An Approach towards Motion-Tolerant PPG-Based Algorithm for Real-Time Heart Rate Monitoring of Moving Pigs
Abstract
1. Introduction
2. Materials and Methods
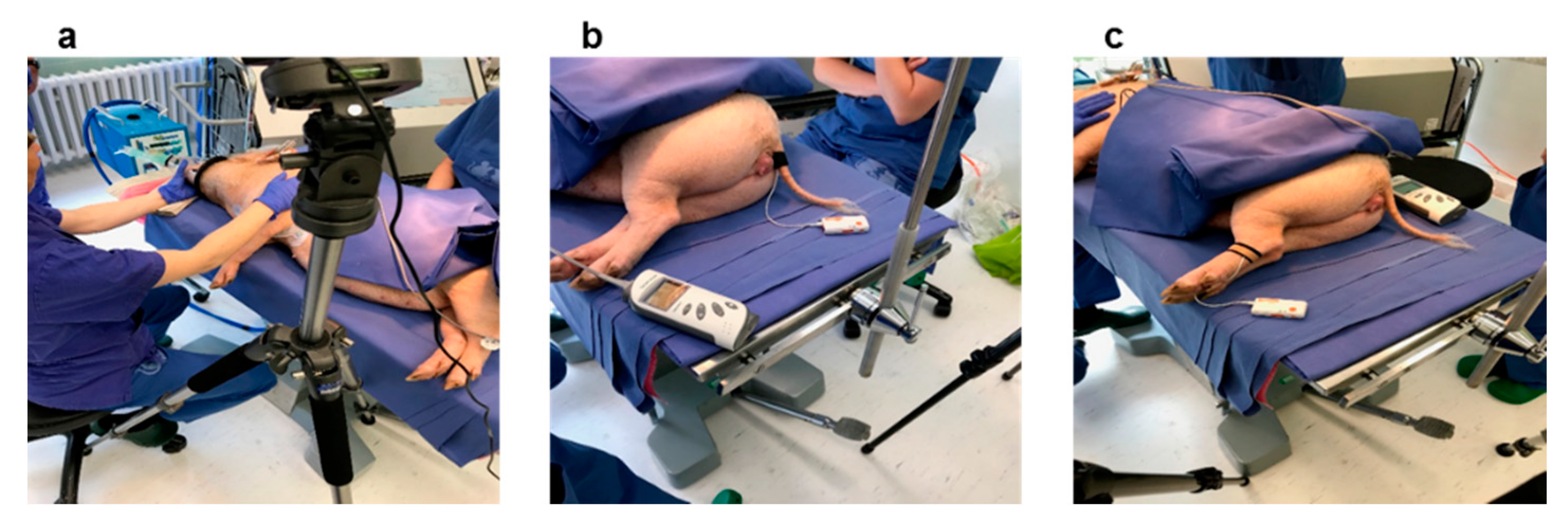
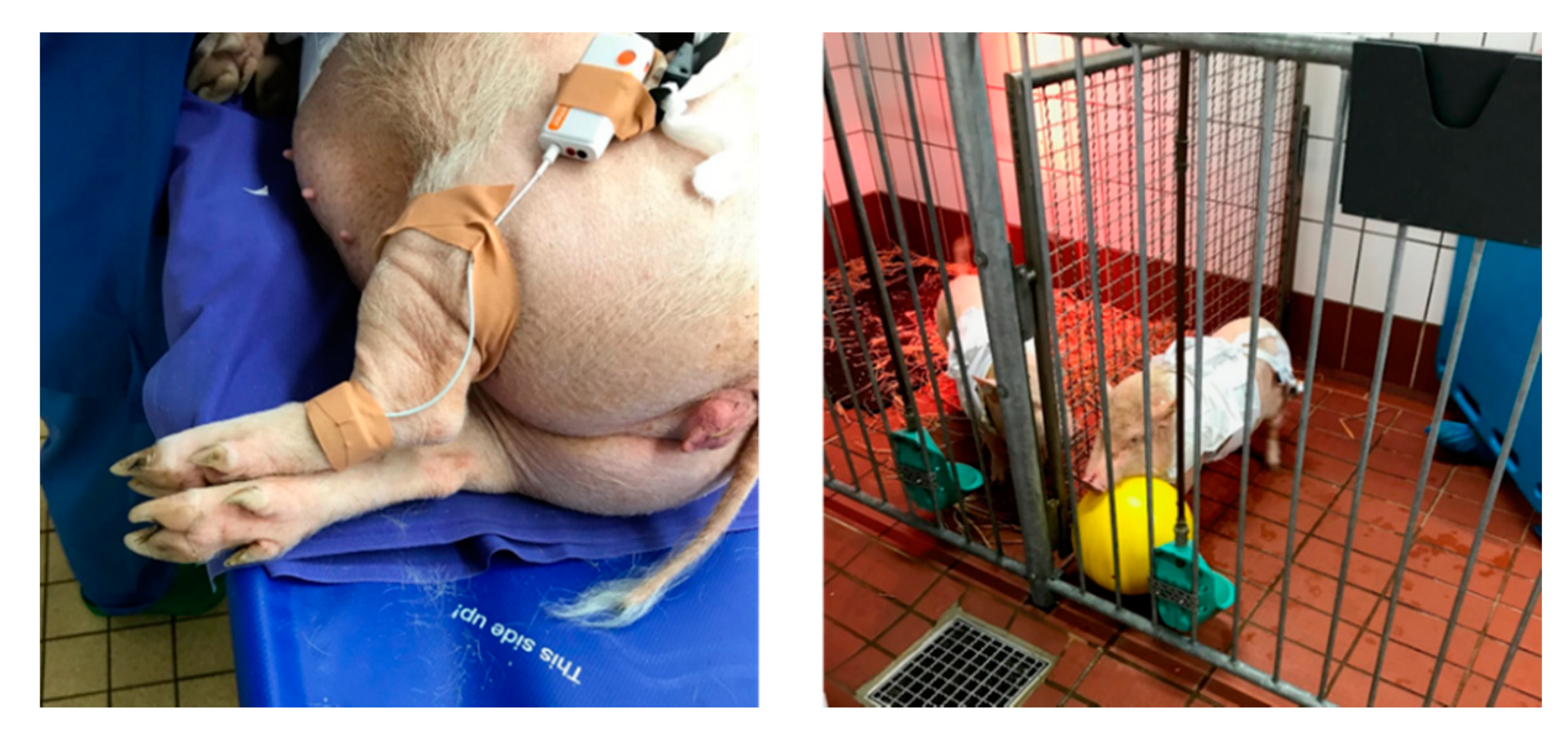
2.1. Experimental Setup and Measurements
2.1.1. Measurements and Sensors
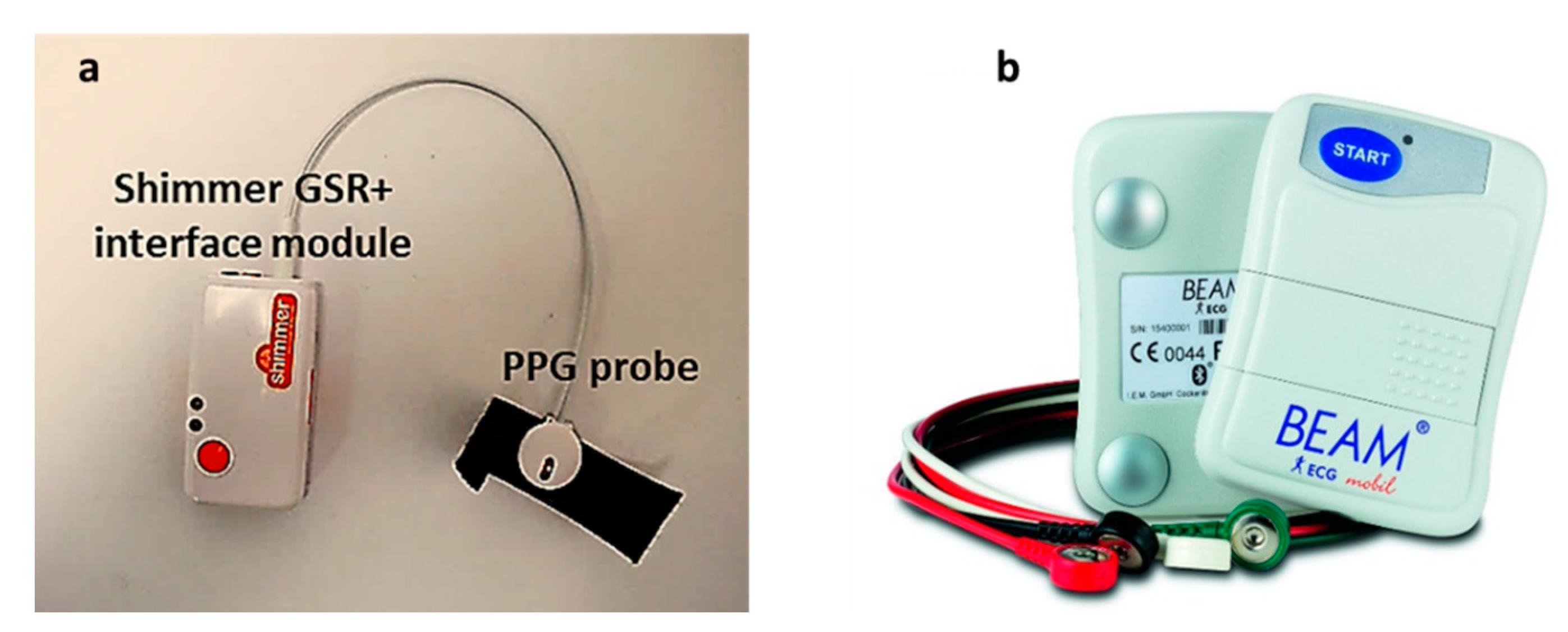
PPG Signal
Gold Standard (ECG Signal)
2.2. Signal Processing and Heart Rate Extraction
2.2.1. Pre-Processing of PPG Signals
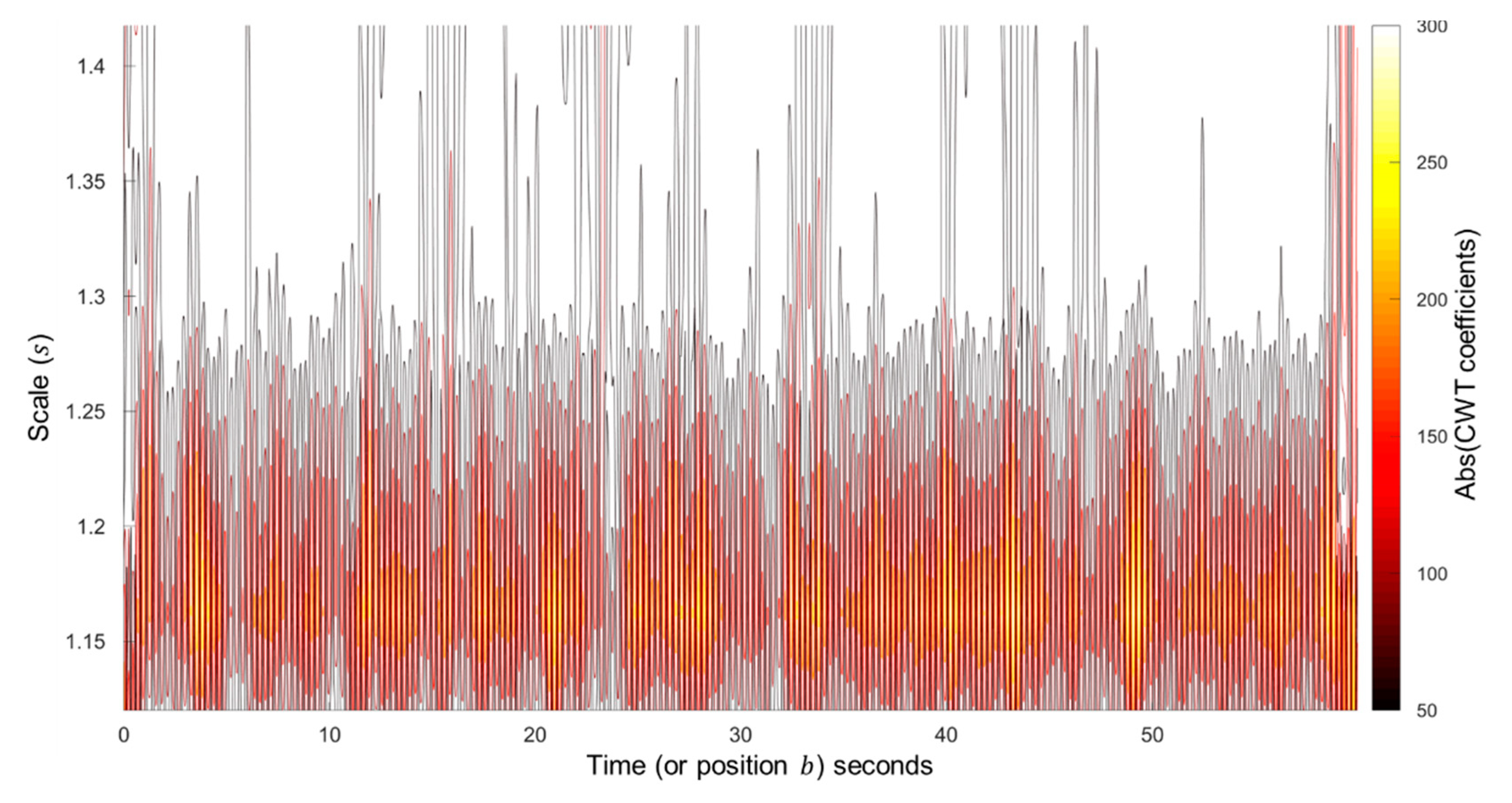
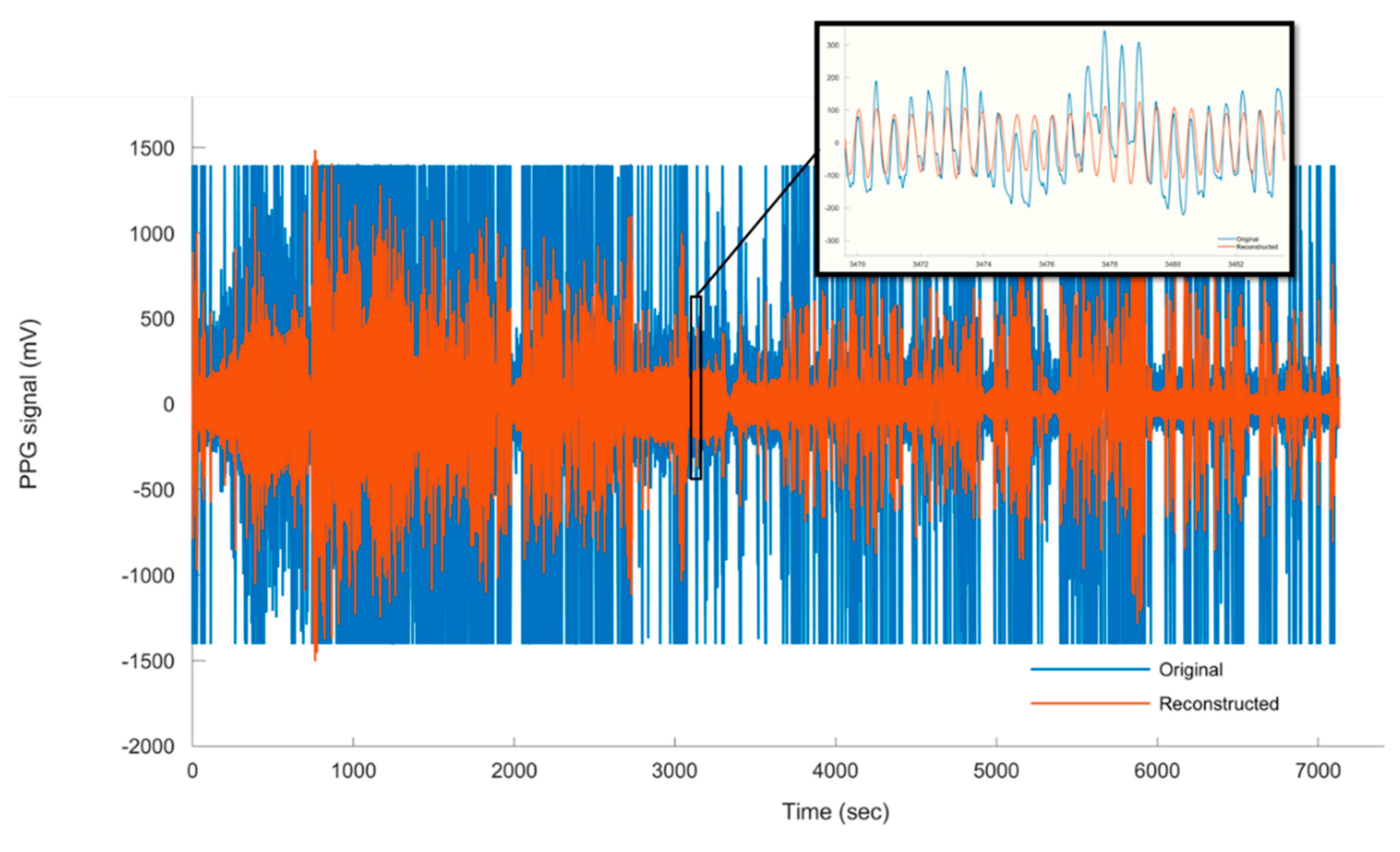
2.2.2. Wavelet Analysis and Cardiogenic Signal Reconstruction
Continuous Wavelet Transform Method
- Compute the discrete Fourier transform (DFT) of the analysed signal , which includes samples, using Fast Fourier Transform (FFT) as follows:where is an index of frequency.
- Obtain the DFT () of the analysing wavelet () at the appropriate angular frequencies as follows:
- Obtain the DFT of the analysing wavelet at different scales.
2.2.3. Signal Quality Indices (SQIs)
- Perfusion index () is defined as the ratio of the pulsatile signal component to the non-pulsatile or static blood flow in the peripheral tissue. In other words, it is the difference in the amount of light absorbed by the pulse when light is transmitted through the finger [9], which can be defined as follows:where is the statistical mean of the x signal (raw PPG signal), and is the filtered PPG signal.
- Skewness index () is a measure of the symmetry/asymmetry of a probability distribution of the signal about its mean, which is defined as:where and are the empirical estimate of the mean and standard deviation of , respectively, and is the number of samples in the PPG signal [9].
- Signal to Noise ratio () compares the level of a desired signal (pulsatile cardiogenic signal) to the level of background noise [9], and is given bywhere is the standard deviation of the absolute value of the PPG signal (x), and is the standard deviation of the noise.
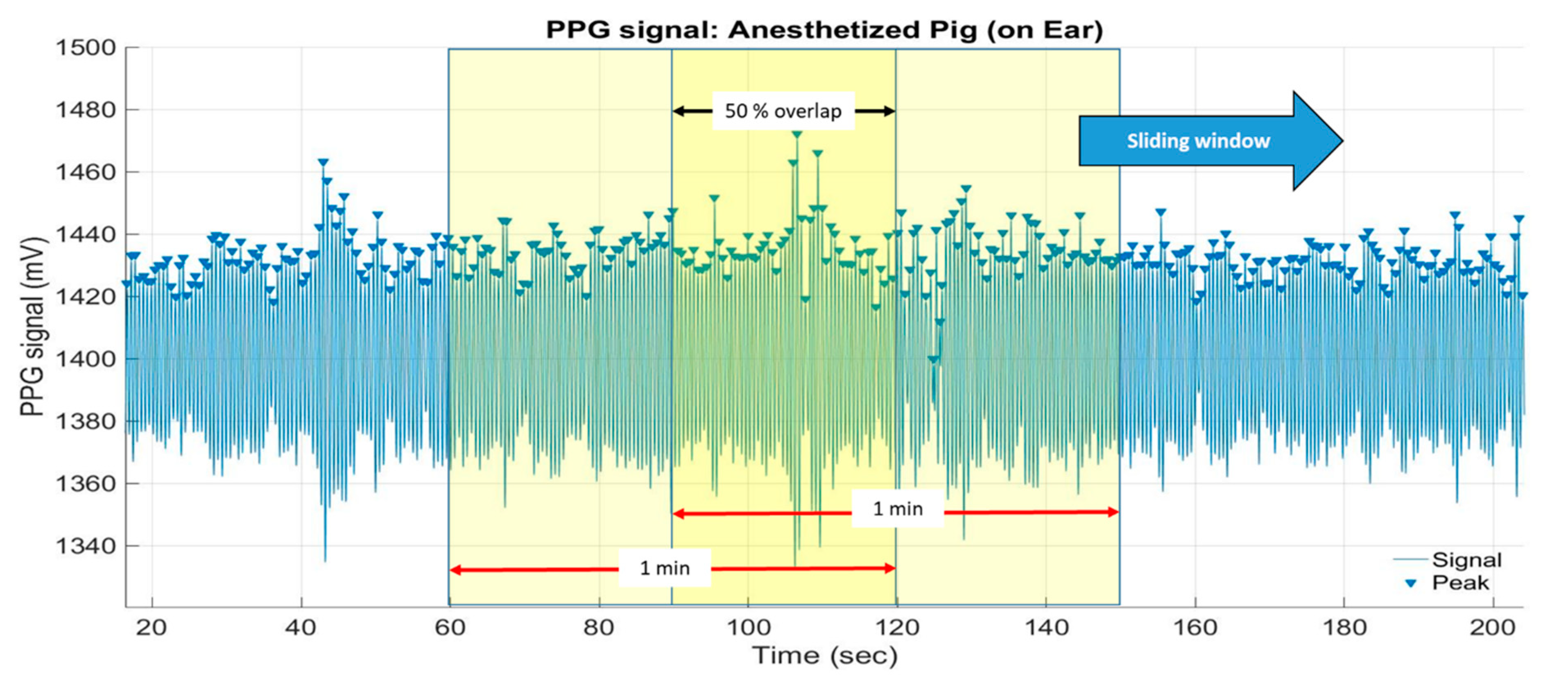
2.2.4. Peak Detection and Heart Rate Calculation
3. Results and Discussion
3.1. Decoupling of the Pulse Wave in the Anaesthetised Pig
3.1.1. Scales Selection
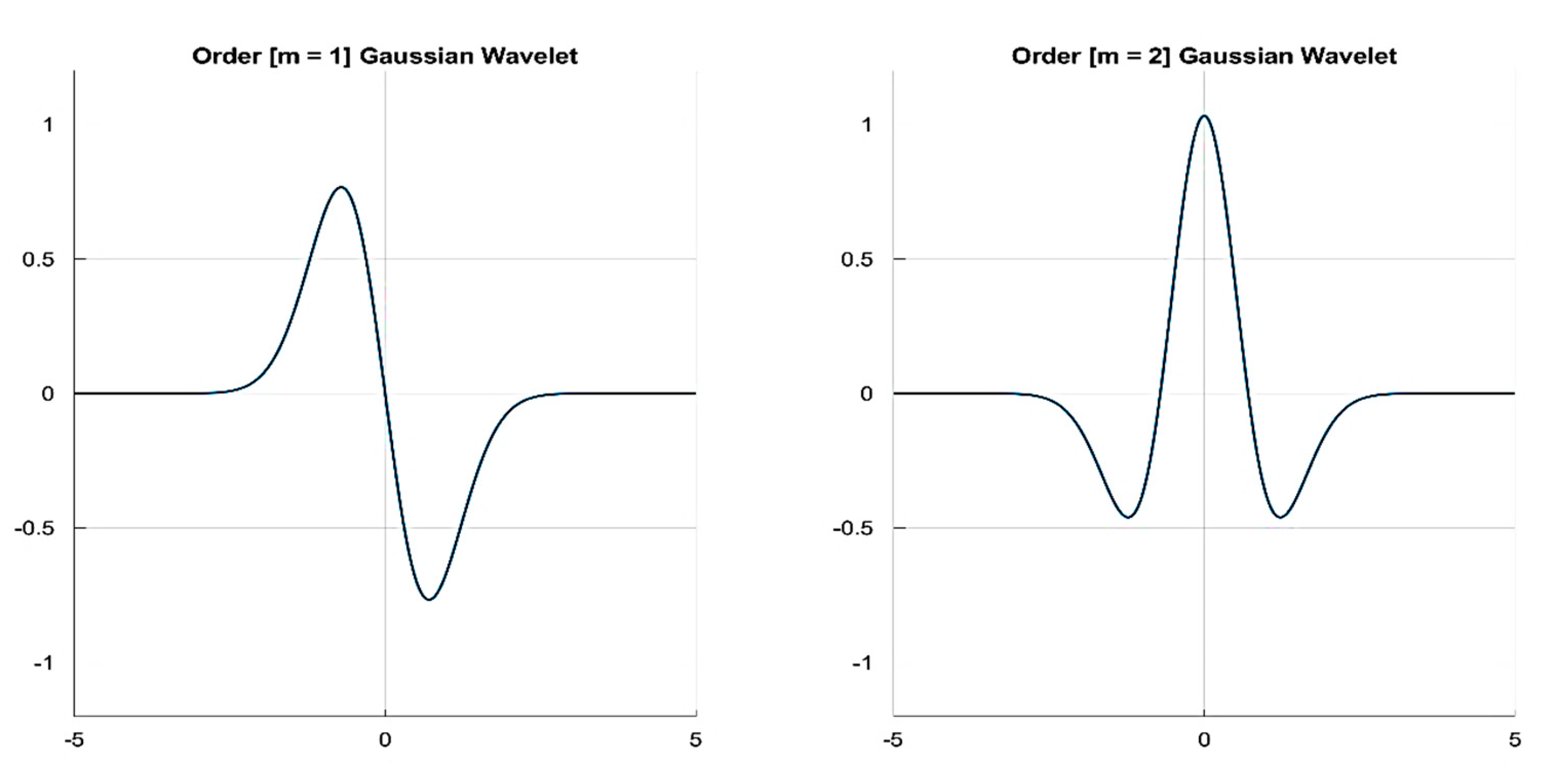
3.1.2. Mother Wavelet Selection
3.1.3. Assessment of PPG Signal Quality
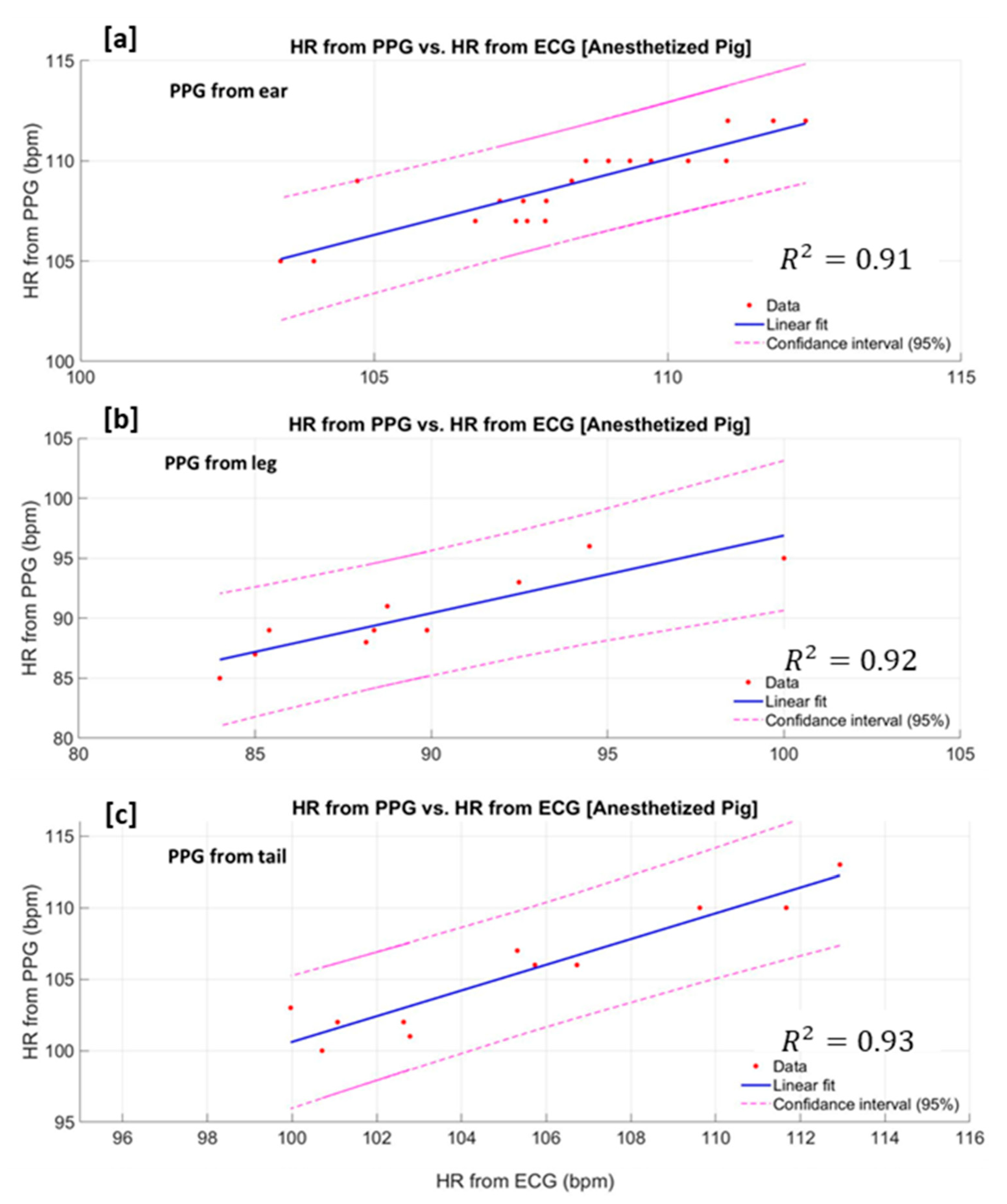
3.2. Heart Rate Estimation Based on Measured PPG From the Non-Anaesthetised (Moving) Pig
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kovacs, L.; Jurkovich, V.; Bakony, M.; Szenci, O.; Potì, P.; Tözsér, J. Welfare implication of measuring heart rate and heart rate variability in dairy cattle: literature review and conclusions for future research. Animal 2013, 8, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Hopster, H.; Blokhuis, H. Validation of a heart-rate monitor for measuring a stress response in dairy cows. Can. J. Anim. Sci. 1994, 74, 465–474. [Google Scholar] [CrossRef]
- Von Holst, D. The Concept of Stress and Its Relevance for Animal Behavior. Adv. Study Behav. 1998, 27, 1–131. [Google Scholar]
- Ivanov, K.P. The development of the concepts of homeothermy and thermoregulation. J. Therm. Boil. 2006, 31, 24–29. [Google Scholar] [CrossRef]
- Whittow, G.C.; Tazawa, H. The Early Development of Thermoregulation in Birds. Physiol. Zool. 1991, 64, 1371–1390. [Google Scholar] [CrossRef]
- Youssef, A.; Exadaktylos, V.; Berckmans, D. Modelling and quantification of the thermoregulatory responses of the developing avian embryo: Electrical analogies of a physiological system. J. Therm. Boil. 2014, 44, 14–19. [Google Scholar] [CrossRef]
- Nie, L.; Berckmans, D.; Wang, C.; Li, B. Is Continuous Heart Rate Monitoring of Livestock a Dream or Is It Realistic? A Review. Sensors 2020, 20, 2291. [Google Scholar] [CrossRef]
- Von Borell, E. Heart rate variability as a measure of autonomic regulation of cardiac activity for assessing stress and welfare in farm animals—A review. Physiol. Behav. 2007, 92, 293–316. [Google Scholar] [CrossRef]
- Elgendi, M. Optimal Signal Quality Index for Photoplethysmogram Signals. Bioengineering 2016, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Viazzi, S.; Exadaktylos, V.; Berckmans, D. Semi-invasive, non-contact measurements of chicken embryo heart rate using video imaging and signal processing. In Proceedings of the 6th European Conference on Precision Livestock Farming, Leuven, Belgium, 10–12 September 2013. [Google Scholar]
- Lee, Y.; Han, H.; Kim, J. Influence of motion artifacts on photoplethysmographic signals for measuring pulse rates. In Proceedings of the 2008 International Conference on Control, Automation and Systems, Seoul, Korea, 14–17 October 2008; pp. 962–965. [Google Scholar]
- Jang, D.-G.; Kwon, U.K.; Yoon, S.K.; Park, C.; Ku, Y.; Noh, S.W.; Kim, Y.H. A Simple and Robust Method for Determining the Quality of Cardiovascular Signals Using the Signal Similarity. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 478–481. [Google Scholar]
- Sabeti, E.; Reamaroon, N.; Mathis, M.; Gryak, J.; Sjoding, M.; Najarian, K. Signal quality measure for pulsatile physiological signals using morphological features: Applications in reliability measure for pulse oximetry. Inform. Med. Unlocked 2019, 16, 100222. [Google Scholar] [CrossRef]
- Tang, S.K.D.; Goh, Y.Y.S.; Wong, M.L.D.; Lew, Y.L.E. PPG signal reconstruction using a combination of discrete wavelet transform and empirical mode decomposition. In Proceedings of the 2016 6th International Conference on Intelligent and Advanced Systems (ICIAS), Kuala Lumpur, Malaysia, 15–17 August 2016; pp. 1–4. [Google Scholar]
- Elgendi, M.; Liang, Y.; Ward, R.K. Toward Generating More Diagnostic Features from Photoplethysmogram Waveforms. Diseases 2018, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Daubechies, I. The wavelet transform, time-frequency localization and signal analysis. IEEE Trans. Inf. Theory 1990, 36, 961–1005. [Google Scholar] [CrossRef]
- Torrence, C.; Compo, G.P. A Practical Guide to Wavelet Analysis. Bull. Am. Meteorol. Soc. 1998, 79, 61–78. [Google Scholar] [CrossRef]
- Komorowski, D.; Pietraszek, S. The Use of Continuous Wavelet Transform Based on the Fast Fourier Transform in the Analysis of Multi-channel Electrogastrography Recordings. J. Med. Syst. 2015, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Montejo, L.A.; E Suarez, L. An improved CWT-based algorithm for the generation of spectrum-compatible records. Int. J. Adv. Struct. Eng. 2013, 5, 26. [Google Scholar] [CrossRef]
- Li, L.-C. A New Method of Wavelet Transform Based on FFT for Signal Processing. In Proceedings of the 2010 Second WRI Global Congress on Intelligent Systems, Wuhan, China, 16–17 December 2010; pp. 203–206. [Google Scholar]
- Sahambi, J.S.; Tandon, S.N.; Bhatt, R. Using wavelet transforms for ECG characterization. An on-line digital signal processing system. IEEE Eng. Med. Boil. Mag. 1997, 16, 77–83. [Google Scholar] [CrossRef]
- Colquhoun, D.A.; Forkin, K.T.; Durieux, M.; Thiele, R.H. Ability of the Masimo pulse CO-Oximeter to detect changes in hemoglobin. J. Clin. Monit. 2012, 26, 69–73. [Google Scholar] [CrossRef]
- Gehring, H.; Hornberger, C.; Matz, H.; Konecny, E.; Schmucker, P. The effects of motion artifact and low perfusion on the performance of a new generation of pulse oximeters in volunteers undergoing hypoxemia. Respir. Care 2002, 47, 48–60. [Google Scholar]
- Cannesson, M.; Delannoy, B.; Morand, A.; Rosamel, P.; Attof, Y.; Bastien, O.; Lehot, J.-J. Does the Pleth Variability Index Indicate the Respiratory-Induced Variation in the Plethysmogram and Arterial Pressure Waveforms? Anesth. Analg. 2008, 106, 1189–1194. [Google Scholar] [CrossRef]
- Krishnan, R.; Natarajan, B.; Warren, S. Two-Stage Approach for Detection and Reduction of Motion Artifacts in Photoplethysmographic Data. IEEE Trans. Biomed. Eng. 2010, 57, 1867–1876. [Google Scholar] [CrossRef]
- Schrøder-Petersen, D.; Simonsen, H. Tail Biting in Pigs. Vet. J. 2001, 162, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, L.T.; Fels, M.; Oczak, M.; Vranken, E.; Ismayilova, G.; Guarino, M.; Viazzi, S.; Bahr, C.; Berckmans, D.; Hartung, J. Tail biting in pigs—causes and management intervention strategies to reduce the behavioural disorder. A review. Berl. Munch. Tierarztl. Wochenschr. 2013, 126, 104–112. [Google Scholar] [PubMed]

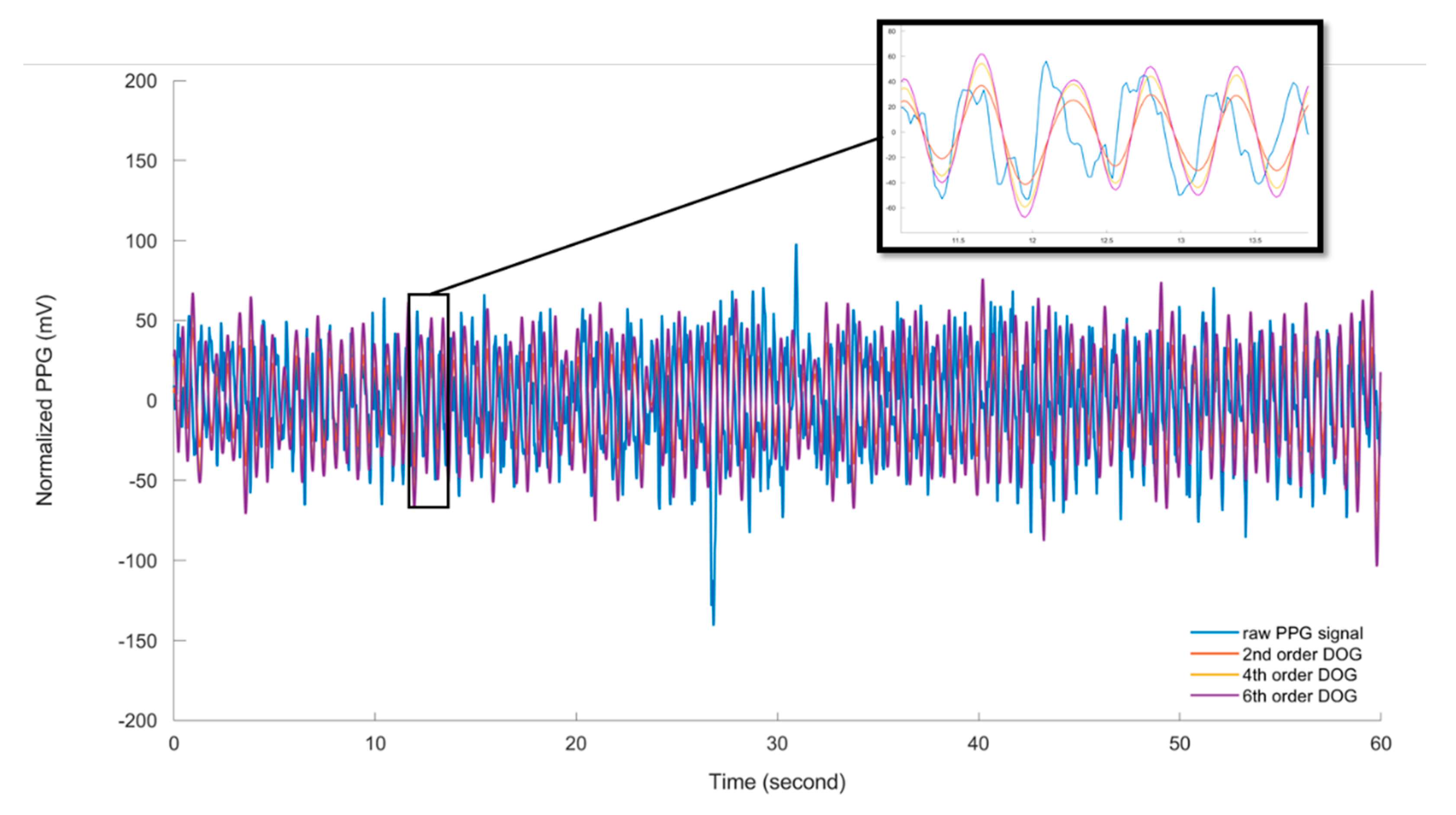

| Wavelet | Ear | Leg | Tail | |
|---|---|---|---|---|
| MAE (± std) | 2nd order DOG | 2.66 (±1.3) * | 1.75 (±1.2) * | 1.38 (±0.8) * |
| 4th order DOG | 2.23 (±0.9) | 1.53 (±0.8) | 1.25 (±0.7) | |
| 6th order DOG | 2.20 (±1.1) | 1.56 (±0.9) | 1.32 (±0.8) | |
| RMSE (± std) | 2nd order DOG | 3.50 (±1.6) * | 2.27 (±1.2) * | 1.45 (±0.9) * |
| 4th order DOG | 3.10 (±1.4) | 1.80 (±1.4) | 1.39 (±0.6) | |
| 6th order DOG | 3.23 (±1.5) | 2.11 (±1.6) | 1.36 (±0.7) |
| SQI | Ear | Leg | Tail |
|---|---|---|---|
| 10 (±2.9) | 8 (±2.2) | 7 (±2.5) | |
| 0.06 (±0.09) | 0.02 (±0.08) | 0.02 (±0.09) | |
| 3.85 (±0.40) | 3.62 (±0.35) | 3.51 (±0.43) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youssef, A.; Peña Fernández, A.; Wassermann, L.; Biernot, S.; Wittauer, E.-M.; Bleich, A.; Hartung, J.; Berckmans, D.; Norton, T. An Approach towards Motion-Tolerant PPG-Based Algorithm for Real-Time Heart Rate Monitoring of Moving Pigs. Sensors 2020, 20, 4251. https://doi.org/10.3390/s20154251
Youssef A, Peña Fernández A, Wassermann L, Biernot S, Wittauer E-M, Bleich A, Hartung J, Berckmans D, Norton T. An Approach towards Motion-Tolerant PPG-Based Algorithm for Real-Time Heart Rate Monitoring of Moving Pigs. Sensors. 2020; 20(15):4251. https://doi.org/10.3390/s20154251
Chicago/Turabian StyleYoussef, Ali, Alberto Peña Fernández, Laura Wassermann, Svenja Biernot, Eva-Maria Wittauer, André Bleich, Joerg Hartung, Daniel Berckmans, and Tomas Norton. 2020. "An Approach towards Motion-Tolerant PPG-Based Algorithm for Real-Time Heart Rate Monitoring of Moving Pigs" Sensors 20, no. 15: 4251. https://doi.org/10.3390/s20154251
APA StyleYoussef, A., Peña Fernández, A., Wassermann, L., Biernot, S., Wittauer, E.-M., Bleich, A., Hartung, J., Berckmans, D., & Norton, T. (2020). An Approach towards Motion-Tolerant PPG-Based Algorithm for Real-Time Heart Rate Monitoring of Moving Pigs. Sensors, 20(15), 4251. https://doi.org/10.3390/s20154251







