Why Not Glycine Electrochemical Biosensors?
Abstract
1. Introduction
2. Main Sources of Clinically Relevant Information
| Sample | Healthy Levels a | Unhealthy Levels a | Ref. |
|---|---|---|---|
| Plasma/Blood b | 147–299 (men) 100–384 (women) | 450–2363 | [35,36] |
| CSF c | 3.8–10 | <3 and 30–1927 | [22,26,35,37] |
| Urine d | 44–300 g | 550–5000 | [31,38] |
| Saliva | 177.80 ± 143.20 | - | [39] |
| Sweat e | 1751 ± 150 (passive) 997–595 (exercise) | - | [40,41] |
| ISF f | 565 ± 92 (adipose) 400 ± 48 (muscle) | - | [42] |
3. Current Analytical Methodologies for the Determination of Glycine
- Chromatographic methods with optical detection seem to lack specificity towards glycine determination [24,47]. As separation and identification are exclusively based on the retention time, there is always a risk of AA coelution [24,45], which could lead to overestimated results. For example, co-elution of glycine, arginine, histidine, and valine is reported for HPLC when the ionic strength of the eluent is not properly chosen [56]. Essentially, a careful selection of the stationary and mobile phases is mandatory to provide appropriate results, which is evidently more expensive, as a particular combination is exclusively used for only one analyte.
- Sample pre-treatments are complicated while inevitable, namely deproteinization and derivatization [47,58,59]. The former process is necessary because of the presence of soluble interferent species, such as peptides and proteins in the fluid, that will encumber the chromatographic column and give elevated backpressure in the instrument [60]. Accordingly, these compounds will disturb both quantitative and qualitative analysis, in addition to generating negative impacts on the instruments. Then, it is essential to include derivatization processes in column-based methods with optical detection because neither chromophore nor fluorophore groups are present in the molecular structure of glycine [47,61]. However, this treatment will bring negative effects to the analysis as well, as a consequence of the presence of derivatized compounds (impurities) [47].
- A typical IEC AA analysis normally takes several hours due to the fact that a low mobile phase flow rate is needed (for example 0.25 mL/min [62,63]) [58], which is quite time-consuming [54,59,64]. Furthermore, there is always extra time spent on mobile phase elution between each measurement for the purposes of removing residual impurities from the previous sample as well as equilibrating the column before the analysis of a new sample. This long analysis results in an important delay between the extraction of the sample and the outcomes’ provision, and therefore, the implementation of any needed medical treatment.
- The instrumentation and maintenance of the IEC instrument (and therefore, the related analyses) are costly [64]. Chromatographic methods require specialized equipment [48] that small-sized hospitals and laboratories might not have access to. Furthermore, these techniques demand for skillful operators that should be capable of implementing testing on the exquisite facilities [59]. The combination of these two aspects implies that glycine analysis is mainly performed in specific centralized laboratories. Thus, after collecting the sample, transportation to external laboratories is many times indispensable, resulting in an even extended overall time of test and data provision [65].
Electrochemical Sensors for the Determination of Glycine
4. Towards the Direct and Decentralized Glycine Electrochemical Detection
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wu, G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010, 1, 31–37. [Google Scholar] [CrossRef]
- Wu, G.Y. Recent advances in swine amino acid nutrition. J. Anim. Sci. Biotechnol. 2010, 1, 118–130. [Google Scholar]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Yu, Y.M.; Yang, R.D.; Matthews, D.E.; Wen, Z.M.; Burke, J.F.; Bier, D.M.; Young, V.R. Quantitative aspects of glycine and alanine nitrogen metabolism in postabsorptive young men: Effects of level of nitrogen and dispensable amino acid intake. J. Nutr. 1985, 115, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Waterlow, J.C.; Golden, M.H.; Garlick, P.J. Protein turnover in man measured with 15N: Comparison of end products and dose regimes. Am. J. Physiol. Endocrinol. Metab. 1978, 235, E165. [Google Scholar] [CrossRef] [PubMed]
- Picou, D.; Taylor-Roberts, T. The measurement of total protein synthesis and catabolism and nitrogen turnover in infants in different nutritional states and receiving different amounts of dietary protein. Clin. Sci. 1969, 36, 283–296. [Google Scholar] [PubMed]
- Matthews, D.E.; Conway, J.M.; Young, V.R.; Bier, D.M. Glycine nitrogen metabolism in man. Metabolism 1981, 30, 886–893. [Google Scholar] [CrossRef]
- Gersovitz, M.; Bier, D.; Matthews, D.; Udall, J.; Munro, H.N.; Young, V.R. Dynamic aspects of whole body glycine metabolism: Influence of protein intake in young adult and elderly males. Metab. Clin. Exp. 1980, 29, 1087–1094. [Google Scholar] [CrossRef]
- Jackson, A.A. The glycine story. Eur. J. Clin. Nutr. 1991, 45, 59–65. [Google Scholar] [PubMed]
- Meléndez-Hevia, E.; de Paz-Lugo, P.; Cornish-Bowden, A.; Cárdenas, M.L. A weak link in metabolism: The metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J. Biosci. 2009, 34, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.D.; Rose, M.L.; Yamashima, S.; Enomoto, N.; Seabra, V.; Madren, J.; Thurman, R.G. Dietary glycine blunts lung inflammatory cell influx following acute endotoxin. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L390–L398. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.L.; Madren, J.; Bunzendahl, H.; Thurman, R.G. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis 1999, 20, 793–798. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.L.; Cattley, R.C.; Dunn, C.; Wong, V.; Li, X.; Thurman, R.G. Dietary glycine prevents the development of liver tumors caused by the peroxisome proliferator WY-14,643. Carcinogenesis 1999, 20, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Thurman, R.G.; Zhong, Z.; von Frankenberg, M.; Stachlewitz, R.F.; Bunzendahl, H. Prevention of cyclosporne-induced nephrotoxicity with dietary glycine. Transplantation 1997, 63, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Bassot, A.; Bulteau, A.L.; Pirola, L.; Morio, B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Wagermaier, W.; Fratzl, P. Collagen. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 9. [Google Scholar]
- Yan, B.X.; Sun, Y.Q. Glycine residues provide flexibility for enzyme active sites. J. Biol. Chem. 1997, 272, 3190–3194. [Google Scholar] [CrossRef]
- Adeva-Andany, M.; Souto-Adeva, G.; Ameneiros-Rodriguez, E.; Fernandez-Fernandez, C.; Donapetry-Garcia, C.; Dominguez-Montero, A. Insulin resistance and glycine metabolism in humans. Amino Acids 2018, 50, 11–27. [Google Scholar] [CrossRef]
- Bannai, M.; Kawai, N. New therapeutic strategy for amino acid medicine: Glycine improves the quality of sleep. J. Pharmacol. Sci. 2012, 118, 145–148. [Google Scholar] [CrossRef]
- Mahbub, M.H.; Yamaguchi, N.; Takahashi, H.; Hase, R.; Amano, H.; Kobayashi-Miura, M.; Kanda, H.; Fujita, Y.; Yamamoto, H.; Yamamoto, M. Alteration in plasma free amino acid levels and its association with gout. Environ. Health Prev. Med. 2017, 22, 7. [Google Scholar] [CrossRef]
- Neeman, G.; Blanaru, M.; Bloch, B.; Kremer, I.; Ermilov, M.; Javitt, D.C.; Heresco-Levy, U. Relation of plasma glycine, serine, and homocysteine levels to schizophrenia symptoms and medication type. Am. J. Psychiatry 2005, 162, 1738–1740. [Google Scholar] [CrossRef]
- Applegarth, D.A.; Toone, J.R. Nonketotic hyperglycinemia (glycine encephalopathy): Laboratory diagnosis. Mol. Genet. Metab. 2001, 74, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, B.; Steinmann, B.; Gitzelmann, R.; Thun-Hohenstein, L.; Mascher, H.; Dumermuth, G. Nonketotic hyperglycinemia: Clinical and electrophysiologic effects of dextromethorphan, an antagonist of the NMDA receptor. Neurology 1993, 43. [Google Scholar] [CrossRef] [PubMed]
- Sandlers, Y. Amino acids profiling for the diagnosis of metabolic disorders. In Biochemical Testing-Clinical Correlation and Diagnosis; Bobbarala, V., Zaman, G.S., Desa, M.N.M., Akim, A.M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Hollak, C.E.M.; Lachmann, R. (Eds.) Inherited Metabolic Disease in Adults: A Clinical Guide; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Korman, S.H.; Gutman, A. Pitfalls in the diagnosis of glycine encephalopathy (non-ketotic hyperglycinemia). Dev. Med. Child Neurol. 2002, 44, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Canovas, R.; Cuartero, M.; Crespo, G.A. Modern creatinine (bio)sensing: Challenges of point-of-care platforms. Biosens. Bioelectron. 2019, 130, 110–124. [Google Scholar] [CrossRef]
- Lim, M.D.; Dickherber, A. Before you analyze a human specimen, think quality, variability, and bias. Anal. Chem. 2011, 83, 8–13. [Google Scholar] [CrossRef]
- Yu, Z.; Kastenmuller, G.; He, Y.; Belcredi, P.; Moller, G.; Prehn, C.; Mendes, J.; Wahl, S.; Roemisch-Margl, W.; Ceglarek, U.; et al. Differences between human plasma and serum metabolite profiles. PLoS ONE 2011, 6, e21230. [Google Scholar] [CrossRef]
- Weng, N.; Jian, W. (Eds.) Targeted Biomarker Quantitation by LC-MS; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef]
- Becker, K.L. Principles and Practice of Endocrinology and Metabolism; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Grier, R.E.; Gahl, W.A.; Cowan, T.; Bernardini, I.; McDowell, G.A.; Rinaldo, P. Revised sections F7.5 (quantitative amino acid analysis) and F7.6 (qualitative amino acid analysis): American College of Medical Genetics Standards and Guidelines for Clinical Genetics Laboratories, 2003. Genet. Med. 2004, 6, 66–68. [Google Scholar] [CrossRef][Green Version]
- McMillan, J.A.; Feigin, R.D.; DeAngelis, C.; Jones, M.D. Oski’s Pediatrics: Principles & Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006. [Google Scholar]
- Shih, V.E. Amino acid analysis. In Physician’s Guide to the Laboratory Diagnosis of Metabolic Diseases; Springer: Berlin/Heidelberg, Germany, 2003; pp. 11–26. [Google Scholar]
- Van Hove, J.L.; Curtis Coughlin, I.; Swanson, M.; Hennermann, J.B. Nonketotic Hyperglycinemia. In GeneReviews® [Internet]; University of Washington: Seattle, WA, USA, 2019. [Google Scholar]
- Mandal, R.; Guo, A.C.; Chaudhary, K.K.; Liu, P.; Yallou, F.S.; Dong, E.; Aziat, F.; Wishart, D.S. Multi-platform characterization of the human cerebrospinal fluid metabolome a comprehensive and quantitative update. Genome Med. 2012, 4, 38. [Google Scholar] [CrossRef]
- Seo, H.-R.; Jeong, E.-S.; Ahmed, M.S.; Lee, H.-K.; Jeon, S.-W. Polymeric membrane silver-ion selective electrodes based on schiff base N, N′-bis (pyridin-2-ylmethylene) benzene-1,2-diamine. Bull. Korean Chem. Soc. 2010, 31, 1699–1703. [Google Scholar] [CrossRef]
- Dame, Z.T.; Aziat, F.; Mandal, R.; Krishnamurthy, R.; Bouatra, S.; Borzouie, S.; Guo, A.C.; Sajed, T.; Deng, L.; Lin, H.; et al. The human saliva metabolome. Metabolomics 2015, 11, 1864–1883. [Google Scholar] [CrossRef]
- Murphy, G.R.; Dunstan, R.H.; Macdonald, M.M.; Borges, N.; Radford, Z.; Sparkes, D.L.; Dascombe, B.J.; Roberts, T.K. Relationships between electrolyte and amino acid compositions in sweat during exercise suggest a role for amino acids and K+ in reabsorption of Na+ and Cl− from sweat. PLoS ONE 2019, 14, e0223381. [Google Scholar] [CrossRef] [PubMed]
- Dunstan, R.H.; Sparkes, D.L.; Dascombe, B.J.; Macdonald, M.M.; Evans, C.A.; Stevens, C.J.; Crompton, M.J.; Gottfries, J.; Franks, J.; Murphy, G.; et al. Sweat Facilitated Amino Acid Losses in Male Athletes during Exercise at 32-34 degrees C. PLoS ONE 2016, 11, e0167844. [Google Scholar] [CrossRef] [PubMed]
- Maggs, D.G.; Jacob, R.; Rife, F.; Lange, R.; Leone, P.; During, M.J.; Tamborlane, W.V.; Sherwin, R.S. Interstitial fluid concentrations of glycerol, glucose, and amino acids in human quadricep muscle and adipose tissue. Evidence for significant lipolysis in skeletal muscle. J. Clin. Investig. 1995, 96, 370–377. [Google Scholar] [CrossRef]
- Fernandes, J.; Saudubray, J.-M.; Van den Berghe, G.; Walter, J.H. Inborn Metabolic Diseases: Diagnosis and Treatment; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Garg, U.; Smith, L.D. Biomarkers in Inborn Errors of Metabolism: Clinical Aspects and Laboratory Determination; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Sharer, J.D.; De Biase, I.; Matern, D.; Young, S.; Bennett, M.J.; Tolun, A.A.; ACMG Laboratory Quality Assurance Committee. Laboratory analysis of amino acids, 2018 revision: A technical standard of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2018, 20, 1499–1507. [Google Scholar] [CrossRef]
- Bowron, A.; Brown, A.; Deverell, D. Metbionet Guidelines for Amino Acid Analysis; National Metabolic Biochemistry Network: Bristol, UK, 2012. [Google Scholar]
- Tang, Y.B.; Teng, L.; Sun, F.; Wang, X.L.; Peng, L.; Cui, Y.Y.; Hu, J.J.; Luan, X.; Zhu, L.; Chen, H.Z. Determination of glycine in biofluid by hydrophilic interaction chromatography coupled with tandem mass spectrometry and its application to the quantification of glycine released by embryonal carcinoma stem cells. J. Chromatogr. B 2012, 905, 61–66. [Google Scholar] [CrossRef]
- Kugimiya, A.; Fukada, R. Chemiluminescence detection of serine, proline, glycine, asparagine, leucine, and histidine by rsing corresponding aminoacyl-tRNA synthetases as recognition elements. Appl. Biochem. Biotechnol. 2015, 176, 1195–1202. [Google Scholar] [CrossRef]
- Yoshida, H.; Kondo, K.; Yamamoto, H.; Kageyama, N.; Ozawa, S.; Shimbo, K.; Muramatsu, T.; Imaizumi, A.; Mizukoshi, T.; Masuda, J.; et al. Validation of an analytical method for human plasma free amino acids by high-performance liquid chromatography ionization mass spectrometry using automated precolumn derivatization. J. Chromatogr. B 2015, 998–999, 88–96. [Google Scholar] [CrossRef]
- Moore, S.; Stein, W.H. Chromatographic determination of amino acids by the use of automatic recording equipment. Methods Enzymol. 1963, 6, 819–831. [Google Scholar] [CrossRef]
- Moore, S.; Spackman, D.H.; Stein, W.H. Chromatography of amino acids on sulfonated polystyrene resins. An improved system. Anal. Chem. 1958, 30, 1185–1190. [Google Scholar] [CrossRef]
- Thomson, A.R.; Miles, B.J. Ion-exchange chromatography of amino-acids: Improvements in the single column system. Nature 1964, 203, 483–484. [Google Scholar] [CrossRef] [PubMed]
- Davey, J.F.; Ersser, R.S. Amino acid analysis of physiological fluids by high-performance liquid chromatography with phenylisothiocyanate derivatization and comparison with ion-exchange chromatography. J. Chromatogr. B 1990, 528, 9–23. [Google Scholar] [CrossRef]
- Li, Q.Z.; Huang, Q.X.; Li, S.C.; Yang, M.Z.; Rao, B. Simultaneous determination of glutamate, glycine, and alanine in human plasma using precolumn derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate and high-performance liquid chromatography. Korean J. Physiol. Pharmacol. 2012, 16, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Applications of the ninhydrin reaction for analysis of amino acids, peptides, and proteins to agricultural and biomedical sciences. J. Agric. Food Chem. 2004, 52, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Fekkes, D. State-of-the-art of high-performance liquid chromatographic analysis of amino acids in physiological samples. J. Chromatogr. B 1996, 682, 3–22. [Google Scholar] [CrossRef]
- Spackman, D.H.; Stein, W.H.; Moore, S. Automatic recording apparatus for use in the chromatography of amino acids. Anal. Chem. 1958, 30, 1191–1206. [Google Scholar] [CrossRef]
- Godel, H.; Graser, T.; Földi, P.; Pfaender, P.; Fürst, P. Measurement of free amino acids in human biological fluids by high-performance liquid chromatography. J. Chromatogr. A 1984, 297, 49–61. [Google Scholar] [CrossRef]
- Tatsumi, M.; Hoshino, W.; Kodama, Y.; Ueatrongchit, T.; Takahashi, K.; Yamaguchi, H.; Tagami, U.; Miyano, H.; Asano, Y.; Mizukoshi, T. Development of a rapid and simple glycine analysis method using a stable glycine oxidase mutant. Anal. Biochem. 2019, 587, 113447. [Google Scholar] [CrossRef]
- Cooper, C.; Packer, N.; Williams, K. Amino Acid Analysis Protocols; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2001; Volume 159. [Google Scholar]
- Azadbakht, A.; Abbasi, A. Fabrication of a high sensitive glycine electrochemical sensor based on immobilization of nanostructured Ni chelidamic acid and bimetallic Au-Pt inorganic-organic hybrid nanocomposite onto glassy carbon modified electrode. Russ. J. Electrochem. 2014, 50, 46–53. [Google Scholar] [CrossRef]
- Fan, J.; Hong, J.; Hu, J.D.; Chen, J.L. Ion chromatography based urine amino acid profiling applied for diagnosis of gastric cancer. Gastroenterol. Res. Pract. 2012, 2012, 474907. [Google Scholar] [CrossRef]
- Ding, Y.; Yu, H.; Mou, S. Direct determination of free amino acids and sugars in green tea by anion-exchange chromatography with integrated pulsed amperometric detection. J. Chromatogr. A 2002, 982, 237–244. [Google Scholar] [CrossRef]
- Bobreshova, O.V.; Parshina, A.V.; Safronova, E.Y.; Titova, T.S.; Yaroslavtsev, A.B. Potentiometric determination of glycine, alanine, and leucine anions and potassium cations in alkaline solutions using zirconia-modified nafion and MF-4SC membranes. Pet. Chem. 2015, 55, 367–372. [Google Scholar] [CrossRef]
- Cuartero, M.; Parrilla, M.; Crespo, G.A. Wearable potentiometric sensors for medical applications. Sensors 2019, 19, 363. [Google Scholar] [CrossRef] [PubMed]
- Walker, V.; Mills, G. Quantitative methods for amino acid analysis in biological fluids. Ann. Clin. Biochem. 1995, 32, 28–57. [Google Scholar] [CrossRef]
- Heinrikson, R.L.; Meredith, S.C. Amino acid analysis by reverse-phase high-performance liquid chromatography: Precolumn derivatization with phenylisothiocyanate. Anal. Biochem. 1984, 136. [Google Scholar] [CrossRef]
- Narayan, S.B.; Ditewig-Meyers, G.; Graham, K.S.; Scott, R.; Bennett, M.J. Measurement of plasma amino acids by Ultraperformance® Liquid Chromatography. Clin. Chem. Lab. Med. 2011, 49, 1177–1185. [Google Scholar] [CrossRef]
- Sigma-Aldrich: Analytical, Biology, Chemistry & Materials. Available online: https://www.sigmaaldrich.com/ (accessed on 6 June 2020).
- Abcam: Antibodies, Proteins, Kits and Reagents for Life Science. Available online: https://www.abcam.com/ (accessed on 6 June 2020).
- Assay Genie. Available online: https://www.assaygenie.com/ (accessed on 6 June 2020).
- CliniSciences: Reagents and Instruments for Immunology, Cell Biology and Molecular Biology. Available online: https://www.clinisciences.com/ (accessed on 6 June 2020).
- BioVision: Life Science Source. Innovation, flexibility, Affordability. Available online: https://www.biovision.com/ (accessed on 6 June 2020).
- Cell Biolabs, Inc.: Creating Solutions for Life Science Research. Available online: https://www.cellbiolabs.com/ (accessed on 6 June 2020).
- Saranya, S.; Jency Feminus, J.; Geetha, B.; Deepa, P.N. Simultaneous detection of glutathione, threonine, and glycine at electrodeposited RuHCF/rGO-modified electrode. Ionics 2019, 25, 5537–5550. [Google Scholar] [CrossRef]
- Alam, M.M.; Asiri, A.M.; Uddin, M.T.; Islam, M.A.; Rahman, M.M. In-situ glycine sensor development based ZnO/Al2O3/Cr2O3 nanoparticles. ChemistrySelect 2018, 3, 11460–11468. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Sadeghi, S.; Bageri, L.; Mokhtarzadeh, A.; Karimzadeh, A.; Shadjou, N.; Mahboob, S. Poly-dopamine-beta-cyclodextrin: A novel nanobiopolymer towards sensing of some amino acids at physiological pH. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 343–357. [Google Scholar] [CrossRef]
- Stadtman, E.R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. 1993, 62, 797–821. [Google Scholar] [CrossRef]
- Saghatforoush, L.; Hasanzadeh, M.; Shadjou, N.; Khalilzadeh, B. Deposition of new thia-containing Schiff-base iron (III) complexes onto carbon nanotube-modified glassy carbon electrodes as a biosensor for electrooxidation and determination of amino acids. Electrochim. Acta 2011, 56, 1051–1061. [Google Scholar] [CrossRef]
- Zheng, L.; Song, J.F. Nickel(II)-baicalein complex modified multiwall carbon nanotube paste electrode and its electrocatalytic oxidation toward glycine. Anal. Biochem. 2009, 391, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Vidotti, M.; de Torresi, S.I.C.; Kubota, L.T. Electrochemical oxidation of glycine by doped nickel hydroxide modified electrode. Sens. Actuators B Chem. 2008, 135, 245–249. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Karim-Nezhad, G.; Shadjou, N.; Hajjizadeh, M.; Khalilzadeh, B.; Saghatforoush, L.; Abnosi, M.H.; Babaei, A.; Ershad, S. Cobalt hydroxide nanoparticles modified glassy carbon electrode as a biosensor for electrooxidation and determination of some amino acids. Anal. Biochem. 2009, 389, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Ràfols, C.; Subirats, X.; Serrano, N.; Diaz-Cruz, J.M. New discrimination tools for harvest year and varieties of white wines based on hydrophilic interaction liquid chromatography with amperometric detection. Talanta 2019, 201, 104–110. [Google Scholar] [CrossRef]
- Toyo’oka, T. Recent advances in separation and detection methods for thiol compounds in biological samples. J. Chromatogr. B 2009, 877, 3318–3330. [Google Scholar] [CrossRef]
- Parshina, A.V.; Titova, T.S.; Safronova, E.Y.; Bobreshova, O.V.; Yaroslavtsev, A.B. Determination of glycine, alanine, and leucine at different solution pH with the aid of donnan potential sensors based on hybrid membranes. J. Anal. Chem. 2016, 71, 259–268. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; Omidinia, E. Mesoporous silica (MCM-41)-Fe2O3 as a novel magnetic nanosensor for determination of trace amounts of amino acids. Colloids Surf. B Biointerfaces 2013, 108, 52–59. [Google Scholar] [CrossRef]
- Roushani, M.; Shamsipur, M.; Pourmortazavi, S.M. Amperometric detection of Glycine, l-Serine, and l-Alanine using glassy carbon electrode modified by NiO nanoparticles. J. Appl. Electrochem. 2012, 42, 1005–1011. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; Chen, S.-T.; Sheikhzadeh, P. MCM-41-NH2 as an advanced nanocatalyst for electrooxidation and determination of amino acids. Catal. Commun. 2012, 19, 21–27. [Google Scholar] [CrossRef]
- St John, A.; Price, C.P. Existing and emerging technologies for point-of-care testing. Clin. Biochem. Rev. 2014, 35, 155–167. [Google Scholar] [PubMed]
- Pundir, C.S.; Lata, S.; Narwal, V. Biosensors for determination of D and L-amino acids: A review. Biosens. Bioelectron. 2018, 117, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Serra, B.; Reviejo, A.J.; Pingarron, J.M. Chiral analysis of amino acids using electrochemical composite bienzyme biosensors. Anal. Biochem. 2001, 298, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Huh, M.H. Development of a biosensor with immobilized l-amino acid oxidase for determination of l-amino acids. J. Food Biochem. 1999, 23, 173–185. [Google Scholar] [CrossRef]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef]
- Lata, S.; Pundir, C.S. L-amino acid biosensor based on L-amino acid oxidase immobilized onto NiHCNFe/c-MWCNT/PPy/GC electrode. Int. J. Biol. Macromol. 2013, 54, 250–257. [Google Scholar] [CrossRef]
- Zain, Z.M.; O’Neill, R.D.; Lowry, J.P.; Pierce, K.W.; Tricklebank, M.; Dewa, A.; Ab Ghani, S. Development of an implantable D-serine biosensor for in vivo monitoring using mammalian D-amino acid oxidase on a poly (o-phenylenediamine) and Nafion-modified platinum-iridium disk electrode. Biosens. Bioelectron. 2010, 25, 1454–1459. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, D.; Shuang, S.; Martin, M.F. A homocysteine biosensor with eggshell membrane as an enzyme immobilization platform. Sens Actuators B: Chem. 2006, 114, 936–942. [Google Scholar] [CrossRef]
- Rosini, E.; Piubelli, L.; Molla, G.; Frattini, L.; Valentino, M.; Varriale, A.; D’Auria, S.; Pollegioni, L. Novel biosensors based on optimized glycine oxidase. FEBS J. 2014, 281, 3460–3472. [Google Scholar] [CrossRef]
- Job, V.; Marcone, G.L.; Pilone, M.S.; Pollegioni, L. Glycine oxidase from Bacillus subtilis. Characterization of a new flavoprotein. J. Biol. Chem. 2002, 277, 6985–6993. [Google Scholar] [CrossRef]
- Job, V.; Molla, G.; Pilone, M.S.; Pollegioni, L. Overexpression of a recombinant wild-type and His-tagged Bacillus subtilis glycine oxidase in Escherichia coli. Eur. J. Biochem. 2002, 269, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Molla, G.; Motteran, L.; Job, V.; Pilone, M.S.; Pollegioni, L. Kinetic mechanisms of glycine oxidase from Bacillus subtilis. Eur. J. Biochem. 2003, 270, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Pedotti, M.; Rosini, E.; Molla, G.; Moschetti, T.; Savino, C.; Vallone, B.; Pollegioni, L. Glyphosate resistance by engineering the flavoenzyme glycine oxidase. J. Biol. Chem. 2009, 284, 36415–36423. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Herde, M.K.; Mitchell, J.A.; Whitfield, J.H.; Wulff, A.B.; Vongsouthi, V.; Sanchez-Romero, I.; Gulakova, P.E.; Minge, D.; Breithausen, B.; et al. Monitoring hippocampal glycine with the computationally designed optical sensor GlyFS. Nat. Chem. Biol. 2018, 14, 861–869. [Google Scholar] [CrossRef]
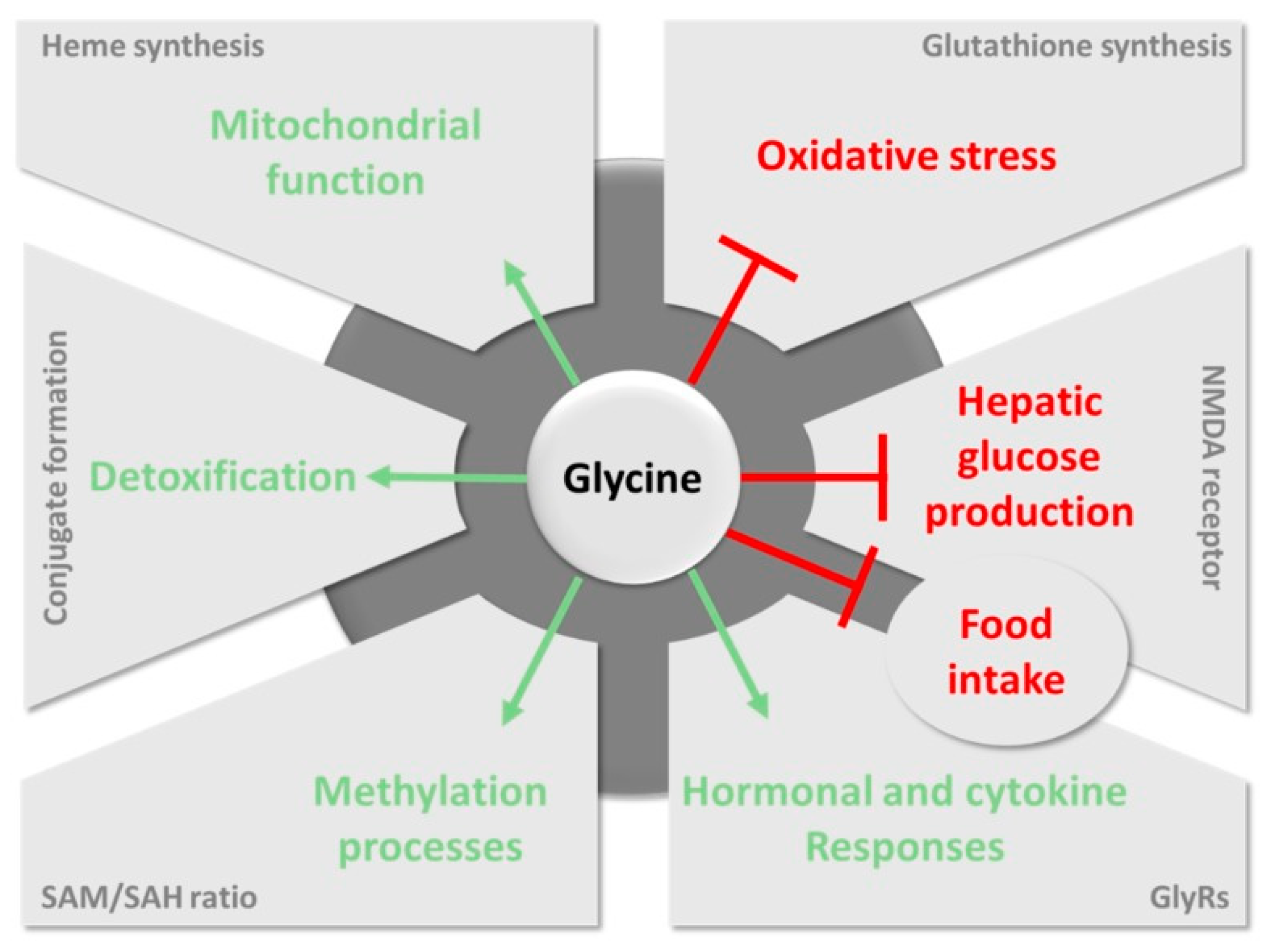

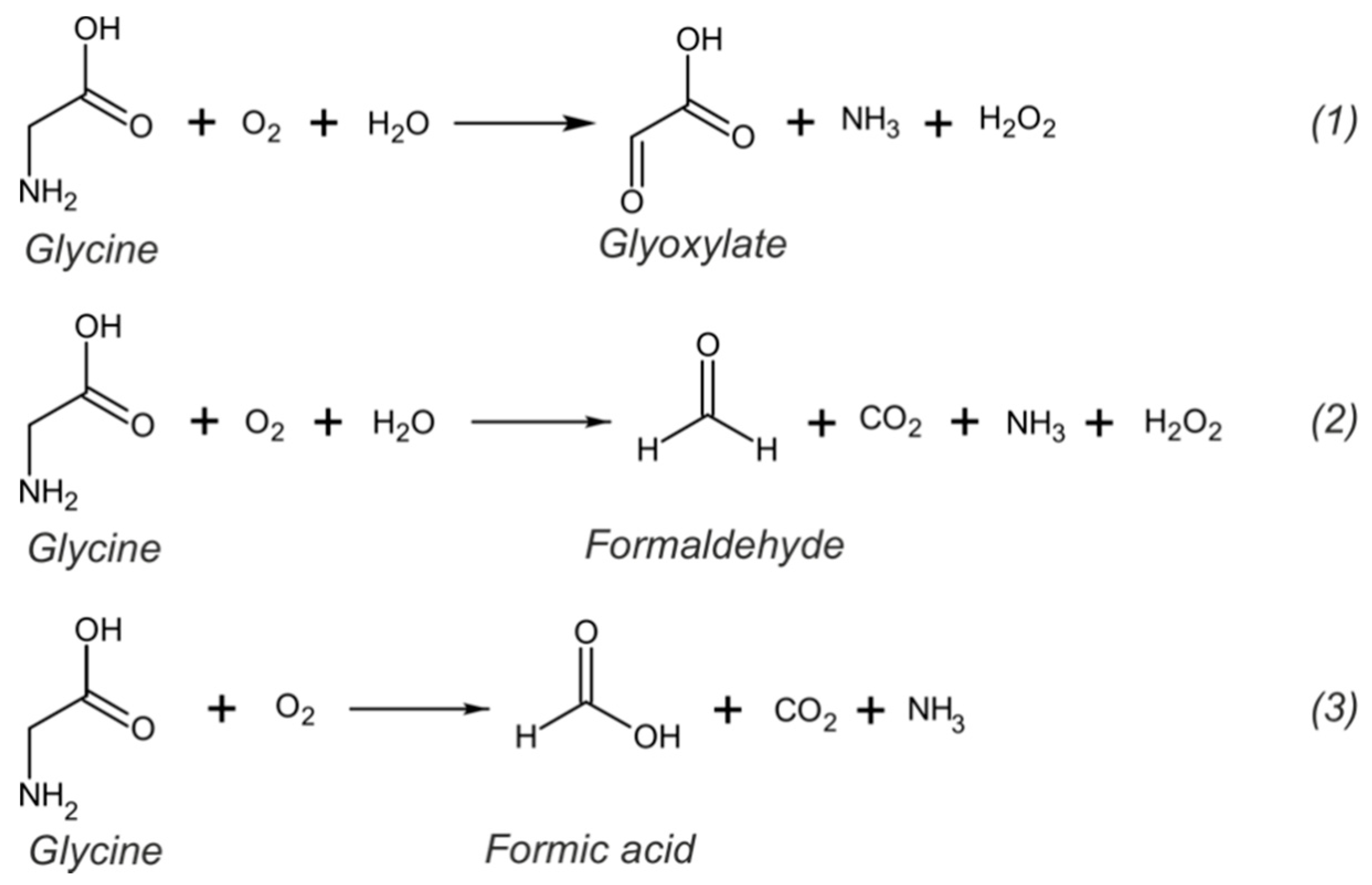
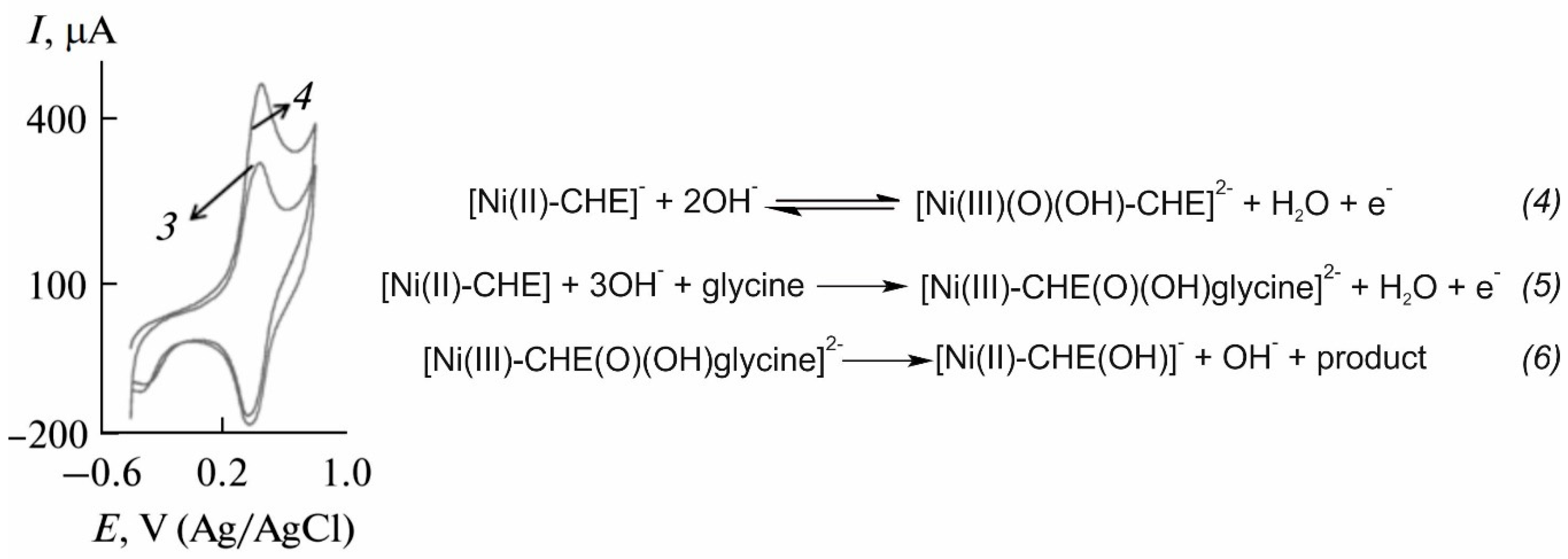

| Sensing Element | Technique | Analytical Parameters | Interferences | Application | Ref. |
|---|---|---|---|---|---|
| RuHCF/rGO | SWV Eox = −1.36 V pH 5 | LOD = 0.4 μM LRR = 1.25–7.49 μM | Able to determine Gly, GSH and Thr simultaneously | Spiked saliva (diluted 100 times) | [75] |
| ZnO/Al2O3/Cr2O3 NPs | Electrometry pH 7 | LOD = 82.25 pM LRR = 0.1–1000 nM | Interference from GSH and Cys. | Spiked human, mouse, and rabbit serum | [76] |
| Polydopamine-β-cyclodextrin | DPV Eox = 0.14 V pH 7.4 | LOD = 0.06 μM LRR = 0.2–70 μM | Interference from Cys, Tyr, Phe. | None | [77] |
| ZrO2 or SiO2 NPs | Potentiometry | LOD = 60 μM | Able to determine Gly, Ala and Leu simultaneously with electrode array | None | [85] |
| ZrO2 NPs | Potentiometry | NS | Able to determine Gly, Ala and Leu simultaneously with electrode array | None | [64] |
| Ni chelidamic acid | Amperometry Eap = 0.35 V pH 13 | LOD = 0.3 μM LRR = 1–750 μM | No interference from Leu, Ala or Glu. | Spiked human serum (diluting and extracting proteins) | [61] |
| MCM-41-Fe2O3 NPs | Amperometry Eap = 0.6 V pH 8.1 | LOD = 145 nM LRR = 0.3–1 μM | Interference from Cys, Val, Phe, Ser, Trp and Tyr | None | [86] |
| NiO NPs | Amperometry Eap = 0.42 V pH 13 | LOD = 0.9 μM LRR = 1–200 μM | Interference from Ser and Ala. No interference from Thr, Asn, His, Gln or Pro | None | [87] |
| MCM-41 functionalized by 3-aminopropyl | DPV Eox = NS pH 7.4 | LOD = 10.11 nM LRR = 0.1–1.2 μM | Interference from Cys, Val, Phe, Ser, Arg, Trp and Tyr | None | [88] |
| Fe(III)–Schiff base complex | DPV Eox = NS pH 2 | LOD = 4.11 μM LRR = 3–12200 μM | Interference from Cys, Val, Phe, Ser, Arg, Trp and Tyr | None | [79] |
| Ni(II)–baicalein complex | Amperometry Eap = 0.55 V pH 13 | LOD = 9.2 μM LRR = 20–1000 μM | Interference from Val, Ser, Trp and His | None | [80] |
| Co(OH)O NPs | DPV Eox = NS pH 13 | LOD = 10.02 μM LRR = 20–1500 μM | Interference from Val, Phe, Ser, Arg, Trp and Tyr | None | [82] |
| Ni(OH)2 | Amperometry Eap = 0.5 V pH 13 | LOD = 30 μM LRR = 0.1–1.2 mM | Interference from Arg. No response to Glu, Leu or Ala | None | [81] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Ràfols, C.; Liu, Y.; Wang, Q.; Cuartero, M.; Crespo, G.A. Why Not Glycine Electrochemical Biosensors? Sensors 2020, 20, 4049. https://doi.org/10.3390/s20144049
Pérez-Ràfols C, Liu Y, Wang Q, Cuartero M, Crespo GA. Why Not Glycine Electrochemical Biosensors? Sensors. 2020; 20(14):4049. https://doi.org/10.3390/s20144049
Chicago/Turabian StylePérez-Ràfols, Clara, Yujie Liu, Qianyu Wang, María Cuartero, and Gastón A. Crespo. 2020. "Why Not Glycine Electrochemical Biosensors?" Sensors 20, no. 14: 4049. https://doi.org/10.3390/s20144049
APA StylePérez-Ràfols, C., Liu, Y., Wang, Q., Cuartero, M., & Crespo, G. A. (2020). Why Not Glycine Electrochemical Biosensors? Sensors, 20(14), 4049. https://doi.org/10.3390/s20144049








