Molecular Imprinted Polymers Coupled to Photonic Structures in Biosensors: The State of Art
Abstract
1. Introduction
2. Molecularly Imprinted Polymers (MIPs)
3. Photonic Structures
3.1. Surface Plasmon Resonance
3.2. Whispering Gallery Modes Resonators
3.3. Optical Waveguide Lightmode Spectroscopy
3.4. Photonic Crystals/Colloidal Crystals
4. MIP Coupled to Photonic Structures
4.1. MIP and SPR
4.1.1. Deposition of MIP Layers
4.1.2. MIP Grafting and In Situ Controlled Polymerization
4.1.3. MIP Films by Lithographic and Printing Techniques
4.1.4. Electropolymerization of MIP Films
4.1.5. Enhancing the Sensor Signal: LSPR and Responsive MIPs
| Configuration | Preparation of the MIP | MIP Thickness | Analyte | LoD | MIP/Analyte (KD) | Sensitivity | Reference |
|---|---|---|---|---|---|---|---|
| Kretschmann | Spin coating | <nm | δ-Opioid G-protein | - | 410 fM | - | [57] |
| Plastic optical fibre | Spin coating | µm | L-Nicotine | 10−4 M | 0.67 μM | 1.3 × 104 nm/M | [58] |
| Optical fibre | Spin coating | µm | Profenofos | 2.5 × 10−6 µg/L | - | 12.7 nm/log @ 10−4 µg/L | [59] |
| Kretschmann | Photografting | 40 nm | Domoic acid | 5 μg/L | EC50 58 μg/L | - | [61] |
| Kretschmann | Photografting | 60 nm | Testosterone | 10−12 M | - | - | [62] |
| Kretschmann | Photografting/RAFT | nm | 17β-Estradiol | 1.15 × 10−15 M | - | - | [63] |
| Kretschmann | Photografting/RAFT | nm | Progesterone | 0.3 × 10−19 M | - | - | [64] |
| Kretschmann | Photografting/ATPR | nm | Ovalbumin | 6.4 ng/mL | - | - | [65] |
| Kretschmann | Photografting/iniferter | nm | Bovine serum albumin | 5.6 × 10−9 M | 0.17 × 10−8 M | 7.4 µRIU | [66] |
| LSPR | Au NPs embedded in MIP | 6 µm | Dopamine | 10−6 M | - | - | [77] |
| LSPR | MIP NPs & Au NPs | nm | Bisphenol A | <10−9 M | 4.7 × 10−9 M | - | [80] |
| LSPR | Au-NPs hybrid MIP-microgels | µm | Glucose | 0.6 × 10−3 M | - | - | [81] |
| LSPR | Fe3O4@polydopamine NPs | - | Chlorpyrifos | 0.76 × 10−9 M | - | - | [82] |
| LSPR | Au-nanostars embedded in MIP | µm | Trinitrotoluene | 2.4 × 10−6 M | 10−5 M | 8.5 × 104 nm/M | [83] |
| LSPR | Ag@AuNPs hexagonal boron nitride (HBN) nanosheets and MIP | 34 nm | Etoposide | 0.4 × 10−12 M | - | - | [84] |
| Kretschmann | Swellable MIP spin coated | µm | Theophylline | 10−6 M | - | - | [85] |
| Kretschmann | Swellable MIP spin coated | - | Dichlorobenzidine | 9 × 10−9 M | 10−12 M | - | [86] |
| Plastic optical fibre | Swellable MIP NPs grafted | 10–50 nm | Serum transferrin | 1.2 × 10−15 M | 10−15 M | - | [41] |
4.2. MIP and Waveguides
| Waveguide Material | Optical Configuration | MIP Thickness | Analyte | LoD or LoQ | Reference |
|---|---|---|---|---|---|
| Polymer | Free-standing filaments | 20 µm | 2,4-Dichlorophenoxy acetic (2,4-D) | LoQ: 0.021 µmol/g | [87] |
| Polymer | Free-standing filaments | <20 µm | Anthracene | LoQ: 1.6 µmol/g | [88] |
| Polymer | Free-standing filaments | 100 µm | Anthracene | - | [89] |
| SiO2/TiO2 | IOW | ∼0.3–1.0 µm | 2,4,6-Trinitrotoluene (TNT) | LoD: 5 ppb | [90] |
| Si3N4/SiO2 | Interferometry | 20–120 nm | TNT | LoD: 2.4 ppt | [91] |
| Si3N4/SiO2 | Diffraction grating | 322 nm | Enrofloxacin | LoQ: <50 µM | [92] |
| Polymer | Young interferometer | - | Melanine | LoQ: <0.1 g/L | [93] |
| Glass | Fabry-Pérot interferometer | 19 µm | Microcystin-LR | LoQ: >1.8 μg/L | [95] |
| Glass | Fiber microarrays | 3.1 µm | Enrofloxacin | LoD: 40 nM | [101] |
| Glass | Fiber | 200 µm | Organophosphates | LoD: <10 ppt | [94] |
| Glass | Fiber | <5 µm | Bisphenol A | LoD: 1.7 ng/mL | [96] |
| Glass | Fiber | - | Cocaine | LoQ: <25 μM | [97] |
| Polymer | Fiber coupled to spectrofluorimeter | few µm to nm | 2,4-D | LoQ: 2.5 nM | [98] |
| ZnO/MoS2 | Lossy mode resonance | 1.2 µm | p-Cresol | LoD: 28 nM | [100] |
4.3. MIP and Photonic Crystals
4.4. MIP and Whispering Gallery Modes Resonators
5. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roda, A.; Michelini, E.; Cevenini, L.; Calabria, D.; Calabretta, M.M.; Simoni, P. Integrating Biochemiluminescence Detection on Smartphones: Mobile Chemistry Platform for Point-of-Need Analysis. Anal. Chem. 2014, 86, 7299–7304. [Google Scholar] [CrossRef]
- Tütüncü, E.; Kokoric, V.; Wilk, A.; Seichter, F.; Schmid, M.; Hunt, W.E.; Manuel, A.M.; Mirkarimi, P.; Alameda, J.B.; Carter, J.C.; et al. Fiber-Coupled Substrate-Integrated Hollow Waveguides: An Innovative Approach to Mid-infrared Remote Gas Sensors. ACS Sens. 2017, 2, 1287–1293. [Google Scholar] [CrossRef]
- Market_Report. Available online: https://www.marketsandmarkets.com/Market-Reports/biosensors-market-798.html (accessed on 25 August 2020).
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef]
- Joe, H.-E.; Yun, H.; Jo, S.-H.; Jun, M.; Min, B.-K. A review on optical fiber sensors for environmental monitoring. Int. J. Precis. Eng. Manuf. Technol. 2018, 5, 173–191. [Google Scholar] [CrossRef]
- Yurish, S. Physical Sensors, Sensor Networks and Remote Sensing. In Advances in Sensors: Reviews; IFSA: Barcelona, Spain, 2018; Volume 5. [Google Scholar]
- Schuller, J.A.; Barnard, E.S.; Cai, W.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for extreme light concentration and manipulation. Nat. Mater. 2010, 9, 193–204. [Google Scholar] [CrossRef]
- Bossi, A.; Bonini, F.; Turner, A.P.F.; Piletsky, S.A. Molecularly imprinted polymers for the recognition of proteins: The state of the art. Biosens. Bioelectron. 2007, 22, 1131–1137. [Google Scholar] [CrossRef]
- Piletsky, S.; Canfarotta, F.; Poma, A.; Bossi, A.M.; Piletsky, S. Molecularly Imprinted Polymers for Cell Recognition. Trends Biotechnol. 2020, 38, 368–387. [Google Scholar] [CrossRef]
- Nicholls, I.A.; Andersson, H.S.; Charlton, C.; Henschel, H.; Karlsson, B.C.G.; Karlsson, J.G.; O’Mahony, J.; Rosengren, A.M.; Rosengren, K.J.; Wikman, S. Theoretical and computational strategies for rational molecularly imprinted polymer design. Biosens. Bioelectron. 2009, 25, 543–552. [Google Scholar] [CrossRef]
- Busato, M.; Distefano, R.; Bates, F.; Karim, K.; Bossi, A.M.; López Vilariño, J.M.; Piletsky, S.; Bombieri, N.; Giorgetti, A. MIRATE: MIps RATional dEsign Science Gateway. J. Integr. Bioinform. 2018, 15. [Google Scholar] [CrossRef]
- Kupai, J.; Razali, M.; Buyuktiryaki, S.; Kecili, R.; Szekely, G. Long-term stability and reusability of molecularly imprinted polymers. Polym. Chem. 2017, 8, 666–673. [Google Scholar] [CrossRef]
- Safaryan, A.H.M.; Smith, A.M.; Bedwell, T.S.; Piletska, E.V.; Canfarotta, F.; Piletsky, S.A. Optimisation of the preservation conditions for molecularly imprinted polymer nanoparticles specific for trypsin. Nanoscale Adv. 2019, 1, 3709–3714. [Google Scholar] [CrossRef]
- MIP Diagnostics Limited. Available online: https://www.mip-dx.com/ (accessed on 25 August 2020).
- AFFINISEP. Available online: https://www.affinisep.com/ (accessed on 25 August 2020).
- MIP Technologies. Available online: http://www.miptechnologies.com/ (accessed on 25 August 2020).
- Arshady, R.; Mosbach, K. Synthesis of substrate-selective polymers by host-guest polymerization. Die Makromol. Chem. 1981, 182, 687–692. [Google Scholar] [CrossRef]
- Wulff, G.; Sarhan, A. Macromolecular Colloquium. Angew. Chem. Int. Ed. Engl. 1972, 11, 334–342. [Google Scholar]
- Rossetti, C.; Świtnicka-Plak, M.A.; Grønhaug Halvorsen, T.; Cormack, P.A.G.; Sellergren, B.; Reubsaet, L. Automated Protein Biomarker Analysis: On-line extraction of clinical samples by Molecularly Imprinted Polymers. Sci. Rep. 2017, 7, 44298. [Google Scholar] [CrossRef]
- Ertürk, G.; Lood, R. Ultrasensitive Detection of Biomarkers by Using a Molecular Imprinting Based Capacitive Biosensor. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Panagiotopoulou, M.; Kunath, S.; Haupt, K.; Bui, B.T.S. Cell and Tissue Imaging with Molecularly Imprinted Polymers. Methods Mol. Biol. 2017, 1575, 399–415. [Google Scholar] [CrossRef]
- Graham, S.P.; El-Sharif, H.F.; Hussain, S.; Fruengel, R.; McLean, R.K.; Hawes, P.C.; Sullivan, M.V.; Reddy, S.M. Evaluation of Molecularly Imprinted Polymers as Synthetic Virus Neutralizing Antibody Mimics. Front. Bioeng. Biotechnol. 2019, 7, 115. [Google Scholar] [CrossRef]
- Wang, S.; Yin, D.; Wang, W.; Shen, X.; Zhu, J.-J.; Chen, H.-Y.; Liu, Z. Targeting and Imaging of Cancer Cells via Monosaccharide-Imprinted Fluorescent Nanoparticles. Sci. Rep. 2016, 6, 22757. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Medina Rangel, P.X.; Bui, B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef]
- Poma, A.; Turner, A.P.F.; Piletsky, S.A. Advances in the manufacture of MIP nanoparticles. Trends Biotechnol. 2010, 28, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.H.; Mosbach, K.; Haupt, K. A Simple Method for Spin-Coating Molecularly Imprinted Polymer Films of Controlled Thickness and Porosity. Adv. Mater. 2004, 16, 719–722. [Google Scholar] [CrossRef]
- Vandevelde, F.; Leïchlé, T.; Ayela, C.; Bergaud, C.; Nicu, L.; Haupt, K. Direct Patterning of Molecularly Imprinted Microdot Arrays for Sensors and Biochips. Langmuir 2007, 23, 6490–6493. [Google Scholar] [CrossRef] [PubMed]
- Linares, A.V.; Falcimaigne-Cordin, A.; Gheber, L.A.; Haupt, K. Patterning Nanostructured, Synthetic, Polymeric Receptors by Simultaneous Projection Photolithography, Nanomolding, and Molecular Imprinting. Small 2011, 7, 2318–2325. [Google Scholar] [CrossRef]
- Fuchs, Y.; Linares, A.V.; Mayes, A.G.; Haupt, K.; Soppera, O. Ultrathin Selective Molecularly Imprinted Polymer Microdots Obtained by Evanescent Wave Photopolymerization. Chem. Mater. 2011, 23, 3645–3651. [Google Scholar] [CrossRef]
- Cui, M.; Zhuang, Y.; Zou, G.; Zhu, B.; Zhang, Q. Fabrication of composite optical fiber taper through “click” polymerization initiated by evanescent field for sensing. Sens. Actuators B Chem. 2019, 284, 243–249. [Google Scholar] [CrossRef]
- Gomez, L.P.C.; Spangenberg, A.; Ton, X.-A.; Fuchs, Y.; Bokeloh, F.; Malval, J.-P.; Bui, B.T.S.; Thuau, D.; Ayela, C.; Haupt, K.; et al. Rapid Prototyping of Chemical Microsensors Based on Molecularly Imprinted Polymers Synthesized by Two-Photon Stereolithography. Adv. Mater. 2016, 28, 5931–5937. [Google Scholar] [CrossRef]
- Pérez-Moral, N.; Mayes, A.G. Noncovalent Imprinting in the Shell of Core-Shell Nanoparticles. Langmuir 2004, 20, 3775–3779. [Google Scholar] [CrossRef]
- Canfarotta, F.; Poma, A.; Guerreiro, A.; Piletsky, S. Solid-phase synthesis of molecularly imprinted nanoparticles. Nat. Protoc. 2016, 11, 443–455. [Google Scholar] [CrossRef]
- Xu, J.; Medina-Rangel, P.X.; Haupt, K.; Bui, B.T.S. Guide to the Preparation of Molecularly Imprinted Polymer Nanoparticles for Protein Recognition by Solid-Phase Synthesis. Methods Enzymol. 2017, 590, 115–141. [Google Scholar] [CrossRef]
- Xing, R.; Wang, S.; Bie, Z.; He, H.; Liu, Z. Preparation of molecularly imprinted polymers specific to glycoproteins, glycans and monosaccharides via boronate affinity controllable-oriented surface imprinting. Nat. Protoc. 2017, 12, 964–987. [Google Scholar] [CrossRef]
- Hoshino, Y.; Kodama, T.; Okahata, Y.; Shea, K.J. Peptide Imprinted Polymer Nanoparticles: A Plastic Antibody. J. Am. Chem. Soc. 2008, 130, 15242–15243. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, A.R.; Chianella, I.; Piletska, E.; Whitcombe, M.J.; Piletsky, S.A. Selection of imprinted nanoparticles by affinity chromatography. Biosens. Bioelectron. 2009, 24, 2740–2743. [Google Scholar] [CrossRef] [PubMed]
- Cenci, L.; Andreetto, E.; Vestri, A.; Bovi, M.; Barozzi, M.; Iacob, E.; Busato, M.; Castagna, A.; Girelli, D.; Bossi, A.M. Surface plasmon resonance based on molecularly imprinted nanoparticles for the picomolar detection of the iron regulating hormone Hepcidin-25. J. Nanobiotechnol. 2015, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Bossi, A.; Piletsky, S.A.; Piletska, E.V.; Righetti, P.G.; Turner, A.P.F. Surface-Grafted Molecularly Imprinted Polymers for Protein Recognition. Anal. Chem. 2001, 73, 5281–5286. [Google Scholar] [CrossRef]
- Cennamo, N.; Maniglio, D.; Tatti, R.; Zeni, L.; Bossi, A.M. Deformable molecularly imprinted nanogels permit sensitivity-gain in plasmonic sensing. Biosens. Bioelectron. 2020, 156, 112126. [Google Scholar] [CrossRef] [PubMed]
- Bertolla, M.; Cenci, L.; Anesi, A.; Ambrosi, E.; Tagliaro, F.; Vanzetti, L.; Guella, G.; Bossi, A.M. Solvent-Responsive Molecularly Imprinted Nanogels for Targeted Protein Analysis in MALDI-TOF Mass Spectrometry. ACS Appl. Mater. Interfaces 2017, 9, 6908–6915. [Google Scholar] [CrossRef]
- Boutureira, O.; Bernardes, G.J.L. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Optical biosensors: An exhaustive and comprehensive review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef]
- Mauriz, E. Low-Fouling Substrates for Plasmonic Sensing of Circulating Biomarkers in Biological Fluids. Biosensors 2020, 10, 63. [Google Scholar] [CrossRef]
- Vollmer, F.; Arnold, S. Whispering-gallery-mode biosensing: Label-free detection down to single molecules. Nat. Methods 2008, 5, 591–596. [Google Scholar] [CrossRef]
- Taitt, C.R.; Anderson, G.P.; Ligler, F.S. Evanescent wave fluorescence biosensors: Advances of the last decade. Biosens. Bioelectron. 2016, 76, 103–112. [Google Scholar] [CrossRef]
- Choi, E.; Choi, Y.; Nejad, Y.H.P.; Shin, K.; Park, J. Label-free specific detection of immunoglobulin G antibody using nanoporous hydrogel photonic crystals. Sens. Actuators B Chem. 2013, 180, 107–113. [Google Scholar] [CrossRef]
- Ho, A.H.P.; Kim, D.; Somekh, M.G. (Eds.) Handbook of Photonics for Biomedical Engineering, 2017 ed.; Springer: Dordrecht, The Netherlands, 2017; ISBN 978-94-007-5051-7. [Google Scholar]
- Righini, G.C.; Soria, S. Biosensing by WGM microspherical resonators. Sensors 2016, 16, 905. [Google Scholar] [CrossRef]
- Cai, L.; Pan, J.; Zhao, Y.; Wang, J.; Xiao, S. Whispering Gallery Mode Optical Microresonators: Structures and Sensing Applications. Phys. Status Solidi 2020, 217, 1900825. [Google Scholar] [CrossRef]
- Gauglitz, G. Critical assessment of relevant methods in the field of biosensors with direct optical detection based on fibers and waveguides using plasmonic, resonance, and interference effects. Anal. Bioanal. Chem. 2020, 412, 3317–3349. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.S. Optical Waveguide Spectroscopy. In Handbook of Spectroscopy; Wiley: Hoboken, NJ, USA, 2014; pp. 1611–1642. [Google Scholar]
- Chiappini, A.; Tran, L.T.N.; Trejo-García, P.M.; Zur, L.; Lukowiak, A.; Ferrari, M.; Righini, G.C. Photonic Crystal Stimuli-Responsive Chromatic Sensors: A Short Review. Micromachines 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Opalux. Available online: https://opalux.com/ (accessed on 25 August 2020).
- Lai, E.P.C.; Fafara, A.; VanderNoot, V.A.; Kono, M.; Polsky, B. Surface plasmon resonance sensors using molecularly imprinted polymers for sorbent assay of theophylline, caffeine, and xanthine. Can. J. Chem. 1998, 76, 265–273. [Google Scholar] [CrossRef]
- Devanathan, S.; Salamon, Z.; Nagar, A.; Narang, S.; Schleich, D.; Darman, P.; Hruby, V.; Tollin, G. Subpicomolar Sensing of δ-Opioid Receptor Ligands by Molecular-Imprinted Polymers Using Plasmon-Waveguide Resonance Spectroscopy. Anal. Chem. 2005, 77, 2569–2574. [Google Scholar] [CrossRef]
- Cennamo, N.; D’Agostino, G.; Pesavento, M.; Zeni, L. High selectivity and sensitivity sensor based on MIP and SPR in tapered plastic optical fibers for the detection of l-nicotine. Sens. Actuators B Chem. 2014, 191, 529–536. [Google Scholar] [CrossRef]
- Verma, R.; Gupta, B.D. Optical fiber sensor for the detection of tetracycline using surface plasmon resonance and molecular imprinting. Analyst 2013, 138, 7254–7263. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Usha, S.P.; Gupta, B.D. Fiber optic profenofos sensor based on surface plasmon resonance technique and molecular imprinting. Biosens. Bioelectron. 2016, 79, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Lotierzo, M.; Henry, O.Y.F.; Piletsky, S.; Tothill, I.; Cullen, D.; Kania, M.; Hock, B.; Turner, A.P.F. Surface plasmon resonance sensor for domoic acid based on grafted imprinted polymer. Biosens. Bioelectron. 2004, 20, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Jing, L.; Ding, Y.; Wei, T. A novel double-layer molecularly imprinted polymer film based surface plasmon resonance for determination of testosterone in aqueous media. Appl. Surf. Sci. 2015, 342, 84–91. [Google Scholar] [CrossRef]
- Wang, Y.; Jiao, S.Q.; Chen, X.L.; Wei, T.X. An efficient grafting technique for producing molecularly imprinted film via reversible addition-fragmentation chain transfer polymerization. Anal. Methods 2017, 9, 5356–5364. [Google Scholar] [CrossRef]
- Nawaz, T.; Ahmad, M.; Yu, J.; Wang, S.; Wei, T. The biomimetic detection of progesterone by novel bifunctional group monomer based molecularly imprinted polymers prepared in UV light. New J. Chem. 2020, 44, 6992–7000. [Google Scholar] [CrossRef]
- Saeki, T.; Sunayama, H.; Kitayama, Y.; Takeuchi, T. Orientationally Fabricated Zwitterionic Molecularly Imprinted Nanocavities for Highly Sensitive Glycoprotein Recognition. Langmuir 2019, 35, 1320–1326. [Google Scholar] [CrossRef]
- Kidakova, A.; Reut, J.; Rappich, J.; Öpik, A.; Syritski, V. Preparation of a surface-grafted protein-selective polymer film by combined use of controlled/living radical photopolymerization and microcontact imprinting. React. Funct. Polym. 2018, 125, 47–56. [Google Scholar] [CrossRef]
- Casey, C.N.; Campbell, S.E.; Gibson, U.J. Phenylalanine detection using matrix assisted pulsed laser evaporation of molecularly imprinted amphiphilic block copolymer films. Biosens. Bioelectron. 2010, 26, 703–709. [Google Scholar] [CrossRef]
- Lautner, G.; Kaev, J.; Reut, J.; Öpik, A.; Rappich, J.; Syritski, V.; Gyurcsányi, R.E. Selective Artificial Receptors Based on Micropatterned Surface-Imprinted Polymers for Label-Free Detection of Proteins by SPR Imaging. Adv. Funct. Mater. 2011, 21, 591–597. [Google Scholar] [CrossRef]
- Sener, G.; Ozgur, E.; Rad, A.Y.; Uzun, L.; Say, R.; Denizli, A. Rapid real-time detection of procalcitonin using a microcontact imprinted surface plasmon resonance biosensor. Analyst 2013, 138, 6422–6428. [Google Scholar] [CrossRef]
- Perçin, I.; Idil, N.; Bakhshpour, M.; Yılmaz, E.; Mattiasson, B.; Denizli, A. Microcontact Imprinted Plasmonic Nanosensors: Powerful Tools in the Detection of Salmonella paratyphi. Sensors 2017, 17, 1375. [Google Scholar] [CrossRef]
- Yu, J.; Lai, E. Interaction of ochratoxin A with molecularly imprinted polypyrrole film on surface plasmon resonance sensor. React. Funct. Polym. 2005, 63, 171–176. [Google Scholar] [CrossRef]
- Pernites, R.B.; Ponnapati, R.R.; Advincula, R.C. Surface Plasmon Resonance (SPR) Detection of Theophylline via Electropolymerized Molecularly Imprinted Polythiophenes. Macromolecules 2010, 43, 9724–9735. [Google Scholar] [CrossRef]
- Pernites, R.; Ponnapati, R.; Felipe, M.J.; Advincula, R. Electropolymerization molecularly imprinted polymer (E-MIP) SPR sensing of drug molecules: Pre-polymerization complexed terthiophene and carbazole electroactive monomers. Biosens. Bioelectron. 2011, 26, 2766–2771. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Pernites, R.B.; Danda, C.; Advincula, R.C. SPR Detection of Dopamine Using Cathodically Electropolymerized, Molecularly Imprinted Poly-p-aminostyrene Thin Films. Macromol. Chem. Phys. 2011, 212, 2439–2451. [Google Scholar] [CrossRef]
- Gupta, G.; Bhaskar, A.S.B.; Tripathi, B.K.; Pandey, P.; Boopathi, M.; Rao, P.V.L.; Singh, B.; Vijayaraghavan, R. Supersensitive detection of T-2 toxin by the in situ synthesized π-conjugated molecularly imprinted nanopatterns. An in situ investigation by surface plasmon resonance combined with electrochemistry. Biosens. Bioelectron. 2011, 26, 2534–2540. [Google Scholar] [CrossRef] [PubMed]
- Baldoneschi, V.; Palladino, P.; Banchini, M.; Minunni, M.; Scarano, S. Norepinephrine as new functional monomer for molecular imprinting: An applicative study for the optical sensing of cardiac biomarkers. Biosens. Bioelectron. 2020, 157, 112161. [Google Scholar] [CrossRef]
- Matsui, J.; Akamatsu, K.; Hara, N.; Miyoshi, D.; Nawafune, H.; Tamaki, K.; Sugimoto, N. SPR Sensor Chip for Detection of Small Molecules Using Molecularly Imprinted Polymer with Embedded Gold Nanoparticles. Anal. Chem. 2005, 77, 4282–4285. [Google Scholar] [CrossRef]
- Özgür, E.; Topçu, A.A.; Yılmaz, E.; Denizli, A. Surface plasmon resonance based biomimetic sensor for urinary tract infections. Talanta 2020, 212, 120778. [Google Scholar] [CrossRef]
- Taguchi, Y.; Takano, E.; Takeuchi, T. SPR Sensing of Bisphenol A Using Molecularly Imprinted Nanoparticles Immobilized on Slab Optical Waveguide with Consecutive Parallel Au and Ag Deposition Bands Coexistent with Bisphenol A-Immobilized Au Nanoparticles. Langmuir 2012, 28, 7083–7088. [Google Scholar] [CrossRef] [PubMed]
- Uchida, A.; Kitayama, Y.; Takano, E.; Ooya, T.; Takeuchi, T. Supraparticles comprised of molecularly imprinted nanoparticles and modified gold nanoparticles as a nanosensor platform. RSC Adv. 2013, 3, 25306–25311. [Google Scholar] [CrossRef]
- Wu, W.; Shen, J.; Li, Y.; Zhu, H.; Banerjee, P.; Zhou, S. Specific glucose-to-SPR signal transduction at physiological pH by molecularly imprinted responsive hybrid microgels. Biomaterials 2012, 33, 7115–7125. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.-H.; Liang, R.-P.; Huang, C.-F.; Wang, Y.; Qiu, J.-D. Surface Plasmon Resonance Sensor Based on Magnetic Molecularly Imprinted Polymers Amplification for Pesticide Recognition. Anal. Chem. 2013, 85, 11944–11951. [Google Scholar] [CrossRef]
- Cennamo, N.; Donà, A.; Pallavicini, P.; D’Agostino, G.; Dacarro, G.; Zeni, L.; Pesavento, M. Sensitive detection of 2,4,6-trinitrotoluene by tridimensional monitoring of molecularly imprinted polymer with optical fiber and five-branched gold nanostars. Sens. Actuators B Chem. 2015, 208, 291–298. [Google Scholar] [CrossRef]
- Özkan, A.; Atar, N.; Yola, M.L. Enhanced surface plasmon resonance (SPR) signals based on immobilization of core-shell nanoparticles incorporated boron nitride nanosheets: Development of molecularly imprinted SPR nanosensor for anticancer drug, etoposide. Biosens. Bioelectron. 2019, 130, 293–298. [Google Scholar] [CrossRef]
- Lavine, B.K.; Westover, D.J.; Kaval, N.; Mirjankar, N.; Oxenford, L.; Mwangi, G.K. Swellable molecularly imprinted polyN-(N-propyl)acrylamide particles for detection of emerging organic contaminants using surface plasmon resonance spectroscopy. Talanta 2007, 72, 1042–1048. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, J.; Zhang, L.; Zhou, J. A 3,3′-dichlorobenzidine-imprinted polymer gel surface plasmon resonance sensor based on template-responsive shrinkage. Anal. Chim. Acta 2014, 812, 129–137. [Google Scholar] [CrossRef]
- Yan, M.; Kapua, A. Fabrication of molecularly imprinted polymer microstructures. Anal. Chim. Acta 2001, 435, 163–167. [Google Scholar] [CrossRef]
- Brazier, J.; Yan, M.; Prahl, S.; Chen, Y.-C. Molecularly Imprinted Polymers Used as Optical Waveguides for the Detection of Fluorescent Analytes. Mater. Res. Soc. Symp. Proc. 2002, 723, 6. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Barzier, J.J.; Yan, M.; Prahl, S.A. Evaluation of molecularly imprinted polyurethane as an optical waveguide for PAH sensing. Nanosens. Mater. Devices 2004, 5593, 513. [Google Scholar] [CrossRef]
- Walker, N.R.; Linman, M.J.; Timmers, M.M.; Dean, S.L.; Burkett, C.M.; Lloyd, J.A.; Keelor, J.D.; Baughman, B.M.; Edmiston, P.L. Selective detection of gas-phase TNT by integrated optical waveguide spectrometry using molecularly imprinted sol-gel sensing films. Anal. Chim. Acta 2007, 593, 82–91. [Google Scholar] [CrossRef]
- Edmiston, P.L.; Campbell, D.P.; Gottfried, D.S.; Baughman, J.; Timmers, M.M. Detection of vapor phase trinitrotoluene in the parts-per-trillion range using waveguide interferometry. Sens. Actuators B Chem. 2010, 143, 574–582. [Google Scholar] [CrossRef]
- Barrios, C.A.; Carrasco, S.; Francesca, M.; Yurrita, P.; Navarro-Villoslada, F.; Moreno-Bondi, M.C. Molecularly imprinted polymer for label-free integrated optical waveguide bio(mimetic)sensors. Sens. Actuators B Chem. 2012, 161, 607–614. [Google Scholar] [CrossRef]
- Aikio, S.; Zeilinger, M.; Hiltunen, J.; Hakalahti, L.; Hiitola-Keinänen, J.; Hiltunen, M.; Kontturi, V.; Siitonen, S.; Puustinen, J.; Lieberzeit, P.; et al. Disposable (bio)chemical integrated optical waveguide sensors implemented on roll-to-roll produced platforms. RSC Adv. 2016, 6, 50414–50422. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Yin, R.; Jensen, J.L. Molecularly imprinted polymer sensors for pesticide and insecticide detection in water. Analyst 2001, 126, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Queirós, R.B.; Silva, S.O.; Sales, M.G.F.; Noronha, J.P.; Frazão, O.; Jorge, P.A.S.; Aguilar, G.G. Optical cavity fibre sensor for detection of microcystin-LR in water. Fourth Eur. Work. Opt. Fibre Sens. 2010, 7653, 76531N. [Google Scholar] [CrossRef]
- Xiong, Y.; Ye, Z.; Xu, J.; Liu, Y.; Zhang, H. A microvolume molecularly imprinted polymer modified fiber-optic evanescent wave sensor for bisphenol A determination. Anal. Bioanal. Chem. 2014, 406, 2411–2420. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Sun, T.; Grattan, K.T.V.; Hardwick, S.A. A fibre optic chemical sensor for the detection of cocaine. Fourth Eur. Work. Opt. Fibre Sens. 2010, 7653, 76531V. [Google Scholar] [CrossRef]
- Ton, X.A.; Acha, V.; Bonomi, P.; Bui, B.T.S.; Haupt, K. A disposable evanescent wave fiber optic sensor coated with a molecularly imprinted polymer as a selective fluorescence probe. Biosens. Bioelectron. 2015, 64, 359–366. [Google Scholar] [CrossRef]
- Ton, X.-A.; Bui, B.T.S.; Resmini, M.; Bonomi, P.; Dika, I.; Soppera, O.; Haupt, K. A versatile fiber-optic fluorescence sensor based on molecularly imprinted microstructures polymerized in situ. Angew. Chem. Int. Ed. Engl. 2013, 52, 8317–8321. [Google Scholar] [CrossRef] [PubMed]
- Usha, S.P.; Gupta, B.D. Urinary p-cresol diagnosis using nanocomposite of ZnO/MoS2 and molecular imprinted polymer on optical fiber based lossy mode resonance sensor. Biosens. Bioelectron. 2018, 101, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, S.; Benito-Peña, E.; Walt, D.R.; Moreno-Bondi, M.C. Fiber-optic array using molecularly imprinted microspheres for antibiotic analysis. Chem. Sci. 2015, 6, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef]
- Chen, H.; Lou, R.; Chen, Y.; Chen, L.; Lu, J.; Dong, Q. Photonic crystal materials and their application in biomedicine. Drug Deliv. 2017, 24, 775–780. [Google Scholar] [CrossRef]
- Chiappini, A.; Pasquardini, L.; Nodehi, S.; Armellini, C.; Bazzanella, N.; Lunelli, L.; Pelli, S.; Ferrari, M.; Pietralunga, S.M. Fluorescent aptamer immobilization on inverse colloidal crystals. Sensors 2018, 18. [Google Scholar] [CrossRef]
- Wang, H.; Gu, H.; Chen, Z.; Shang, L.; Zhao, Z.; Gu, Z.; Zhao, Y. Enzymatic Inverse Opal Hydrogel Particles for Biocatalyst. ACS Appl. Mater. Interfaces 2017, 9, 12914–12918. [Google Scholar] [CrossRef]
- Lee, W.S.; Kang, T.; Kim, S.-H.; Jeong, J. An Antibody-Immobilized Silica Inverse Opal Nanostructure for Label-Free Optical Biosensors. Sensors 2018, 18. [Google Scholar] [CrossRef]
- Chen, W.; Meng, Z.; Xue, M.; Shea, K.J. Molecular imprinted photonic crystal for sensing of biomolecules. Mol. Impr. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Dai, J.; Vu, D.; Nagel, S.; Lin, C.H.; Fidalgo de Cortalezzi, M. Colloidal crystal templated molecular imprinted polymer for the detection of 2-butoxyethanol in water contaminated by hydraulic fracturing. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cui, Y.; Bai, J.; Sun, Z.; Ning, B.; Li, S.; Wang, J.; Peng, Y.; Gao, Z. Novel Biomimic Crystalline Colloidal Array for Fast Detection of Trace Parathion. ACS Sens. 2017, 2, 1013–1019. [Google Scholar] [CrossRef]
- Huang, C.; Cheng, Y.; Gao, Z.; Zhang, H.; Wei, J. Portable label-free inverse opal photonic hydrogel particles serve as facile pesticides colorimetric monitoring. Sens. Actuators B Chem. 2018, 273, 1705–1712. [Google Scholar] [CrossRef]
- Hou, J.; Zhang, H.; Yang, Q.; Li, M.; Jiang, L.; Song, Y. Hydrophilic-Hydrophobic Patterned Molecularly Imprinted Photonic Crystal Sensors for High-Sensitive Colorimetric Detection of Tetracycline. Small 2015, 11, 2738–2742. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Q.; Lin, F.-Y.; Yu, L. A molecularly imprinted photonic polymer sensor with high selectivity for tetracyclines analysis in food. Analyst 2012, 137, 3502–3509. [Google Scholar] [CrossRef] [PubMed]
- Sai, N.; Wu, Y.; Yu, G.; Sun, Z.; Huang, G. A novel enrichment imprinted crystalline colloidal array for the ultratrace detection of chloramphenicol. Talanta 2016, 161, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, H.; Huang, Z.; Jin, Z.; Fang, S.; He, J.; Liu, Y.; Zhang, Y.; Lai, J. The visual detection of anesthetics in fish based on an inverse opal photonic crystal sensor. RSC Adv. 2019, 9, 16831–16838. [Google Scholar] [CrossRef]
- Kadhem, A.J.; Xiang, S.; Nagel, S.; Lin, C.H.; de Cortalezzi, M.F. Photonic molecularly imprinted polymer film for the detection of testosterone in aqueous samples. Polymers 2018, 10, 349. [Google Scholar] [CrossRef]
- Dabrowski, M.; Cieplak, M.; Sharma, P.S.; Borowicz, P.; Noworyta, K.; Lisowski, W.; D’Souza, F.; Kuhn, A.; Kutner, W. Hierarchical templating in deposition of semi-covalently imprinted inverse opal polythiophene film for femtomolar determination of human serum albumin. Biosens. Bioelectron. 2017, 94, 155–161. [Google Scholar] [CrossRef]
- Chen, W.; Lei, W.; Xue, M.; Xue, F.; Meng, Z.H.; Zhang, W.B.; Qu, F.; Shea, K.J. Protein recognition by a surface imprinted colloidal array. J. Mater. Chem. A 2014, 2, 7165–7169. [Google Scholar] [CrossRef]
- Vollmer, F.; Braun, D.; Libchaber, A.; Khoshsima, M.; Teraoka, I.; Arnold, S. Protein detection by optical shift of a resonant microcavity. Appl. Phys. Lett. 2002, 80, 4057–4059. [Google Scholar] [CrossRef]
- Vollmer, F.; Arnold, S.; Braun, D.; Teraoka, I.; Libchaber, A. Multiplexed DNA quantification by spectroscopic shift of two microsphere cavities. Biophys. J. 2003, 85, 1974–1979. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, J.; Yang, Z.; Wilkinson, J.S.; Zhou, X. Optical biosensors based on refractometric sensing schemes: A review. Biosens. Bioelectron. 2019, 144, 111693. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Shen, X.; Chang, Z.; Tang, L.; Dong, W.F.; Li, M.; He, J.J. Ultrasensitive detection of testosterone using microring resonator with molecularly imprinted polymers. Sensors 2015, 15, 31558–31565. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Cao, Z.; Liu, Y.; Zhang, Q.; Zou, J.; Shao, L.; Wang, Y.; He, J.; Li, M. Highly-sensitive optical biosensor based on equal FSR cascaded microring resonator with intensity interrogation for detection of progesterone molecules. Opt. Express 2017, 25, 33193–33201. [Google Scholar] [CrossRef]
- Eisner, L.; Wilhelm, I.; Flachenecker, G.; Hürttlen, J.; Schade, W. Molecularly imprinted sol-gel for tnt detection with optical micro-ring resonator sensor chips. Sensors 2019, 19, 3909. [Google Scholar] [CrossRef] [PubMed]
- Hammond, G.D.; Vojta, A.L.; Grant, S.A.; Hunt, H.K. Integrating nanostructured artificial receptors with whispering gallery mode optical microresonators via inorganic molecular imprinting techniques. Biosensors 2016, 6. [Google Scholar] [CrossRef]
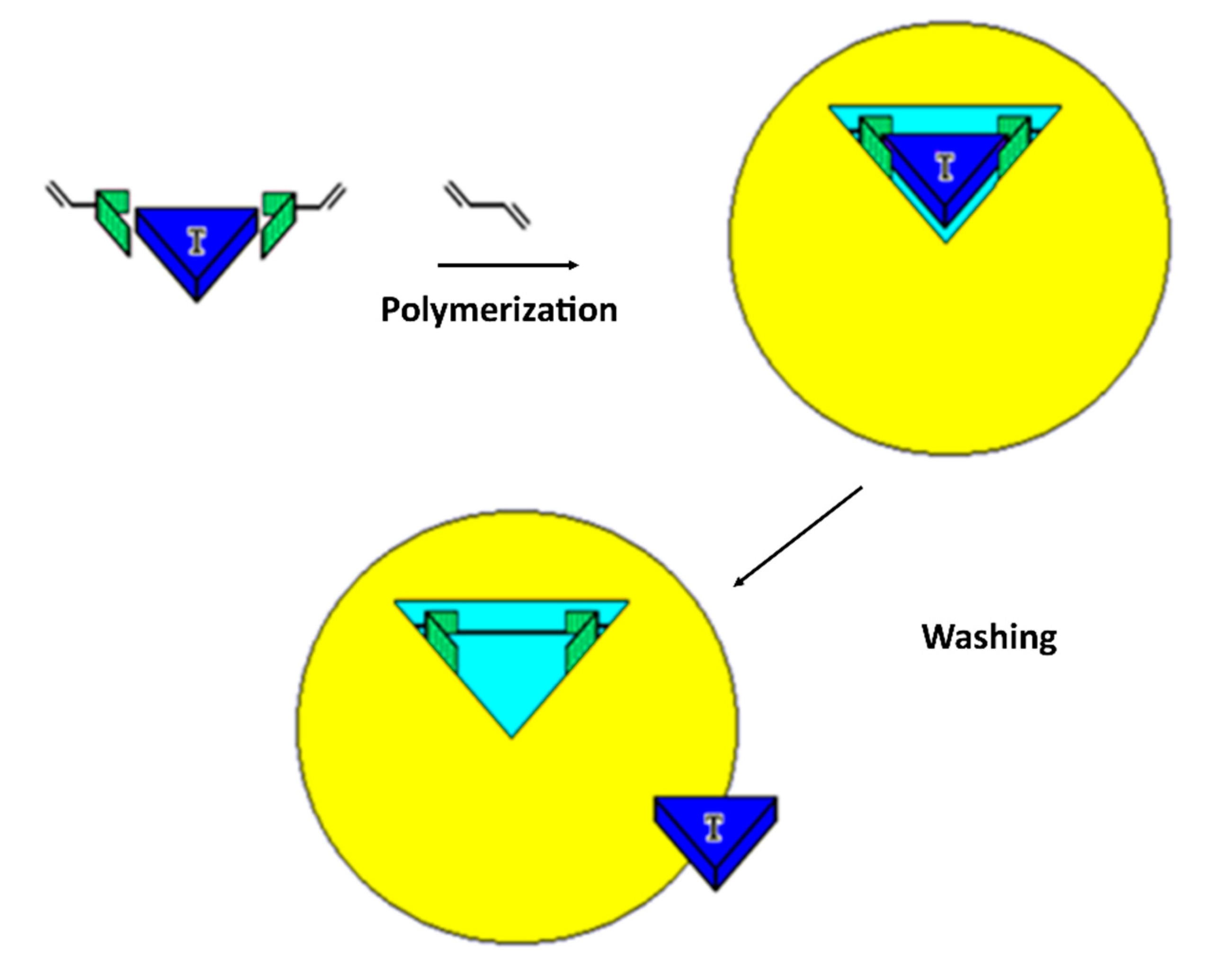
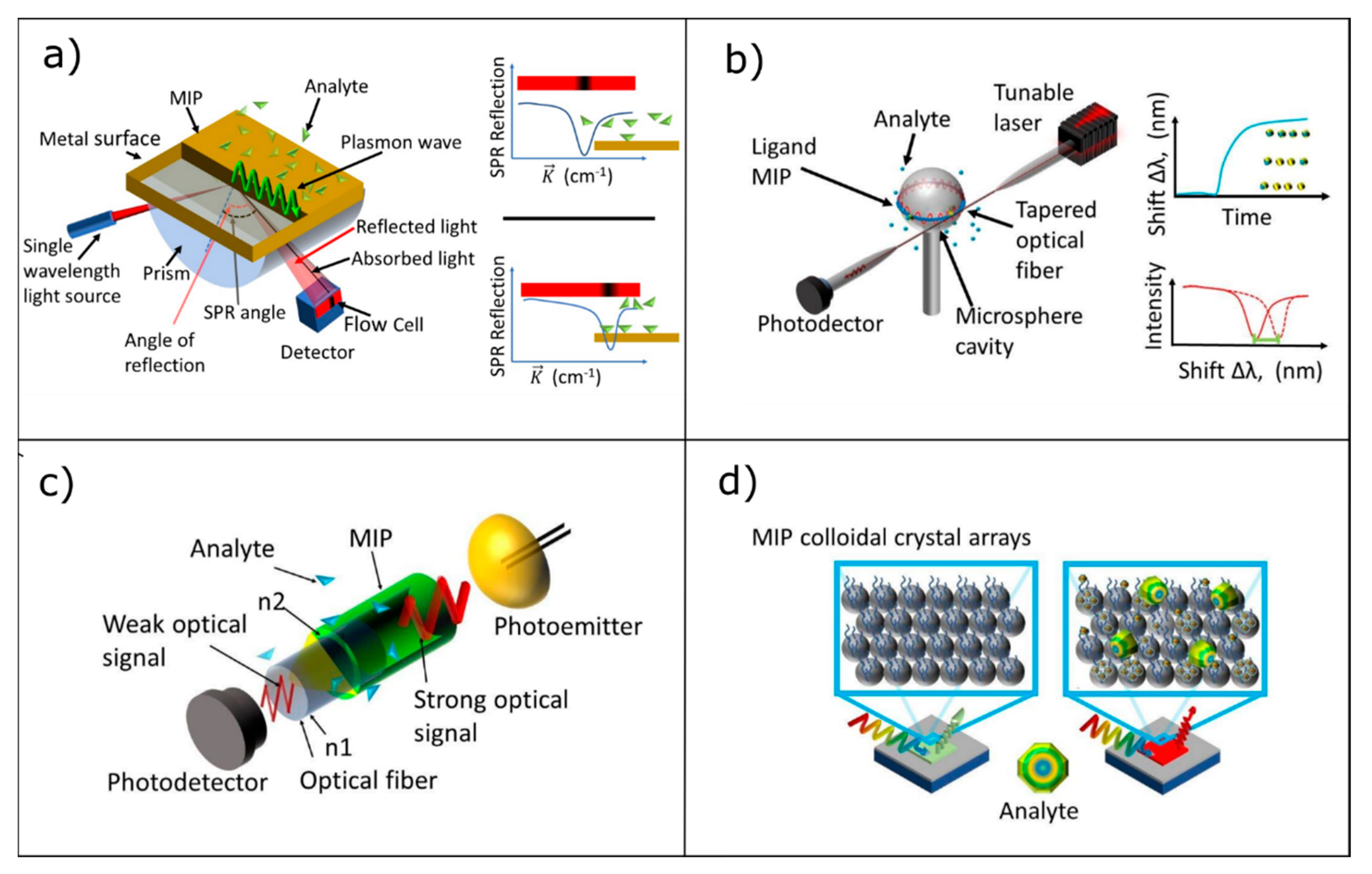

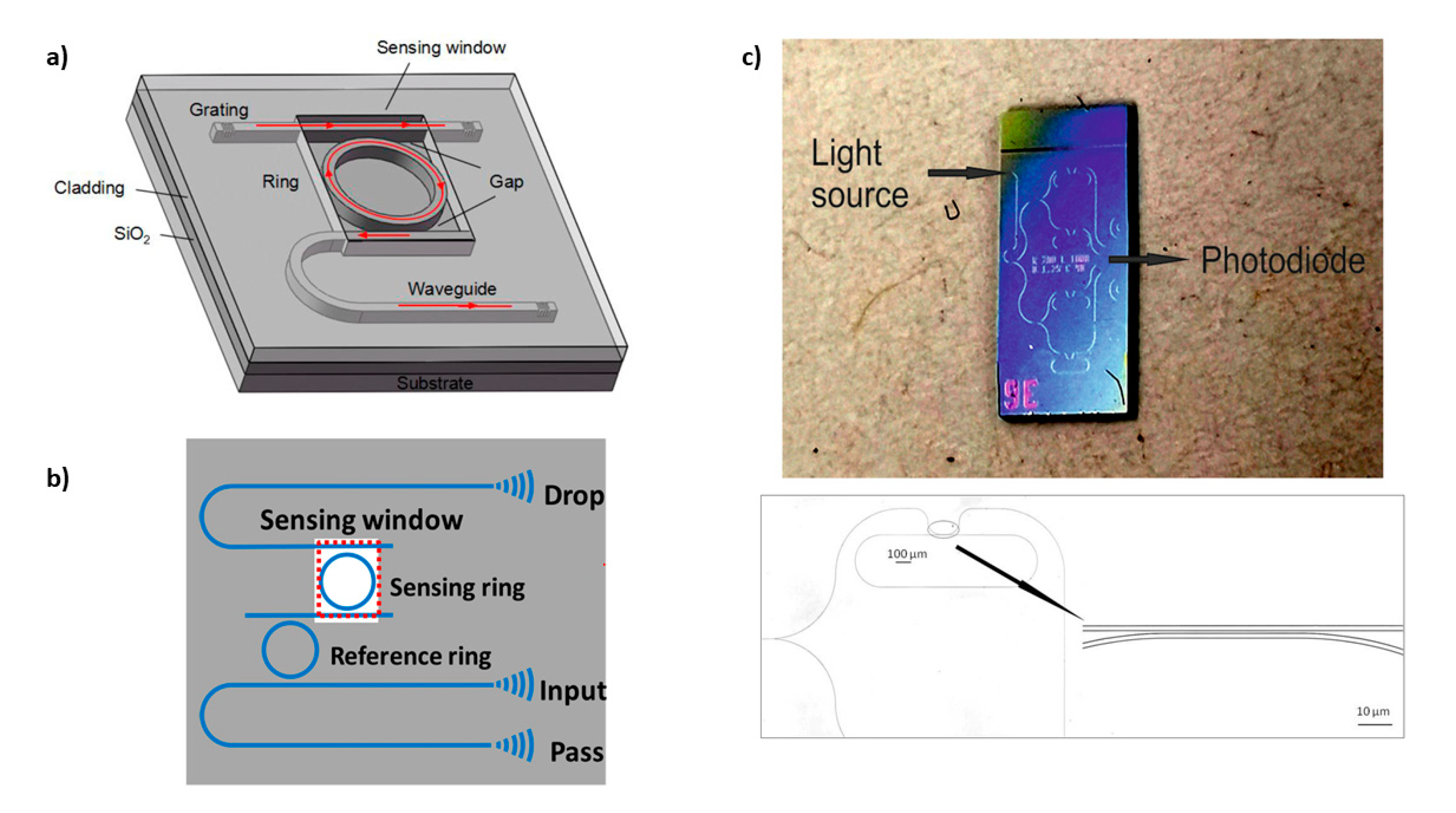
| Photonic Crystal Configuration | Analyte | LoD or LoQ | Reference |
|---|---|---|---|
| Film | 2-Butoxyethanol (2BE) | LoD: 3.4 ppb | [109] |
| Film | Parathion | LoQ: <0.01 ng/mL | [110] |
| Hydrogel | Methanephosphonic acid (MPA) | LoD: <1.0 µM | [111] |
| Film | Tetracycline | LoD: <2 nM | [112] |
| Film | Tetracycline | LoQ: <80 nM | [113] |
| Film | Chloramphenicol (Cm) | LoD: 1.5 nM | [114] |
| Film | Benzocaine | LoD: 0.1 mM | [115] |
| Film | Testosterone | LoD: 4.2 ppb | [116] |
| Film | Human Serum Albumin (HSA) | LoD: 13 fM | [117] |
| Microspheres | Hemoglobin bovine (Hb) | LoQ: <0.1 mg/mL | [118] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiappini, A.; Pasquardini, L.; Bossi, A.M. Molecular Imprinted Polymers Coupled to Photonic Structures in Biosensors: The State of Art. Sensors 2020, 20, 5069. https://doi.org/10.3390/s20185069
Chiappini A, Pasquardini L, Bossi AM. Molecular Imprinted Polymers Coupled to Photonic Structures in Biosensors: The State of Art. Sensors. 2020; 20(18):5069. https://doi.org/10.3390/s20185069
Chicago/Turabian StyleChiappini, Andrea, Laura Pasquardini, and Alessandra Maria Bossi. 2020. "Molecular Imprinted Polymers Coupled to Photonic Structures in Biosensors: The State of Art" Sensors 20, no. 18: 5069. https://doi.org/10.3390/s20185069
APA StyleChiappini, A., Pasquardini, L., & Bossi, A. M. (2020). Molecular Imprinted Polymers Coupled to Photonic Structures in Biosensors: The State of Art. Sensors, 20(18), 5069. https://doi.org/10.3390/s20185069







