; Volume 3, pp. 31–52. [Google Scholar]
- Han, D.; Zhai, L.; Gu, F.; Wang, Z. Highly sensitive NO2 gas sensor of ppb-level detection based on In2O3 nanobricks at low temperature. Sens. Actuator B-Chem. 2018, 262, 655–663. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.; Xue, Y.; Zhang, M.; Li, P.; Lian, K.; Zhuiykov, S.; Zhang, W.; Chen, Y. Highly sensitive and ultra-fast gas sensor based on CeO2-loaded In2O3 hollow spheres for ppb-level hydrogen detection. Sens. Actuator B-Chem. 2018, 257, 124–135. [Google Scholar] [CrossRef]
- Zhang, S.C.; Huang, Y.W.; Kuang, Z.; Wang, S.Y.; Song, W.L.; Ao, D.Y.; Liu, W.; Li, Z.J. Solvothermal Synthesized In2O3 Nanoparticles for ppb Level H2S Detection. Nanosci. Nanotechnol. Lett. 2015, 7, 455–461. [Google Scholar] [CrossRef]
- Li, Z.; Yan, S.; Wu, Z.; Li, H.; Wang, J.; Shen, W.; Wang, Z.; Fu, Y. Hydrogen gas sensor based on mesoporous In2O3 with fast response/recovery and ppb level detection limit. Int. J. Hydrogen Energy 2018, 43, 22746–22755. [Google Scholar] [CrossRef]
- Patil, V.L.; Vanalakar, S.A.; Patil, P.S.; Kim, J.H. Fabrication of nanostructured ZnO thin films based NO2 gas sensor via SILAR technique. Sens. Actuator B-Chem. 2017, 239, 1185–1193. [Google Scholar] [CrossRef]
- Marcu, A.; Viespe, C. Laser-grown ZnO nanowires for room-temperature SAW-sensor applications. Sens. Actuator B-Chem. 2015, 208, 1–6. [Google Scholar] [CrossRef]
- Li, H.; Wei, Y.; Zhao, Y.; Zhang, Y.; Yin, F.; Zhang, C.; Bakenov, Z. Simple One-Pot Synthesis of Hexagonal ZnO Nanoplates as Anode Material for Lithium-Ion Batteries. J. Nanomater. 2016, 2016, 4675960. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, T.; Deng, J.; Zhang, R.; Lou, Z.; Wang, L. P-type Co3O4 nanomaterials based gas sensor: Preparation and acetone sensing performance. Sens. Actuator B-Chem. 2017, 242, 369–377. [Google Scholar] [CrossRef]
- Shen, S.F.; Xu, M.L.; Lin, D.B.; Pan, H.B. The growth of urchin-like Co3O4 directly on sensor substrate and its gas sensing properties. Appl. Surf. Sci. 2017, 396, 327–332. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Wen, Z.; Ye, Z. Controllable synthesis of Co3O4 crossed nanosheet arrays toward an acetone gas sensor. Sens. Actuator B-Chem. 2017, 238, 1052–1059. [Google Scholar] [CrossRef]
- Lai, T.Y.; Fang, T.H.; Hsiao, Y.J.; Chan, C.A. Characteristics of Au-doped SnO2–ZnO heteronanostructures for gas sensing applications. Vacuum 2019, 166, 155–161. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Fonseca, V.S.; Andrade Neto, N.F.; Ribeiro, R.A.P.; Longo, E.; Lazaro, S.R.; Motta, F.V.; Bomio, M.R.D. Connecting theory with experiment to understand the photocatalytic activity of CuO–ZnO heterostructure. Ceram. Int. 2020, 46, 9446–9454. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor. Nanomaterials 2020, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.C.; Chen, H.I.; Liu, I.P.; Chen, C.C.; Liou, J.K.; Hsu, K.S.; Liu, W.C. On the ammonia gas sensing performance of a RF sputtered NiO thin-film sensor. IEEE Sens. J. 2015, 15, 3711–3715. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.; Zhang, S.; Chen, W.; Kuang, Z.; Ao, D.; Liu, W.; Fu, Y. A fast response & recovery H2S gas sensor based on α-Fe2O3 nanoparticles with ppb level detection limit. J. Hazard. Mater. 2015, 300, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Yang, Z.; Navale, S.T.; Han, S.; Liu, X.; Liu, W.; Lu, Y.; Stadler, F.J.; Zhu, D. Ethanol sensing behavior of Pd-nanoparticles decorated ZnO-nanorod based chemiresistive gas sensors. Sens Actuator B-Chem. 2019, 298, 126850. [Google Scholar] [CrossRef]
- Miu, D.; Birjega, R.; Viespe, C. Surface Acoustic Wave Hydrogen Sensors bsed on Nanostructured Pd/WO3 Bilayers. Sensors 2018, 18, 3636. [Google Scholar] [CrossRef]
- Selvaraj, B.; Rauappan, J.B.B.; Babu, K.J. Influence of calcination temperature on the growth of electrospun multi-junction ZnO nanowires: A room temperature ammonia sensor. Mater. Sci. Semicond. Process. 2020, 112, 105006. [Google Scholar] [CrossRef]
- Jakubik, W.; Urbanczyk, M.; Maciak, E. SAW hydrogen gas sensor based on WO3 and Pd nanostructures. Procedia Chem. 2019, 1, 200–203. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Chou, T.W.; Xu, C.Y.; Huang, W.C.; Lin, C.F.; Wu, Y.S.; Lin, Y.S.; Chen, H. ZnO/ZnS core-shell nanostructures for hydrogen gas sensing performances. Ceram. Int. 2019, 45, 17751–17757. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, X.; Wang, C.; Ma, J.; Yan, B.; Du, Z.; Li, M.; Wang, W.; Fan, H. 3D porous flower-like ZnO microstructures loaded by large-size Ag and their ultrahigh sensitivity to ethanol. J. Alloys Compd. 2020, 829, 154453. [Google Scholar] [CrossRef]
- Bharathi, P.; Mohan, M.K.; Shalini, V.; Harish, S.; Navaneethan, M.; Archanaa, J.; Kumar, M.G.; Dhivya, P.; Ponnusamy, S.; Shimomura, M.; et al. Growth and influence of Gd doping on ZnO nanostructures for enhanced optical, structural properties and gas sensing applications. Appl. Surf. Sci. 2020, 499, 143857. [Google Scholar] [CrossRef]
- Shi, W.; Shang, Y.; Ahmed, M.M.; Zhao, R.; Li, S.; Du, J.; Li, J. A facile controllable self-assembly of 3D elliptical ZnO microspheres from 1D nanowires for effective detection of acetone. Mater. Lett. 2020, 270, 127706. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Liu, Z.; Li, X.; Geng, Y.; Xiaoqing, T.; Du, Y.; Qian, Z. Enhanced acetone-sensing properties to ppb detection level using Au/Pd doped ZnO nanorod. Sens. Actuator B-Chem. 2020, 310, 127129. [Google Scholar] [CrossRef]
- Nakate, U.T.; Ahmad, R.; Patil, P.; Bhat, K.S.; Wang, Y.; Mahmoudi, T.; Yu, Y.T.; Suh, E.; Hahn, Y.B. High response and low concentration hydrogen gas sensing properties using hollow ZnO particles transformed from polystyrene@ZnO core-shell structures. Int. J. Hydrogen Energy 2019, 44, 15677–15688. [Google Scholar] [CrossRef]
- Wang, M.; Luo, Q.; Hussain, S.; Liu, G.; Qiao, G.; Kim, E.J. Sharply-precipitated spherical assembly of ZnO nanosheets for low temperature H2S gas sensing performances. Mater. Sci. Semicond. Process. 2019, 100, 283–289. [Google Scholar] [CrossRef]
- Choi, K.S.; Chang, S.P. Effect of structure morphologies on hydrogen gas sensing by ZnO nanotubes. Mater. Lett. 2018, 230, 48–52. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Kong, J.; Jia, H.; Yu, M. ZnO microspheres: Controllable preparation and optical properties. Superlattices Microstruct. 2015, 86, 228–235. [Google Scholar] [CrossRef]
- Arasu, M.V.; Madankumarb, A.; Theerthagiric, J.; Sallad, S.; Prabub, S.; Kime, H.S.; Al-Dhabia, N.A.; Arokiyarajf, S.; Duraipandiyana, V. Synthesis and characterization of ZnO nanoflakes anchored carbon nanoplates for antioxidant and anticancer activity in MCF7 cell lines. Mater. Sci. Eng. C 2019, 102, 536–540. [Google Scholar] [CrossRef]
- Zhao, X.; Nagashima, K.; Zhang, G.; Hosomi, T.; Yoshida, H.; Akihiro, Y.; Kanai, M.; Mizukami, W.; Zhu, Z.; Takahashi, T.; et al. Synthesis of Monodispersedly Sized ZnO Nanowires from Randomly Sized Seeds. Nano Lett. 2020, 20, 599–605. [Google Scholar] [CrossRef]
- DiMauro, A.; Zimbone, M.; Fragalà, M.E.; Impellizzeri, G. Synthesis of ZnO nanofibers by the electrospinning process. Mater. Sci. Semicond. Process. 2016, 42, 98–101. [Google Scholar] [CrossRef]
- Liau, L.C.K.; Huang, J.S. Energy-level variations of Cu-doped ZnO fabricated through sol-gel processing. J. Alloy. Compd. 2017, 702, 153–160. [Google Scholar] [CrossRef]
- Hui, A.; Ma, J.; Liu, J.; Bao, Y.; Zhang, J. Morphological evolution of Fe doped sea urchin-shaped ZnO nanoparticles with enhanced photocatalytic activity. J. Alloy. Compd. 2017, 696, 639–647. [Google Scholar] [CrossRef]
- Xu, K.; Liu, C.; Chen, R.; Fang, X.; Wu, X.; Liu, J. Structural and room temperature ferromagnetic properties of Ni doped ZnO nanoparticles via low-temperature hydrothermal method. Physica B 2016, 502, 155–159. [Google Scholar] [CrossRef]
- Xing, X.; Deng, D.; Li, Y.; Chen, N.; Liu, X.; Wang, Y. Macro-/nanoporous Al-doped ZnO via self-sustained decomposition of metal-organic complexes for application in degradation of Congo red. Ceram. Int. 2016, 42, 18914–18924. [Google Scholar] [CrossRef]
- Lu, W.C.; Kumar, S.S.; Chen, Y.C.; Hsu, C.M.; Lin, H.N. Au/Cu2O/ZnO ternary nanocomposite for low concentration NO2 gas sensing at room temperature. Mater. Lett. 2019, 256, 126657. [Google Scholar] [CrossRef]
- Li, F.; Asadi, H. DFT study of the effect of platinum on the H2 gas sensing performance of ZnO nanotube: Explaining the experimental observations. J. Mol. Liq. 2020, 309, 113139. [Google Scholar] [CrossRef]
- Shakya, V.; Pandey, N.K.; Misra, S.K.; Roy, A. Electrical and optical properties of ZnOWO3 nanocomposite and its application as a solid-state humidity sensor. Bull. Mater. Sci. 2017, 40, 253–262. [Google Scholar] [CrossRef]
- Wang, Z.; Song, C.; Yin, H.; Zhang, J. Capacitive humidity sensors based on zinc oxide nanorods grown on silicon nanowires arrays at room temperature. Sens. Actuator A-Phys. 2015, 235, 234–239. [Google Scholar] [CrossRef]
- Feng, M.H.; Li, X.J. Capacitive humidity-sensing properties of ZnO nanorods/silicon nanoporous pillar array enhanced by LiCl incorporation. Sens. Actuator B-Chem. 2018, 272, 543–549. [Google Scholar] [CrossRef]
- Li, B.; Tian, Q.; Su, H.; Wang, X.; Wang, T.; Zhang, D. High sensitivity protable capacitive humidity sensor based on In2O3 nanocubes-decorated GO nanosheets and its wearable application in respiration detection. Sens. Actuator B-Chem. 2019, 299, 126973. [Google Scholar] [CrossRef]
- Shruthi, J.; Jayababu, N.; Reddy, M.V.R. Synthesis of Y2O3-ZnO nanocomposites for the enhancement of room temperature 2-methoxyethanol gas sensing performance. J. Alloys Compd. 2019, 798, 438–445. [Google Scholar] [CrossRef]
- Marcu, A.; Viespe, C. Surface AcousticWave Sensors for Hydrogen and Deuterium Detection. Sensors 2017, 17, 1417. [Google Scholar] [CrossRef]
- Li, W.; Guo, Y.; Tang, Y.; Zu, X.; Ma, J.; Wang, L.; Fu, Y.Q. Room-Temperature Ammonia Sensor Based on ZnO Nanorods Deposited on ST-Cut Quartz Surface Acoustic Wave Devices. Sensors 2017, 17, 1142. [Google Scholar] [CrossRef] [PubMed]
- Shu, L.; Jiang, T.; Xia, Y.; Wang, X.; Yan, D.; Wu, W. The Investigation of a SAW Oxygen Gas Sensor Operated at Room Temperature, Based on Nanostructured ZnxFeyO Films. Sensors 2019, 19, 3025. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; He, Y.; Wen, C.; Ma, K. Surface acoustic wave ultraviolet detector based on zinc oxide nanowire sensing layer. Sens. Actuator B-Chem. 2012, 184, 34–40. [Google Scholar] [CrossRef]
- Phan, D.T.; Chung, G.S. Surface acoustic wave hydrogen sensors based on ZnO nanoparticles incorporated with a Pt catalyst. Sens. Actuator B-Chem. 2012, 161, 341–348. [Google Scholar] [CrossRef]
- Kumar, S.S.; Venkateswarlu, P.; Rao, V.R.; Rao, G.N. Synthesis, characterization and optical properties of zinc oxide nanoparticles. Int. Nano Lett. 2013, 3, 30. [Google Scholar] [CrossRef]
- Sundaram, P.S.; Inbanathan, S.S.R.; Arivazhagan, G. Structural and Optical Properties of Mn doped ZnO Nanoparticles prepared by co-precipitation method. Physica B 2019, 574, 411668. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, A.; Tomar, M.; Gupta, V. Growth of highly porous ZnO nanostructures for carbon monoxide gas sensing. Surf. Coat. Technol. 2018, 343, 49–56. [Google Scholar] [CrossRef]
- Van Khai, T.; Van Thu, L.; Ha, L.T.T.; Thanh, V.M.; Lam, T.D. Structural, optical and gas sensing properties of vertically well-aligned ZnO nanowires grown on graphene/Si substrate by thermal evaporation method. Mater. Charact. 2018, 141, 296–317. [Google Scholar] [CrossRef]
- Xu, L.; Guo, Y.; Liao, Q.; Zhang, J.; Xu, D. Morphological control of ZnO nanostructures by electrodeposition morphological control of ZnO nanostructures by electrodeposition. J. Phys. Chem. B 2005, 109, 13519–13522. [Google Scholar] [CrossRef]
- Wu, H.; Pan, W. Preparation of zinc oxide nanofibers by electrospinning. J. Am. Ceram. Soc. 2006, 89, 699–701. [Google Scholar] [CrossRef]
- Wei, S.; Zhao, J.; Hu, B.; Wu, K.; Du, W.; Zhou, M. Hydrothermal synthesis and gas sensing properties of hexagonal and orthorhombic WO3nanostructures. Ceram. Int. 2017, 43, 2579–2585. [Google Scholar] [CrossRef]
- Pál, E.; Hornok, V.; Kun, R.; Oszkó, A.; Seemann, T.; Dékány, I.; Busse, M. Hydrothermal synthesis and humidity sensing property of ZnO nanostructures and ZnOIn(OH)3 nanocomposites. J. Colloid Interface Sci. 2012, 378, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, A.; Nunes, D.; Duarte, P.; Rodrigues, J.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Synthesis of long ZnO nanorods under microwave irradiation or conventional heating. J. Phys. Chem. C 2014, 118, 14629–14639. [Google Scholar] [CrossRef]
- Hjiri, M.; Dhahri, R.; El Mir, L.; Bonavita, A.; Donato, N.; Leonardi, S.G.; Neri, G. CO sensing properties of Ga-doped ZnO prepared by sol-gel route. J. Alloys Compd. 2015, 634, 187–192. [Google Scholar] [CrossRef]
- Song, L.; Yue, H.; Li, H.; Liu, L.; Li, Y.; Du, L.; Duan, H.; Klyui, N.I. Hierarchical porous ZnO microflowers with ultra-high ethanol gas-sensing at low concentration. Chem. Phys. Lett. 2018, 699, 1–7. [Google Scholar] [CrossRef]
- Gao, F.; Boussaid, F.; Xuan, W.; Tsui, C.Y.; Bermak, A. Dual Transduction Surface Acoustic Wave Gas Sensor for VOC Discrimination. IEEE Electron Device Lett. 2018, 39, 1920–1923. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Zhang, Y.; Pan, Y.; Liang, Y.; Li, J. Enhanced Sensitivity of a Hydrogen Sulfide Sensor Based on Surface Acoustic Waves at Room Temperature. Sensors 2018, 18, 3796. [Google Scholar] [CrossRef]
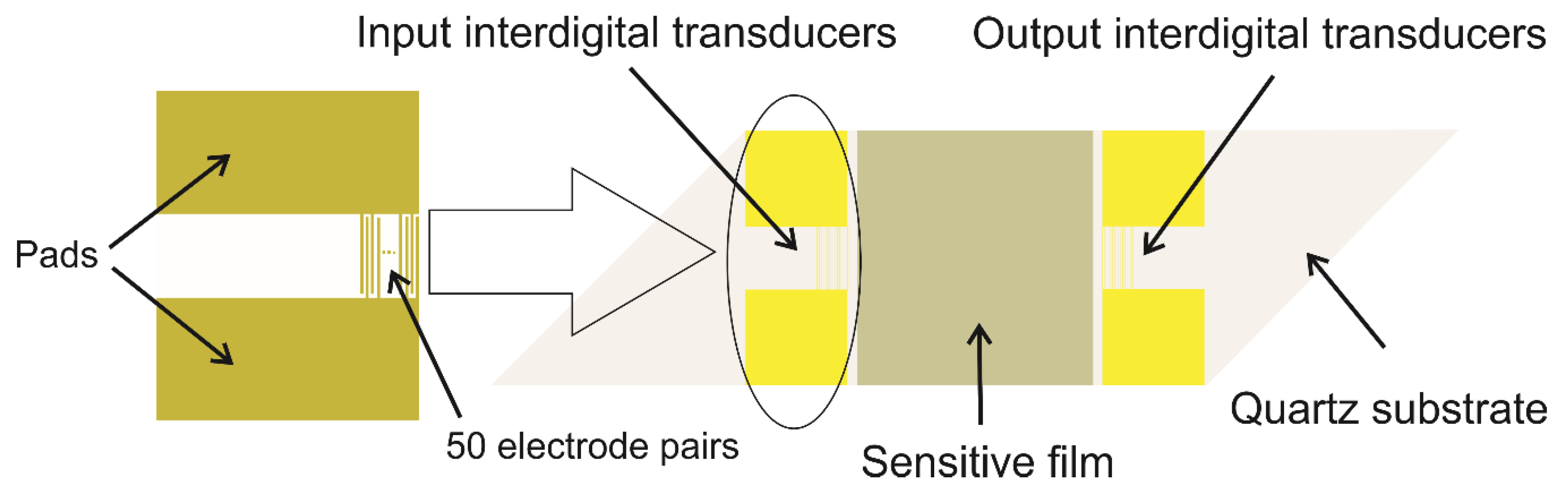

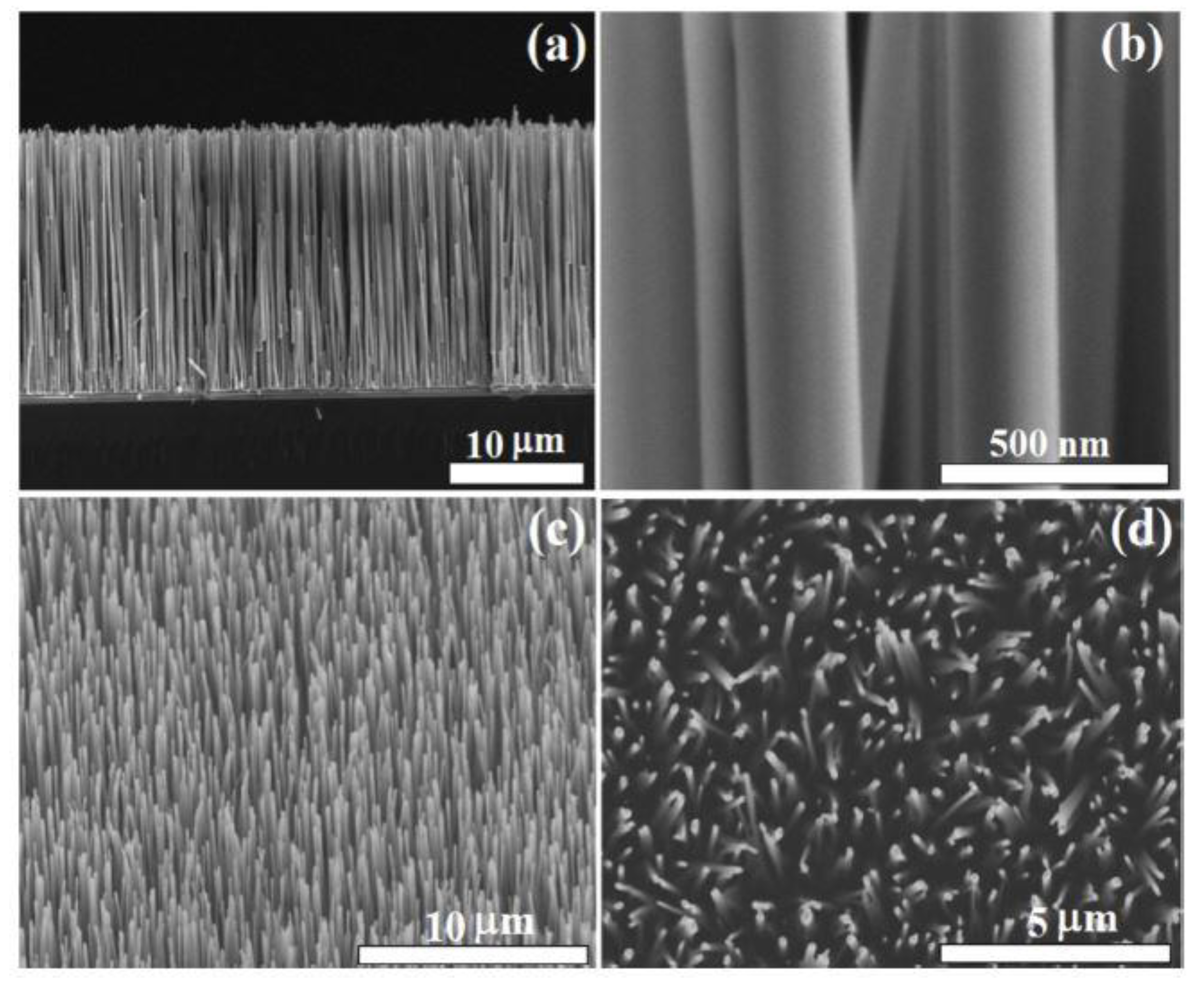

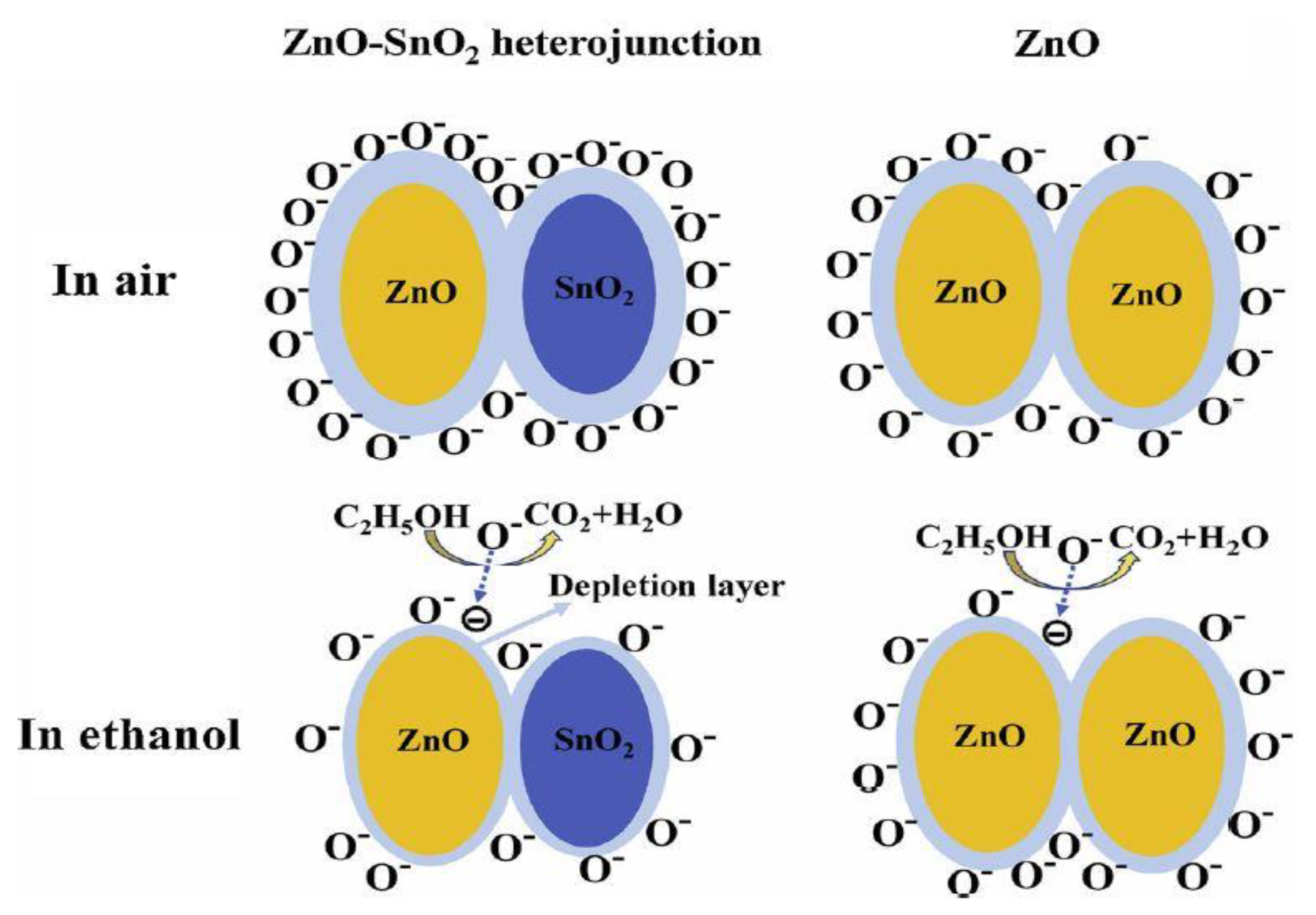

| Nr. crt | Sensor Type | Sensitive Material | Analyte | Minimum ConcentratIon Detected | Operating Condition | References |
|---|---|---|---|---|---|---|
| 1 | Resistive | ZnO nanoflowers | Ethanol | 5 ppm | 250 °C | [84] |
| Nitrogen dioxide | 250 ppb | 200 °C | ||||
| Benzene | 2.5 ppm | 250 °C | ||||
| 2 | Resistive | ZnO microspheres | Acetone | 100 ppm | 280 °C | [129] |
| 3 | Optoelectronic | ZnO/Pd | Nitrogen dioxide | 2.5 ppb | RT | [71] |
| 4 | Chemiresistive | Pd/ZnO | Ethanol | 500 ppm | 260 °C | [122] |
| 5 | Resistive | C/ZnO/ZnO | Ethanol | 100 ppm | 300 °C | [90] |
| 6 | Resistive | Au doped ZnO | Acetone | 5 ppb | 150 °C | [130] |
| Pd doped ZnO | ||||||
| 7 | Chemiresistive | ZnO nanowire-integrated film | Nitrogen dioxide | 50 ppb | RT, UV activation | [11] |
| 8 | Resistive | ZnO microspheres | Hydrogen | 100 ppm | 225 °C | [131] |
| 9 | Chemiresistive | Polyvinyl pyrrolidine-ZnO nanofibers | Ammonia | 20 ppm | RT | [124] |
| 10 | SAW | ZnO-Al2O3 composite | Hydrogen sulphide | 500 ppb | RT | [81] |
| Nr. Crt. | Sensitive Material | Analyte | Sensitivity | Operating Condition | Response/Recovery Time | References |
|---|---|---|---|---|---|---|
| 1 | ZnO | Hydrogen | 0.15 Hz/ppm | RT | 12–16 s/- | [45] |
| Pd/ZnO | 0.51 Hz/ppm | |||||
| 2 | ZnO-Al2O3 composite | Hydrogen sulfide | 15 kHz/ppm | RT | - | [82] |
| 3 | ZnO nanowires | Hydrogen | 0.062 Hz/ppm | RT | - | [112] |
| ZnO thin film | 0.010 Hz/ppm | - | ||||
| 4 | ZnO nanofilm | Ammonia | 3 Hz/ppm | RT | 50 s/34 s | [150] |
| ZnO nanorods | 11 Hz/ppm | 226 s/431 s | ||||
| 5 | ZnO/CuO | 2-propanol | 200.26 kHz/100 ppm | RT | - | [100] |
| Acetone | 107.23 kHz/100 ppm | - | ||||
| Ethanol | 100.69 kHz/100 ppm | - | ||||
| 6 | ZnXFeyO | Oxygen | -258.85 Hz/1%O2 | RT | 200 s/- | [151] |
| 7 | ZnO nanowire (600 nm) | Hydrogen | 0.015 Hz/ppm | RT | 9–15 s/6–9 s | [149] |
| Deuterium | 0.09 Hz/ppm | 9–15 s/6–9 s | ||||
| ZnO Film (100 nm) | Hydrogen | 0.005 Hz/ppm | 11–18 s/7–11 s | |||
| Deuterium | 0.026 Hz/ppm | 11–18 s/7–11 s | ||||
| 8 | ZnO/SiO2 | Ammonia | 0.1132 kHz/ppm | - | - | [80] |
| 9 | Multi-crystal ZnO | UV | 15.790 kHz | - | [152] | |
| ZnO nanowire | 101.340 kHz | - | ||||
| 10 | ZnO nanoparticle film | Hydrogen | 55 kHz/1% H2 | RT | - | [153] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantinoiu, I.; Viespe, C. ZnO Metal Oxide Semiconductor in Surface Acoustic Wave Sensors: A Review. Sensors 2020, 20, 5118. https://doi.org/10.3390/s20185118
Constantinoiu I, Viespe C. ZnO Metal Oxide Semiconductor in Surface Acoustic Wave Sensors: A Review. Sensors. 2020; 20(18):5118. https://doi.org/10.3390/s20185118
Chicago/Turabian StyleConstantinoiu, Izabela, and Cristian Viespe. 2020. "ZnO Metal Oxide Semiconductor in Surface Acoustic Wave Sensors: A Review" Sensors 20, no. 18: 5118. https://doi.org/10.3390/s20185118
APA StyleConstantinoiu, I., & Viespe, C. (2020). ZnO Metal Oxide Semiconductor in Surface Acoustic Wave Sensors: A Review. Sensors, 20(18), 5118. https://doi.org/10.3390/s20185118






