Polarizer-Free AOTF-Based SWIR Hyperspectral Imaging for Biomedical Applications
Abstract
1. Introduction
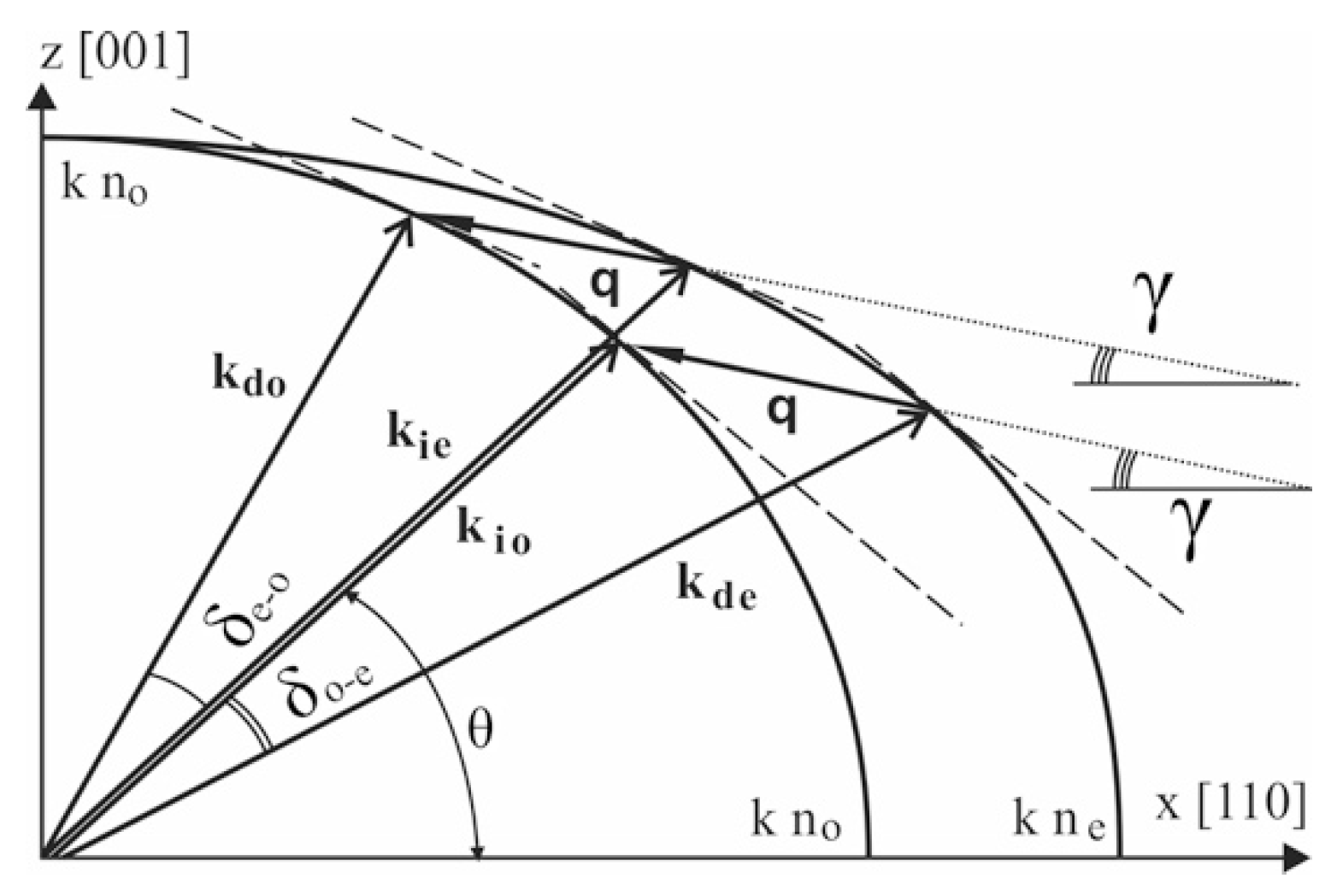
2. Theoretical Background
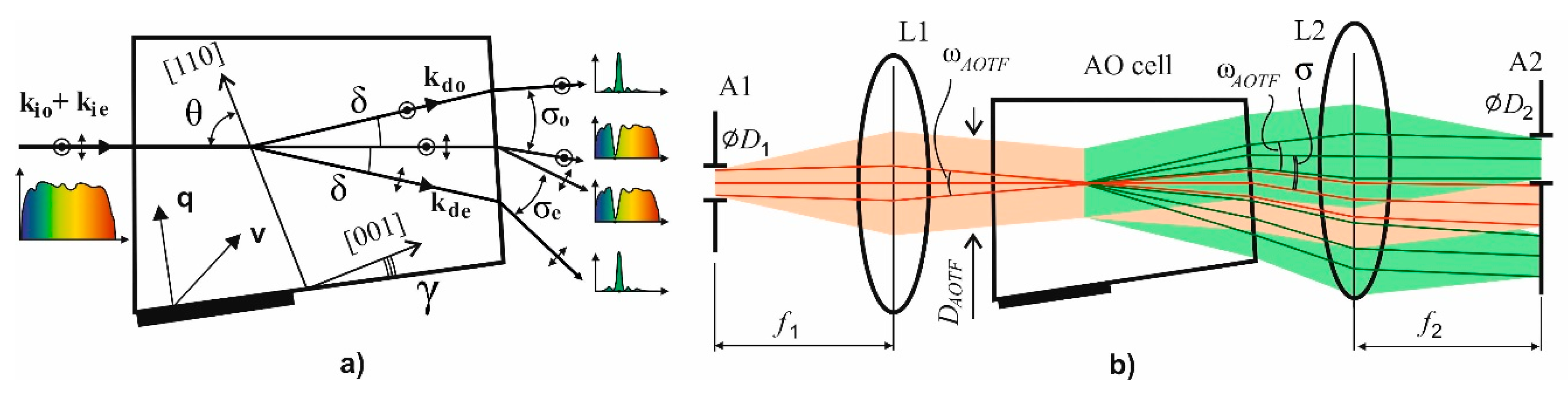
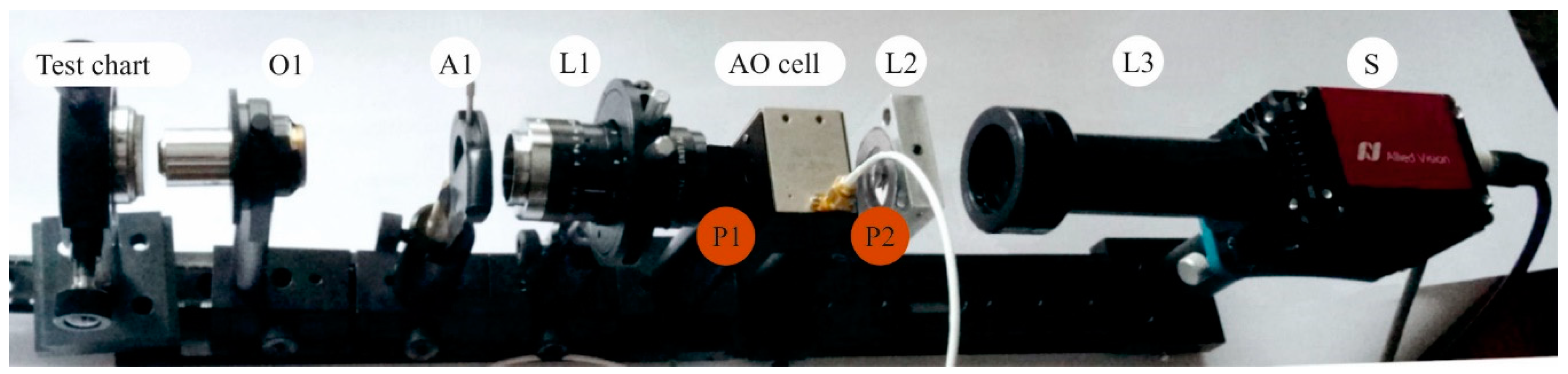
3. Optical System Design and Optimization
4. Polarizer-Free AOTF Imager Schemes
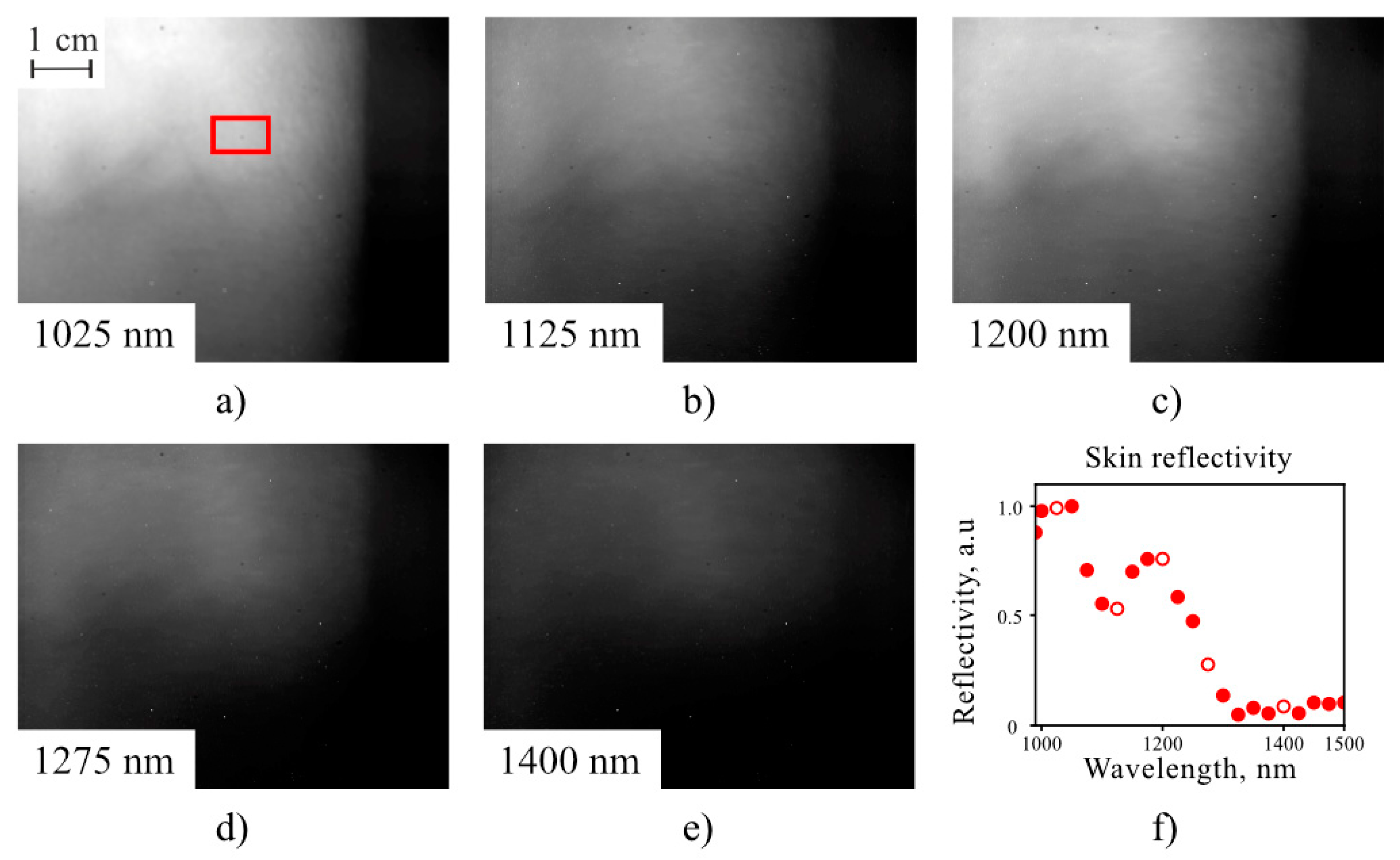
5. Results and Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lu, G.; Fei, B. Medical hyperspectral imaging: A review. J. Biomed. Opt. 2014, 19, 010901. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gutiérrez, J.A.; Pardo, A.; Real, E.; López-Higuera, J.M.; Conde, O.M. Custom Scanning Hyperspectral Imaging System for Biomedical Applications: Modeling, Benchmarking, and Specifications. Sensors 2019, 19, 1692. [Google Scholar] [CrossRef] [PubMed]
- Halicek, M.; Fabelo, H.; Ortega, S.; Callico, G.M.; Fei, B. In-Vivo and Ex-Vivo Tissue Analysis through Hyperspectral Imaging Techniques: Revealing the Invisible Features of Cancer. Cancers 2019, 11, 756. [Google Scholar] [CrossRef] [PubMed]
- Fabelo, H.; Ortega, S.; Lazcano, R.; Madroñal, D.; M. Callicó, G.; Juárez, E.; Salvador, R.; Bulters, D.; Bulstrode, H.; Szolna, A.; et al. An Intraoperative Visualization System Using Hyperspectral Imaging to Aid in Brain Tumor Delineation. Sensors 2018, 18, 430. [Google Scholar] [CrossRef]
- Milanic, M.; Paluchowski, L.A.; Randeberg, L.L. Hyperspectral imaging for detection of arthritis: Feasibility and prospects. J. Biomed. Opt. 2015, 20, 096011. [Google Scholar] [CrossRef]
- Yoon, J.; Joseph, J.; Waterhouse, D.J.; Luthman, A.S.; Gordon, G.S.D.; di Pietro, M.; Januszewicz, W.; Fitzgerald, R.C.; Bohndiek, S.E. A clinically translatable hyperspectral endoscopy (HySE) system for imaging the gastrointestinal tract. Nat. Commun. 2019, 10, 1902. [Google Scholar] [CrossRef]
- Beaulieu, R.J.; Goldstein, S.D.; Singh, J.; Safar, B.; Banerjee, A.; Ahuja, N. Automated diagnosis of colon cancer using hyperspectral sensing. Int. J. Med. Robot. Comput. Assist. Surg. 2018, 14, e1897. [Google Scholar] [CrossRef]
- Golovynskyi, S.; Golovynska, I.; Stepanova, L.I.; Datsenko, O.I.; Liu, L.; Qu, J.; Ohulchanskyy, T.Y. Optical windows for head tissues in near-infrared and short-wave infrared regions: Approaching transcranial light applications. J. Biophotonics 2018, 11, e201800141. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Yakovliev, A.; Ziniuk, R.; Wang, D.; Xue, B.; Vretik, L.O.; Nikolaeva, O.A.; Tan, M.; Chen, G.; Slominskii, Y.L.; Qu, J.; et al. Hyperspectral Multiplexed Biological Imaging of Nanoprobes Emitting in the Short-Wave Infrared Region. Nanoscale Res. Lett. 2019, 14, 243. [Google Scholar] [CrossRef]
- Wilson, R.H.; Nadeau, K.P.; Jaworski, F.B.; Tromberg, B.J.; Durkin, A.J. Review of short-wave infrared spectroscopy and imaging methods for biological tissue characterization. J. Biomed. Opt. 2015, 20, 030901. [Google Scholar] [CrossRef] [PubMed]
- Hagen, N.; Kudenov, M.W. Review of snapshot spectral imaging technologies. Opt. Eng. 2013, 52, 090901. [Google Scholar] [CrossRef]
- Li, Q.; He, X.; Wang, Y.; Liu, H.; Xu, D.; Guo, F. Review of spectral imaging technology in biomedical engineering: Achievements and challenges. J. Biomed. Opt. 2013, 18, 100901. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-I. Hyperspectral Imaging: Techniques for Spectral Detection and Classification; Springer: Berlin, Germany, 2003; ISBN 978-0-306-47483-5. [Google Scholar]
- Suhre, D.R.; Denes, L.J.; Gupta, N. Telecentric confocal optics for aberration correction of acousto-optic tunable filters. Appl. Opt. 2004, 43, 1255. [Google Scholar] [CrossRef]
- Machikhin, A.; Batshev, V.; Pozhar, V. Aberration analysis of AOTF-based spectral imaging systems. J. Opt. Soc. Am. A 2017, 34, 1109. [Google Scholar] [CrossRef]
- Voloshinov, V.B.; Yushkov, K.B.; Linde, B.B.J. Improvement in performance of a TeO2 acousto-optic imaging spectrometer. J. Opt. A: Pure Appl. Opt. 2007, 9, 341–347. [Google Scholar] [CrossRef]
- He, Z.; Shu, R.; Wang, J. Imaging spectrometer based on AOTF and its prospects in deep-space exploration application. In Proc. SPIE 8196, International Symposium on Photoelectronic Detection and Imaging 2011: Space Exploration Technologies and Applications; Zarnecki, J.C., Nardell, C.A., Shu, R., Yang, J., Zhang, Y., Eds.; SPIE: Bellingham, WA, USA, 2011. [Google Scholar]
- Gupta, N. Development of spectropolarimetric imagers from 400 to 1700 nm. In Proceedings of the Polarization: Measurement, Analysis, and Remote Sensing XI; SPIE: Bellingham, WA, USA, 2014. [Google Scholar]
- Korablev, O.I.; Belyaev, D.A.; Dobrolenskiy, Y.S.; Trokhimovskiy, A.Y.; Kalinnikov, Y.K. Acousto-optic tunable filter spectrometers in space missions [Invited]. Appl. Opt. 2018, 57, C103. [Google Scholar] [CrossRef]
- Gao, Z.; Zeng, L.; Wu, Q. AOTF-based near-infrared imaging spectrometer for rapid identification of camouflaged target. In Proc. SPIE 9298, International Symposium on Optoelectronic Technology and Application 2014: Imaging Spectroscopy; and Telescopes and Large Optics; Rolland, J.P., Yan, C., Kim, D.W., Ma, W., Zheng, L., Eds.; SPIE: Bellingham, WA, USA, 2014; p. 92980R. [Google Scholar]
- Gupta, N.; Suhre, D.R. Notch filtering using a multiple passband AOTF in the SWIR region. Appl. Opt. 2016, 55, 7855–7860. [Google Scholar] [CrossRef]
- Epikhin, V.M.; Vizen, F.L.; Magomedov, Z.A.; Pal’tsev, L.L. Polarizer-Free Acousto-Optic Monochromators. Tech. Phys. 2018, 63, 1040–1043. [Google Scholar] [CrossRef]
- Voloshinov, V.B.; Molchanov, V.Y.; Babkina, T.M. Acousto-optic filter of nonpolarized electromagnetic radiation. Tech. Phys. 2000, 45, 1186–1191. [Google Scholar] [CrossRef]
- Voloshinov, V.B.; Molchanov, V.Y.; Mosquera, J.C. Spectral and polarization analysis of optical images by means of acousto-optics. Opt. Laser Technol. 1996, 28, 119–127. [Google Scholar] [CrossRef]
- Chang, I.C. Noncollinear acousto-optic filter with large angular aperture. Appl. Phys. Lett. 1974, 25, 370–372. [Google Scholar] [CrossRef]
- Pozhar, V.; Machihin, A. Image aberrations caused by light diffraction via ultrasonic waves in uniaxial crystals. Appl. Opt. 2012, 51, 4513. [Google Scholar] [CrossRef] [PubMed]
- Randeberg, L.L.; Hernandez-Palacios, J. Hyperspectral imaging of bruises in the SWIR spectral region. In Proceedings SPIE 8207, Photonic Therapeutics and Diagnostics VIII; SPIE: Bellingham, WA, USA, 2012; Volume 8207, p. 82070N-9. [Google Scholar]




| λ, μm | |||
|---|---|---|---|
| 1.0 | 180 × 210 | 170 × 200 | 160 × 200 |
| 1.2 | 170 × 200 | 170 × 200 | 150 × 200 |
| 1.4 | 160 × 180 | 150 × 180 | 140 × 160 |
| 1.6 | 150 × 170 | 140 × 160 | 120 × 140 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batshev, V.; Machikhin, A.; Martynov, G.; Pozhar, V.; Boritko, S.; Sharikova, M.; Lomonov, V.; Vinogradov, A. Polarizer-Free AOTF-Based SWIR Hyperspectral Imaging for Biomedical Applications. Sensors 2020, 20, 4439. https://doi.org/10.3390/s20164439
Batshev V, Machikhin A, Martynov G, Pozhar V, Boritko S, Sharikova M, Lomonov V, Vinogradov A. Polarizer-Free AOTF-Based SWIR Hyperspectral Imaging for Biomedical Applications. Sensors. 2020; 20(16):4439. https://doi.org/10.3390/s20164439
Chicago/Turabian StyleBatshev, Vladislav, Alexander Machikhin, Grigoriy Martynov, Vitold Pozhar, Sergey Boritko, Milana Sharikova, Vladimir Lomonov, and Alexander Vinogradov. 2020. "Polarizer-Free AOTF-Based SWIR Hyperspectral Imaging for Biomedical Applications" Sensors 20, no. 16: 4439. https://doi.org/10.3390/s20164439
APA StyleBatshev, V., Machikhin, A., Martynov, G., Pozhar, V., Boritko, S., Sharikova, M., Lomonov, V., & Vinogradov, A. (2020). Polarizer-Free AOTF-Based SWIR Hyperspectral Imaging for Biomedical Applications. Sensors, 20(16), 4439. https://doi.org/10.3390/s20164439






