Wearable Epileptic Seizure Prediction System with Machine-Learning-Based Anomaly Detection of Heart Rate Variability
Abstract
1. Introduction
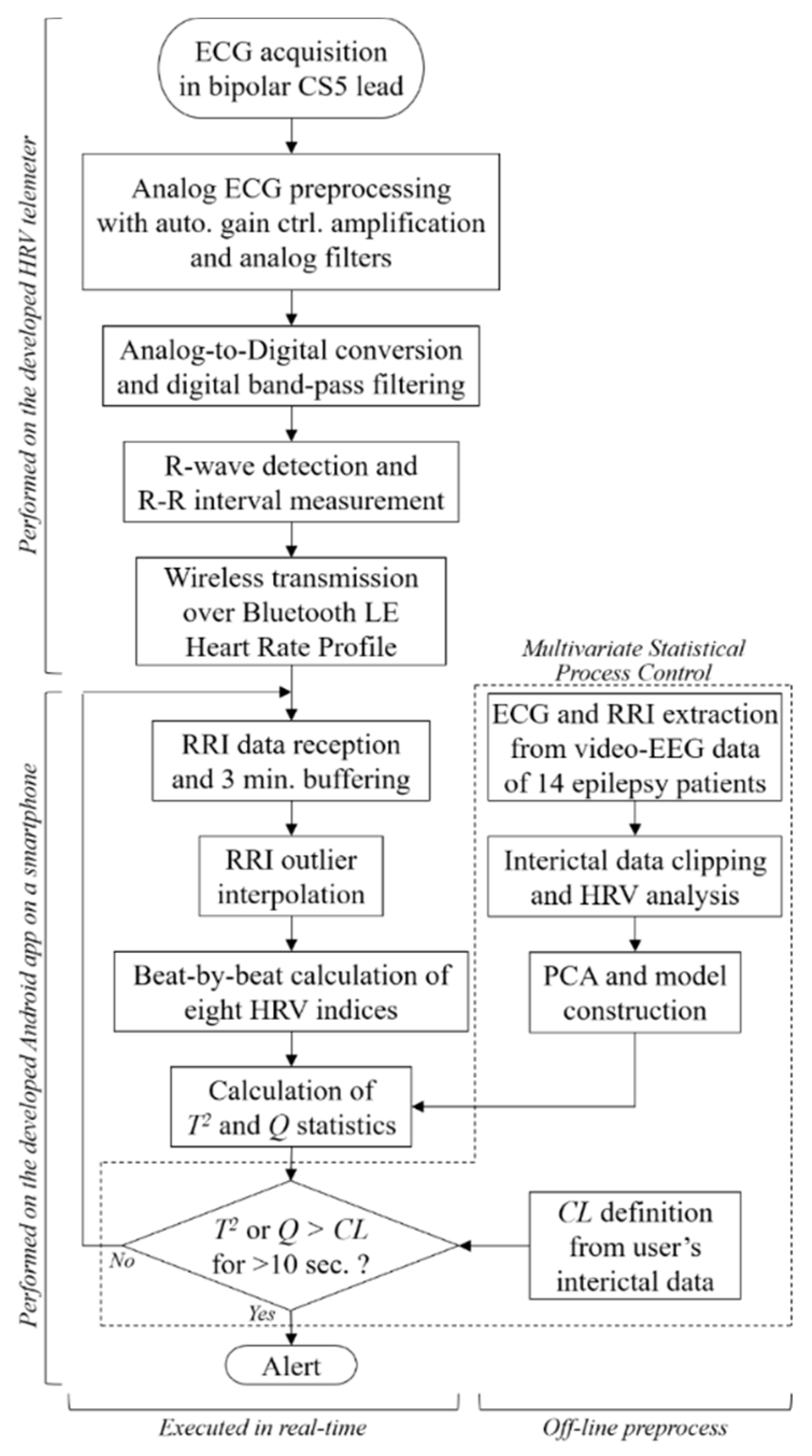
2. Epileptic Seizure Prediction System
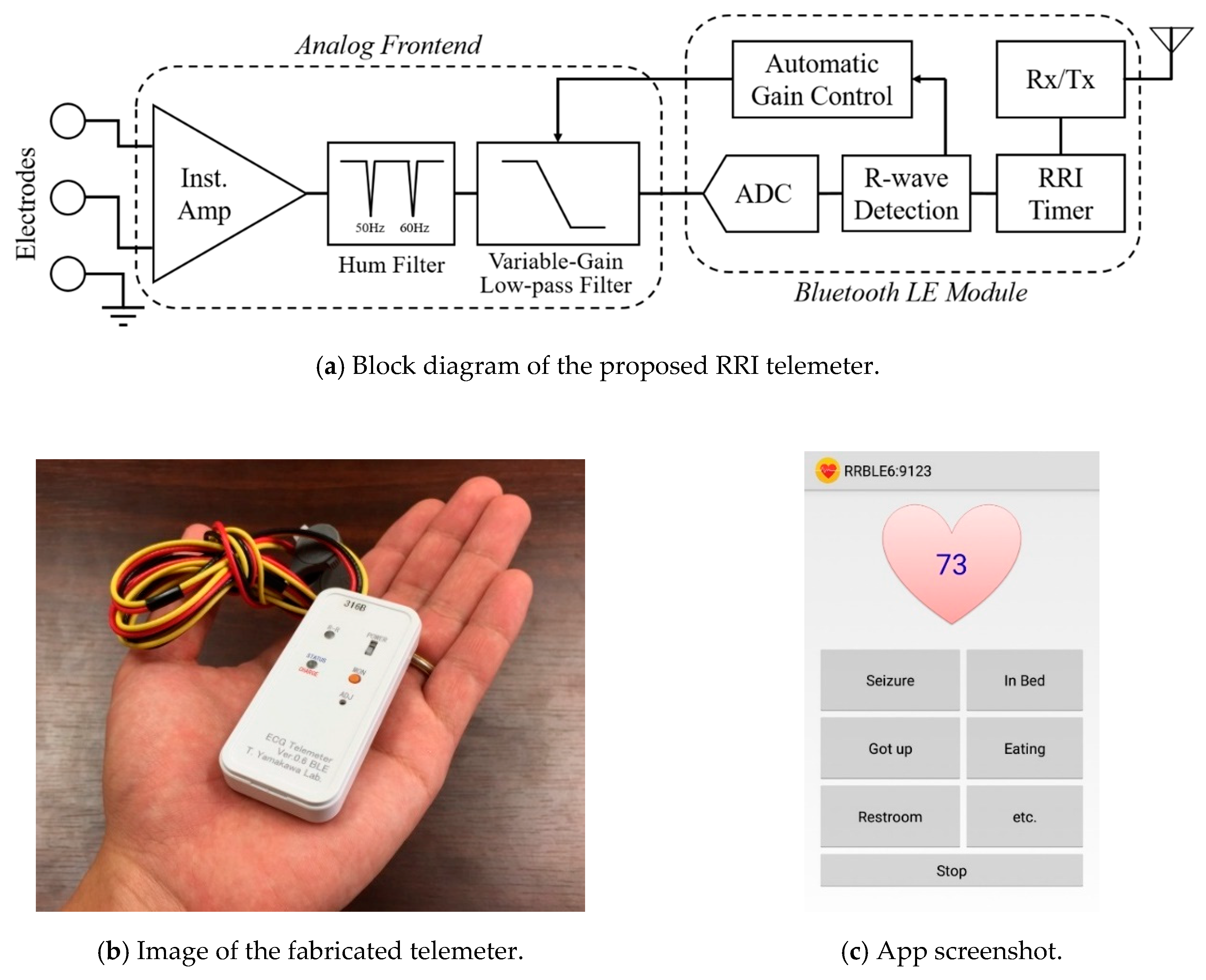
2.1. System Composition
2.2. HRV Analysis
2.3. Anomaly Detection Prior to Epileptic Seizure
| Algorithm 1 Seizure prediction algorithm | ||
| 1 | set | τ [0] ← 0, C [0] ← Ɲ. |
| 2 | while do | |
| 3 | Collect the newly measured t-th RRI y[t]. | |
| 4 | Extract and preprocess the HRV indices x[t]. | |
| 5 | Calculate the t-th T2 [t] and Q[t] from x[t] by using Equations (5) and (6). | |
| 6 | if | |
| 7 | then | |
| 8 | else | |
| 9 | end if | |
| 10 | if | |
| 11 | then | |
| 12 | end if | |
| 13 | Wait until the next RRI data y [t + 1] are measured. | |
| 14 | end while | |
3. Experimental Methods
3.1. MSPC Model Construction
3.2. Measurement Setup and Protocols
4. Results
4.1. Patient Attribution
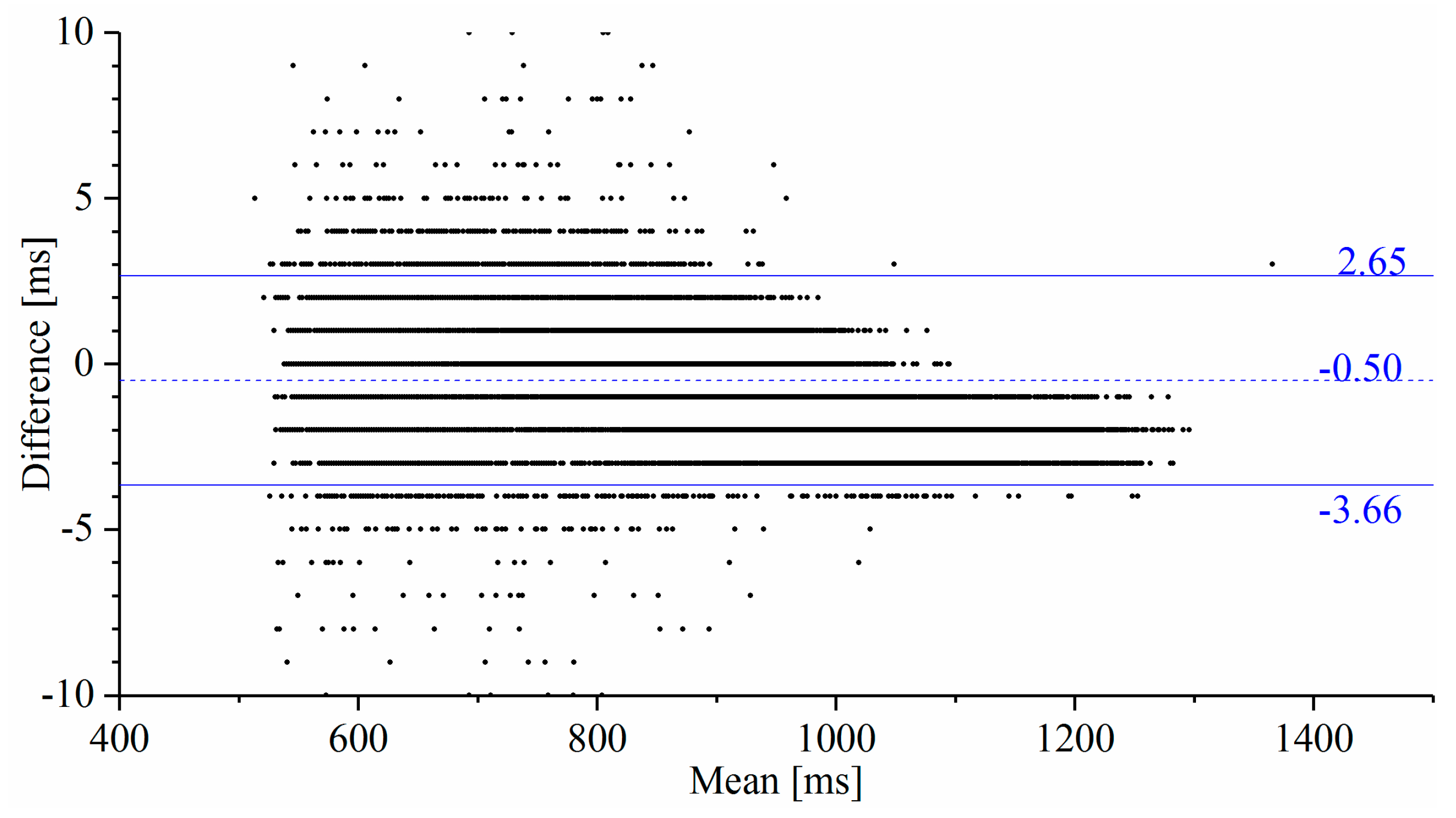
4.2. Measurement Accuracy and Reliability
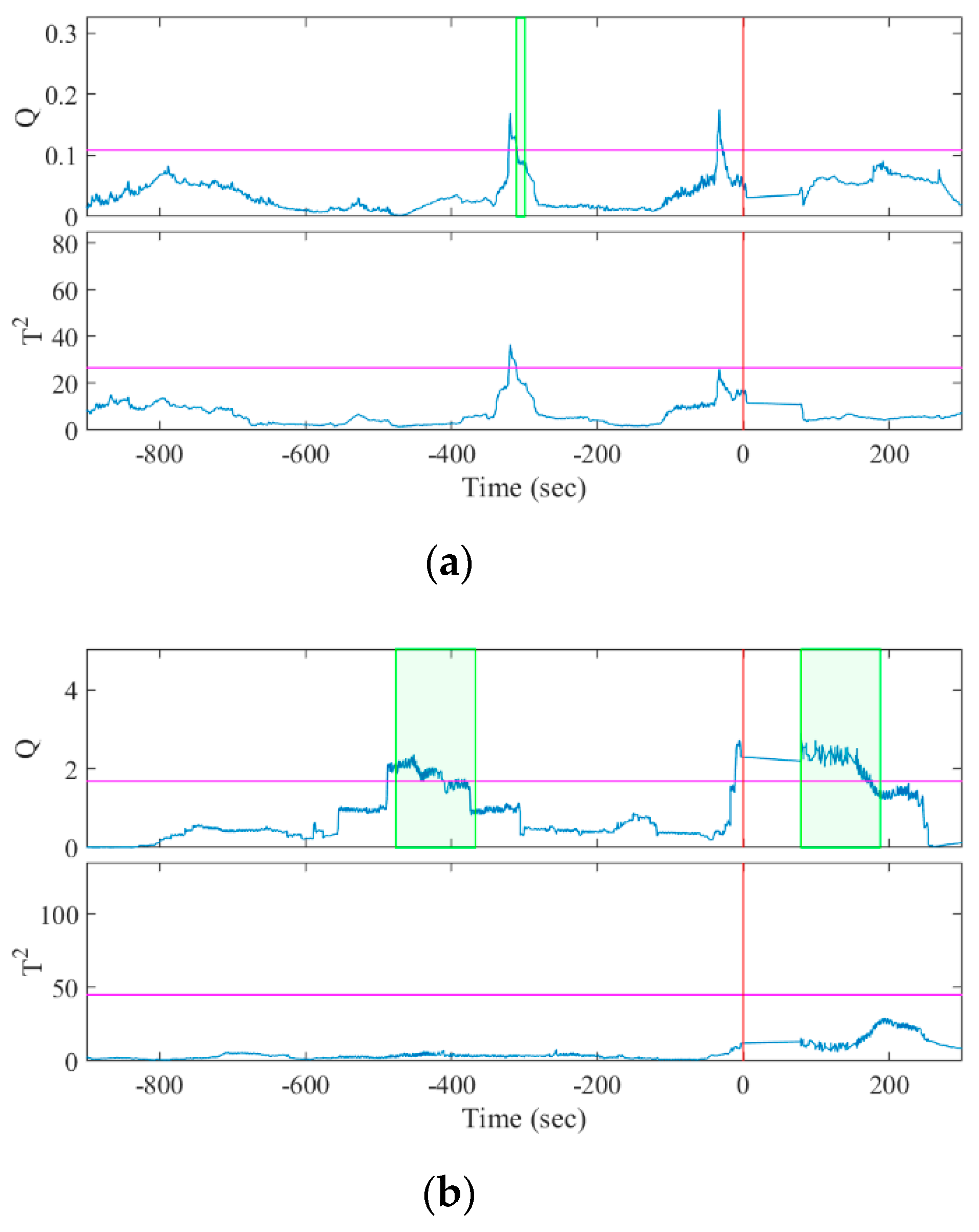
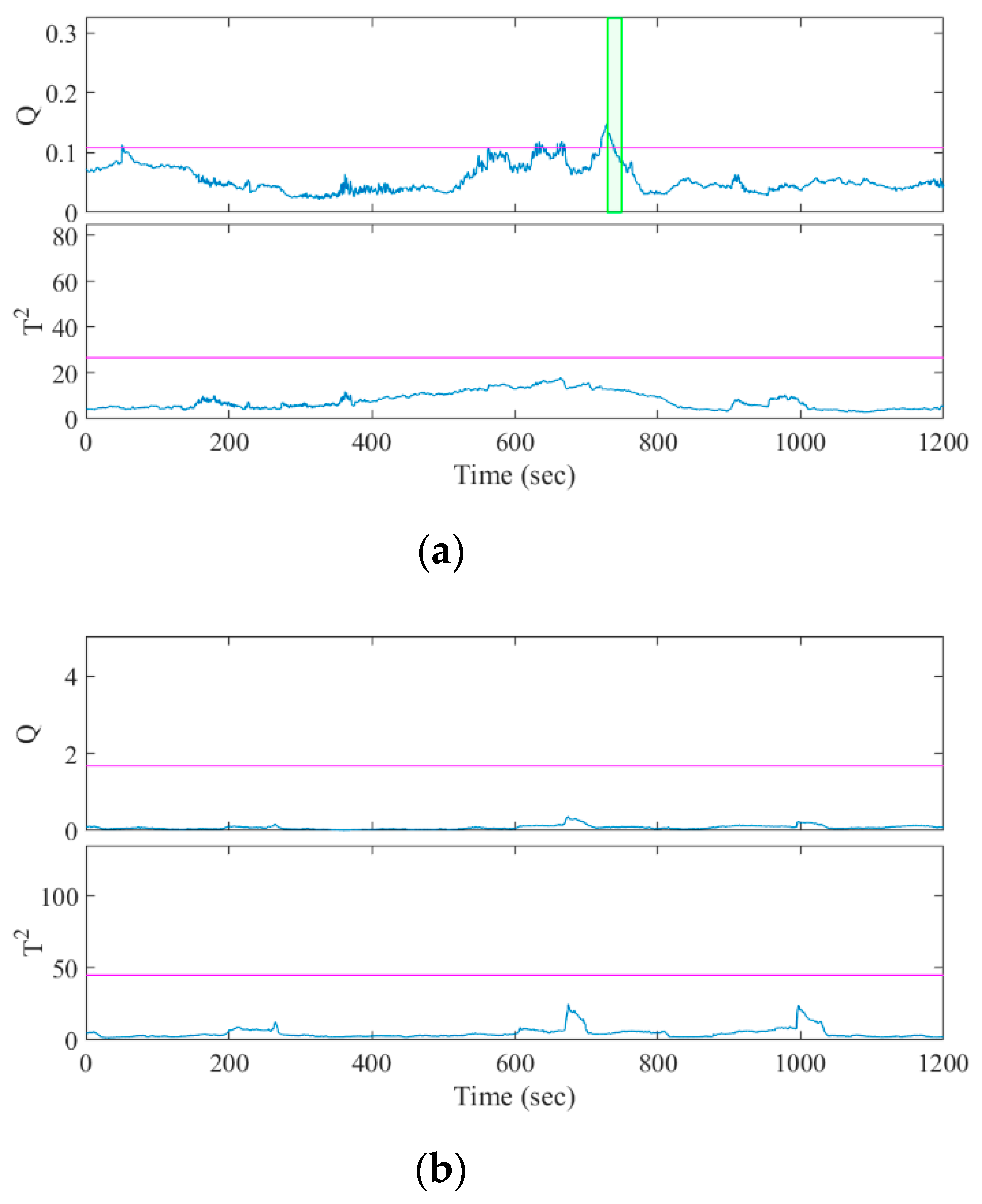
4.3. Seizure Prediction
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thurman, D.J.; Beghi, E.; Begley, C.E.; Berg, A.T.; Buchhalter, J.R.; Ding, D.; Hesdorffer, D.C.; Hauser, W.A.; Kazis, L.; Kobau, R.; et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia 2011, 52, 2–26. [Google Scholar] [CrossRef]
- Chang, B.S.; Lowenstein, D.H. Epilepsy. N. Engl. J. Med. 2003, 349, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Bonhage, A.; Sales, F.; Wagner, K.; Teotonio, R.; Carius, A.; Schelle, A.; Ihle, M. Views of patients with epilepsy on seizure prediction devices. Epilepsy Behav. 2010, 18, 388–396. [Google Scholar] [CrossRef]
- Iasemidis, L.D. Epileptic seizure prediction and control. IEEE Trans. Biomed. Eng. 2003, 50, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Carney, P.R.; Myers, S.; Geyer, J.D. Seizure prediction: Methods. Epilepsy Behav. 2011, 22, S94–S101. [Google Scholar] [CrossRef] [PubMed]
- Kiral-Kornek, I.; Roy, S.; Nurse, E.; Mashford, B.; Karoly, P.; Carroll, T.; Payne, D.; Saha, S.; Baldassano, S.; O’Brien, T.; et al. Epileptic Seizure Prediction Using Big Data and Deep Learning: Toward a Mobile System. EBioMedicine 2018, 27, 103–111. [Google Scholar] [CrossRef]
- Karoly, P.J.; Ung, H.; Grayden, D.B.; Kuhlmann, L.; Leyde, K.; Cook, M.J.; Freestone, D.R. The circadian profile of epilepsy improves seizure forecasting. Brain 2017, 140, 2169–2182. [Google Scholar] [CrossRef]
- Zijlmans, M.; Flanagan, D.; Gotman, J. Heart rate changes and ECG abnormalities during epileptic seizures: Prevalence and definition of an objective clinical sign. Epilepsia 2002, 43, 847–854. [Google Scholar] [CrossRef]
- Brotherstone, R.; McLellan, A. Parasympathetic alteration during sub-clinical seizures. Seizure 2012, 21, 391–398. [Google Scholar] [CrossRef][Green Version]
- Jansen, K.; Varon, C.; Van Huffel, S.; Lagae, L. Peri-ictal ECG changes in childhood epilepsy: Implications for detection systems. Epilepsy Behav. 2013, 29, 72–76. [Google Scholar] [CrossRef]
- Galimberti, C.A.; Marchioni, E.; Barzizza, F.; Manni, R.; Sartori, I.; Tartara, A. Partial epileptic seizures of different origin variably affect cardiac rhythm. Epilepsia 1996, 37, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Leutmezer, F.; Schernthaner, C.; Lurger, S.; Pötzelberger, K.; Baumgartner, C. Electrocardiographic changes at the onset of epileptic seizures. Epilepsia 2003, 44, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Benninger, F.; Urak, L.; Plattner, B.; Geldner, J.; Feucht, M. EKG abnormalities in children and adolescents with symptomatic temporal lobe epilepsy. Neurology 2004, 63, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Jin, K.; Itabashi, H.; Iwasaki, M.; Kakisaka, Y.; Aoki, M.; Nakasato, N. Earlier tachycardia onset in right than left mesial temporal lobe seizures. Neurology 2014, 83, 1332–1336. [Google Scholar] [CrossRef]
- Malik, M.; Camm, A.J.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Quintana, D.; Alvares, G.; Heathers, J. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef]
- Lotufo, P.A.; Valiengo, L.; Benseñor, I.M.; Brunoni, A.R. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia 2012, 53, 272–282. [Google Scholar] [CrossRef]
- Jeppesen, J.; Beniczky, S.; Johansen, P.; Sidenius, P.; Fuglsang-Frederiksen, A. Detection of epileptic seizures with a modified heart rate variability algorithm based on Lorenz plot. Seizure 2015, 24, 1–7. [Google Scholar] [CrossRef]
- Novak, V.; Reeves, L.; Novak, P.; Low, A.; Sharbrough, W. Time-frequency mapping of R-R interval during complex partial seizures of temporal lobe origin. J. Auton. Nerv. Syst. 1999, 77, 195–202. [Google Scholar] [CrossRef]
- Kerem, D.H.; Geva, A.B. Forecasting epilepsy from the heart rate signal. Med. Biol. Eng. Comput. 2005, 43, 230–239. [Google Scholar] [CrossRef]
- Billeci, L.; Marino, D.; Insana, L.; Vatti, G.; Varanini, M. Patient-specific seizure prediction based on heart rate variability and recurrence quantification analysis. PLoS ONE 2018, 13, e0204339. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, K.; Miyajima, M.; Yamakawa, T.; Abe, E.; Suzuki, Y.; Sawada, Y.; Kano, M.; Maehara, T.; Ohta, K.; Sasai-Sakuma, T.; et al. Epileptic Seizure Prediction Based on Multivariate Statistical Process Control of Heart Rate Variability Features. IEEE Trans. Biomed. Eng. 2016, 63, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.; Ryvlin, P. Standards for testing and clinical validation of seizure detection devices. Epilepsia 2018, 59, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, T.; Inoue, T.; Harada, M.; Tsuneda, A. Design of a CMOS heartbeat spike-pulse detection circuit integrable in an RFID tag for heart rate signal sensing. IEICE Trans. Electron. 2007, 90, 1336–1343. [Google Scholar] [CrossRef]
- Yamakawa, T.; Matsumoto, G.; Aoki, T. A Low-Cost Long-Life RR Interval Telemeter with Automatic Gain Control for Various ECG Amplitudes. J. Adv. Res. Phys. 2012, 3, 011205. [Google Scholar]
- Yamakawa, T.; Fujiwara, K.; Kano, M.; Miyajima, M.; Suzuki, Y.; Maehara, T.; Ohta, K.; Sasano, T.; Matsuura, M.; Matsushima, E. Development of a wearable HRV telemetry system to be operated by non-experts in daily life. In Proceedings of the 2013 Asia-Pacific Signal and Information Association Annual Summit and Conference, Kaohsiung, Taiwan, 29 October–1 November 2013. [Google Scholar]
- Nomikos, P.; Macgregor, J.F. Monitoring Batch Processes Using Multiway Principal Component Analysis. AIChE J. 1994, 40, 1361–1375. [Google Scholar] [CrossRef]
- MacGregor, J.F.; Kourti, T. Statistical process control of multivariate processes. Control Eng. Pract. 1995, 3, 403–414. [Google Scholar] [CrossRef]
- Kano, M.; Nakagawa, Y. Data-based process monitoring, process control, and quality improvement: Recent developments and applications in steel industry. Comput. Chem. Eng. 2008, 32, 12–24. [Google Scholar] [CrossRef]
- Jackson, J.E.; Mudholkar, G.S. Control Procedures for Residuals Associated With Principal Component Analysis. Technometrics 1979, 21, 341–349. [Google Scholar] [CrossRef]
- Lockman, J.; Fisher, R.S.; Olson, D.M. Detection of seizure-like movements using a wrist accelerometer. Epilepsy Behav. 2011, 20, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Kramer, U.; Kipervasser, S.; Shlitner, A.; Kuzniecky, R. A Novel Portable Seizure Detection Alarm System: Preliminary Results. J. Clin. Neurophysiol. 2011, 28, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Onorati, F.; Regalia, G.; Caborni, C.; Migliorini, M.; Bender, D.; Poh, M.Z.; Frazier, C.; Kovitch Thropp, E.; Mynatt, E.D.; Bidwell, J.; et al. Multicenter clinical assessment of improved wearable multimodal convulsive seizure detectors. Epilepsia 2017, 58, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Beniczky, S.; Conradsen, I.; Wolf, P. Detection of convulsive seizures using surface electromyography. Epilepsia 2018, 59, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sforza, E.; Jouny, C.; Ibanez, V. Cardiac activation during arousal in humans: Further evidence for hierarchy in the arousal response. Clin. Neurophysiol. 2000, 111, 1611–1619. [Google Scholar] [CrossRef]
- Gosselin, N.; Michaud, M.; Carrier, J.; Lavigne, G.; Montplaisir, J. Age difference in heart rate changes associated with micro-arousals in humans. Clin. Neurophysiol. 2002, 113, 1517–1521. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef]
- Gibbons, J.D.; Chakraborti, S. Nonparametric Statistical Inference. In International Encyclopedia of Statistical Science; Lovric, M., Ed.; Springer: Berlin/Heidelberg, German, 2011; pp. 977–979. ISBN 978-3-642-04898-2. [Google Scholar]
- Snyder, D.E.; Echauz, J.; Grimes, D.B.; Litt, B. The statistics of a practical seizure warning system. J. Neural Eng. 2008, 5, 392–401. [Google Scholar] [CrossRef]
- Ramgopal, S.; Thome-Souza, S.; Jackson, M.; Kadish, N.E.; Sánchez Fernández, I.; Klehm, J.; Bosl, W.; Reinsberger, C.; Schachter, S.; Loddenkemper, T. Seizure detection, seizure prediction, and closed-loop warning systems in epilepsy. Epilepsy Behav. 2014, 37, 291–307. [Google Scholar] [CrossRef]
- Delamont, R.S.; Walker, M.C. Pre-ictal autonomic changes. Epilepsy Res. 2011, 97, 267–272. [Google Scholar] [CrossRef]
- Salo, M.A.; Huikuri, H.V.; Seppänen, T. Ectopic beats in heart rate variability analysis: Effects of editing on time and frequency domain measures. Ann. Noninvasive Electrocardiol. 2001, 6, 5–17. [Google Scholar] [CrossRef]
- Chihara, Y.; Yamakawa, T. Evaluation of a developed multichannel R-R interval telemeter and garment-type electrode. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 4173–4176. [Google Scholar]
- Mormann, F.; Andrzejak, R.G.; Elger, C.E.; Lehnertz, K. Seizure prediction: The long and winding road. Brain 2007, 130, 314–333. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, P.; Concato, J.; Feinstein, A.R.; Holford, T.R. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J. Clin. Epidemiol. 1995, 48, 1503–1510. [Google Scholar] [CrossRef]
- Bou Assi, E.; Nguyen, D.K.; Rihana, S.; Sawan, M. Towards accurate prediction of epileptic seizures: A review. Biomed. Signal Process. Control 2017, 34, 144–157. [Google Scholar] [CrossRef]
- Jeppesen, J.; Fuglsang-Frederiksen, A.; Johansen, P.; Christensen, J.; Wüstenhagen, S.; Tankisi, H.; Qerama, E.; Hess, A.; Beniczky, S. Seizure detection based on heart rate variability using a wearable electrocardiography device. Epilepsia 2019, 60, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Pavei, J.; Heinzen, R.G.; Novakova, B.; Walz, R.; Serra, A.J.; Reuber, M.; Ponnusamy, A.; Marques, J.L.B. Early seizure detection based on cardiac autonomic regulation dynamics. Front. Physiol. 2017, 8, 765. [Google Scholar] [CrossRef]
- Ansakorpi, H.; Korpelainen, J.T.; Suominen, K.; Tolonen, U.; Myllylä, V.V.; Isojärvi, J.I. Interictal cardiovascular autonomic responses in patients with temporal lobe epilepsy. Epilepsia 2000, 41, 42–47. [Google Scholar] [CrossRef]
- Ansakorpi, H.; Korpelainen, J.T.; Huikuri, H.V.; Tolonen, U.; Myllylä, V.V.; Isojärvi, J.I.T. Heart rate dynamics in refractory and well controlled temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatry 2002, 72, 26–30. [Google Scholar] [CrossRef]
- Abe, E.; Fujiwara, K.; Hiraoka, T.; Yamakawa, T.; Kano, M. Development of Drowsiness Detection Method by Integrating Heart Rate Variability Analysis and Multivariate Statistical Process Control. SICE J. Control. Meas. Syst. Integr. 2016, 9, 10–17. [Google Scholar] [CrossRef]
| Patient | Sex | Age | Seizure Foci | Medication (mg/day) |
|---|---|---|---|---|
| A | F | 31 | Right temporal lobe | CBZ 200, GBP 1200 |
| B | M | 54 | Left mesial temporal lobe | LEV 500, VPA 1000 |
| C | M | 20 | Left temporal lobe | LEV 1000 |
| D | F | 25 | Undefined | RFN 600, LTG 150, LEV 2500, VPA 400 |
| E | F | 42 | Occipital lobe (undefined lateralization) | LEV 2000, GBP 600, CZP 1, ZNS 300 |
| F | M | 9 | Right frontal lobe | VPA 400, CBZ 200 |
| G | F | 14 | Undefined | LEV 1750, LCM 50 |
| Patient | Seizures | Total Duration (h:min) | Interictal Duration (h:min) | Control (age/gender) | Total Duration (h:min) |
|---|---|---|---|---|---|
| A | 3 FIAS | 70:14 | 0:53 | 31/F | 5:08 |
| B | FIAS→FBTCS | 40:35 | 13:47 | 57/M | 4:48 |
| C | 2 FIAS | 32:18 | 9:21 | 20/M | 7:17 |
| D | FIAS | 105:52 | 2:57 | 25/F | 7:08 |
| E | 2 FAS | 85:03 | 2:43 | 45/F | 11:21 |
| F | 2 FIAS | 28:39 | 8:07 | 9/M | 6:07 |
| G | 3 FAS | 86:45 | 2:28 | 16/F | 7:14 |
| Patient | Total RRIs | RRI Outliers | Failure Rate (%) |
|---|---|---|---|
| A | 245,920 | 11,564 | 4.7 |
| B | 163,520 | 577 | 0.4 |
| C | 159,240 | 5805 | 3.6 |
| D | 474,630 | 31,665 | 6.7 |
| E | 343,770 | 14,668 | 4.3 |
| F | 144,530 | 2446 | 1.7 |
| G | 419,440 | 11,866 | 2.8 |
| Seizure | Duration (min:s to min:s) | |
|---|---|---|
| Q | T2 | |
| A1 | −05:10 to −04:58 | NA |
| A2 | −05:16 to −02:19 | NA |
| A3 | NA | NA |
| B1 | −07:06 to −05:16 | NA |
| C1 | −14:40 to −14:25, −12:41 to −11:40 | NA |
| C2 | NA | −09:56 to −08:16 |
| D1 | −13:05 to −11:15 | NA |
| E1 | −16:09 to −14:44, −09:36 to −06:39 | NA |
| E2 | −14:43 to −10:36 | NA |
| F1 | −12:43 to −12:06 | −13:18 to −10:28, −06:34 to −02:52 |
| F2 | −15:41 to −12:42, −10:13 to −08:44 | NA |
| G1 | −14:23 to −13:39, −08:37 to −04:54, −03:38 to −01:05 | NA |
| G2 | −03:52 to −02:59 | NA |
| G3 | −12:18 to −09:33 | NA |
| Sen | 85.7% | 14.3% |
| Patient | False Positive Rate (times/h) | False Positive Rate of Healthy Control | ||
|---|---|---|---|---|
| Q | T2 | Q | T2 | |
| A | 3.34 | 1.11 | 0 | 2.14 |
| B | 0.29 | 1.16 | 0 | 1.46 |
| C | 1.62 | 1.62 | 0.69 | 1.92 |
| D | 0.43 | 1.71 | 0.14 | 0.56 |
| E | 0.67 | 0.34 | 1.32 | 0.59 |
| F | 0.73 | 4.76 | 1.30 | 0.65 |
| G | 0.74 | 0.37 | 0.97 | 1.82 |
| Total | 0.62 | 1.34 | 0.93 | 1.02 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamakawa, T.; Miyajima, M.; Fujiwara, K.; Kano, M.; Suzuki, Y.; Watanabe, Y.; Watanabe, S.; Hoshida, T.; Inaji, M.; Maehara, T. Wearable Epileptic Seizure Prediction System with Machine-Learning-Based Anomaly Detection of Heart Rate Variability. Sensors 2020, 20, 3987. https://doi.org/10.3390/s20143987
Yamakawa T, Miyajima M, Fujiwara K, Kano M, Suzuki Y, Watanabe Y, Watanabe S, Hoshida T, Inaji M, Maehara T. Wearable Epileptic Seizure Prediction System with Machine-Learning-Based Anomaly Detection of Heart Rate Variability. Sensors. 2020; 20(14):3987. https://doi.org/10.3390/s20143987
Chicago/Turabian StyleYamakawa, Toshitaka, Miho Miyajima, Koichi Fujiwara, Manabu Kano, Yoko Suzuki, Yutaka Watanabe, Satsuki Watanabe, Tohru Hoshida, Motoki Inaji, and Taketoshi Maehara. 2020. "Wearable Epileptic Seizure Prediction System with Machine-Learning-Based Anomaly Detection of Heart Rate Variability" Sensors 20, no. 14: 3987. https://doi.org/10.3390/s20143987
APA StyleYamakawa, T., Miyajima, M., Fujiwara, K., Kano, M., Suzuki, Y., Watanabe, Y., Watanabe, S., Hoshida, T., Inaji, M., & Maehara, T. (2020). Wearable Epileptic Seizure Prediction System with Machine-Learning-Based Anomaly Detection of Heart Rate Variability. Sensors, 20(14), 3987. https://doi.org/10.3390/s20143987





