Abstract
In this article, a zigzag graphene nanoribbon (ZGNR)-based sensor was built utilizing the Atomistic ToolKit Virtual NanoLab (ATK-VNL), and used to detect nitric oxide (NO), nitrogen dioxide (NO2), and ammonia (NH3). The successful adsorption of these gases on the surface of the ZGNR was investigated using adsorption energy (Eads), adsorption distance (D), charge transfer (∆Q), density of states (DOS), and band structure. Among the three gases, the ZGNR showed the highest adsorption energy for NO with −0.273 eV, the smallest adsorption distance with 2.88 Å, and the highest charge transfer with −0.104 e. Moreover, the DOS results reflected a significant increase of the density at the Fermi level due to the improvement of ZGNR conductivity as a result of gas adsorption. The surface of ZGNR was then modified with an epoxy group (-O-) once, then with a hydroxyl group (-OH), and finally with both (-O-) and (-OH) groups in order to improve the adsorption capacity of ZGNR. The adsorption parameters of ZGNR were improved significantly after the modification. The highest adsorption energy was found for the case of ZGNR-O-OH-NO2 with −0.953 eV, while the highest charge transfer was found for the case of ZGNR-OH-NO with −0.146 e. Consequently, ZGNR-OH and ZGNR-O-OH can be considered as promising gas sensors for NO and NO2, respectively.
1. Introduction
Graphene-based nanomaterials (G-NMs) have been subjected to intensive investigations in recent years due to their remarkable properties and promising application for electronics [1,2,3,4]. Nevertheless, it has been reported that graphene has some limitations in its sensing properties to some kinds of gas molecules such as CO, CO2, CH4, N2, NO2, NH3, and H2, affecting its usage for applications in the field of gas sensors [5,6,7,8]. These limitations can be solved either by functionalizing the surface of graphene or generating a G-NM which exhibits a tunable band gap that is referred to as a graphene nanoribbon (GNR) [9,10]. GNRs can be produced either by etching or patterning graphene along a specific direction [11,12,13]. In addition, practical challenges are still to be resolved in order to utilize graphene for practical sensors, such as its purity degree, trace metal content, application tests, and practical deposition of graphene-based materials on surfaces [14]. GNRs are considered a promising candidate for the field of gas sensors as compared with other C-NMs, thanks to their long and reactive edges that facilitate the adsorption of gas molecules [15,16,17,18,19,20]. Furthermore, GNRs have been reported in different forms of devices for utilization as a gas sensor, including field-effect transistors (FETs), chemiresistors, and capacitance sensors, etc. [21,22].
Although there have been great benefits to humans as a result of the continuous developments in technology and industry, there have been serious effects on human health due to the release of hazardous radiation [23,24,25] and substances such as toxic gases [26,27] and volatile organic compounds [28,29]. The presence of some of these gases, even at low concentrations, is very dangerous to human life [30]. Monitoring the level of nitrogen-based gases such as NO, NO2, and NH3 is of great interest for industry as well as medical applications. For instance, detecting NO molecules in the exhalation process can aid in the diagnosis of some respiratory diseases such as asthma [31,32]. Moreover, one of the main serious effects of NO gas is its easy transformation to the highly toxic NO2 gas through oxidation [33,34,35,36]. NO2 is a toxic gas which can be produced from diesel engines and combustion of fuel in industry [37]. Consequently, NO2-based gas sensors are of great importance to humans due to the high toxicity of NO2. These sensors have been used mainly in detecting pollutants in atmospheric air as well as the detection of explosive vapors [38,39]. On the other hand, monitoring and detecting NH3 gas molecules is a must in semiconductor and optoelectronic applications as well as for environmental purposes [40,41]. It has been found that annihilation of NH3 gas molecules at the 50–100 ppm level affects human health negatively through irritation of the throat, nose, and eyes [41,42]. Consequently, monitoring and detecting NO, NO2, and NH3 gas molecules are of great importance for overcoming some serious human problems such as air pollution.
Therefore, the main concern of the current study is to improve the adsorption capacity of the zigzag graphene nanoribbon (ZGNR) towards NO, NO2, and NH3 gases by functionalizing its surface with epoxy and hydroxyl functional groups (-O- and -OH) to facilitate the adsorption of the gas molecules. Density functional theory (DFT), with the aid of the Atomistic ToolKit Virtual NanoLab (ATK-VNL), has been used to build bare ZGNR and to investigate its capacity to adsorb NO, NO2, and NH3 gases. The results reflected good adsorption parameters, indicating the ability of pure ZGNR to detect the three gases. To achieve the goal of this study, three new functionalized ZGNR systems were built: ZGNR-O, ZGNR-OH, and ZGNR-O-OH. The results showed a significant improvement in the adsorption energy, adsorption distance, and charge transfer between the gases and the three modified systems upon surface functionalization.
2. Computational Method
Four different ZGNR systems (bare ZGNR, ZGNR-O, ZGNR-OH, and ZGNR-O-OH) have been investigated by DFT calculations based on the ATK-VNL package in this study. Herein, DFT was utilized to relax and to optimize all atoms and systems until the convergence of force was 0.01 eV/Å. The resulting systems were then used as gas sensors to detect NO, NO2, and NH3 gases. The calculations were performed based on the generalized gradient approximation (GGA) of the Perdew–Burke–Ernzerhof (PBE) functional [43,44]. The density mesh cutoff was taken to be 125 Hartree and the force tolerance to be 0.01 eV/Å. Monkhorst–Pack k point sampling of 4 × 2 × 1 was used during all calculations. The successful adsorption of NO, NO2, NH3 gases on the surface of ZGNR systems was explored based on the adsorption energy (), adsorption distance (D), charge transfer (), band structure, and density of states (DOS). The adsorption energy of the gas molecules on ZGNR systems was calculated using the following formula [45,46,47]:
where is the total energy of the optimized structure of any gas molecules adsorbed on any of the different ZGNR systems. is the total energy of each of the ZGNR systems without gas adsorption, and is the total energy of each of the optimized NO, NO2, and NH3 gases. Increasing the value of with a negative sign indicates that the adsorption of NO, NO2, and NH3 gases on the surface of ZGNR systems is stronger [26,48]. Moreover, the charge transfer between the NO, NO2, and NH3 gases and the different ZGNR systems was calculated using Mulliken population analysis [49,50].
3. Results
3.1. ZGNR System
The bare ZGNR system was prepared based on ATK-VNL and investigated as a gas sensor to detect NO, NO2, and NH3 gases. The optimized structures of ZGNR before and after adsorption of the three gases are given in Figure 1. The results show that the average C-C bond length of ZGNR is 1.425 Å. The adsorption of NO, NO2, and NH3 gases on the surface of ZGNR was explored based on the adsorption energy, adsorption distance, charge transfer, band structure, and DOS. The calculations show that the adsorption energy of NO on ZGNR was −0.273 eV and the adsorption distance was 2.88 Å, as shown in Table 1. Moreover, the Mulliken charge analysis showed −0.104 e transfer between NO and ZGNR during the adsorption process. On the other hand, the adsorption energy, adsorption distance, and charge transfer values between NO2 and ZGNR were −0.225 eV, 3.11 Å, and 0.040 e, respectively. The positive value of charge transfer in the case of NO2 gas indicates that the electrons transfer from ZGNR to NO2. The results also reflect the capability of ZGNR to adsorb NH3 with −0.091 eV adsorption energy and −0.018 e charge transfer.

Figure 1.
Top and side views of the optimized (a) ZGNR, (b) ZGNR-NO, (c) ZGNR-NO2, and (d) ZGNR-NH3.

Table 1.
Adsorption parameters of the optimized NO, NO2, and NH3 gases adsorbed on the zigzag graphene nanoribbon (ZGNR) system. : adsorption energy; D: adsorption distance; : charge transfer.
The band structures of ZGNR before and after adsorption of NO, NO2, and NH3 gases are shown in Figure 2a–d. The band structure results show that the band gap of ZGNR is 0 eV, reflecting its metallic nature, which has been reported [51,52]. Although no changes in the band gap were observed after the adsorption of NO, NO2, and NH3 gases, considerable changes were detected in the DOS results in Figure 3.

Figure 2.
Band structures of (a) ZGNR, (b) ZGNR-NO, (c) ZGNR-NO2, and (d) ZGNR-NH3.

Figure 3.
Density of states of the ZGNR system before and after the adsorption of (a) NO, (b) NO2, and (c) NH3.
The DOS results show that the DOS at the Fermi level increases significantly after adsorption of NO and NO2 and slightly for the case of NH3. In addition to the increase of DOS at the Fermi level, a significant increase was also observed around −12.3, −7.4, 9.9, and 17.3 eV for the case of NO in Figure 3a. For the case of NO2 in Figure 3b, considerable increases of the DOS around −10.6, −7.4, −2.8, −1.9, 2.8, 9.9, and 19.2 eV were observed after gas adsorption. Meanwhile, increases of DOS for the case of NH3 were observed around −5.2, 6.5, 13.5, 15.8, and 19.2 eV, as shown in Figure 3c.
3.2. ZGNR-O System
Figure 4a–d shows the optimized structure of the ZGNR-O system before and after adsorption of the gas molecules. The results show that the C-O bond length was 1.39 Å. The adsorption energy, adsorption distance, and charge transfer between the gas molecules and ZGNR-O are shown in Table 2. The adsorption distances between NO, NO2, and NH3 gases and ZGNR-O were 2.66, 3.15, and 3.15 Å, respectively. Although no considerable changes were observed in the adsorption parameters after the adsorption of NO2 gas, after the functionalization of ZGNR with -O- group, the adsorption energy increased to −0.318 and −0.124 eV for the cases of NO and NH3. Moreover, the charge transfer between NH3 gas and ZGNR-O system increased significantly to −0.128 e. Figure 5a–d shows the band structures of ZGNR-O before and after adsorption of NO, NO2, and NH3 gases. No significant changes were observed in the band gap after the adsorption of the gas molecules in the band structure results. Nevertheless, a remarkable increase in the DOS at the Fermi level was observed after the adsorption of the three gases on the surface of ZGNR-O, as shown in Figure 6. Figure 6a shows that DOS values around −12.3, −9.0, −7.1, 9.6, 13.5, 16.0, and 22.3 eV increased significantly after adsorption of NO gas. Due to the adsorption of NO2, a new peak around −21.7 eV was detected, as shown in Figure 6b. In addition, considerable increases were observed around −10.9, −7.1, −2.6, 2.5, 9.6, 13.5, 16.0, 17.5, 18.8, and 22.3 eV, confirming the adsorption of NO2 gas. For the case of NH3 in Figure 6c, considerable increases in DOS values around −15.4, −5.2, 6.5, 13.5, 16.0, and 17.5 eV were observed. Moreover, the peaks around 20.8 and 22.4 eV were combined to form a new peak around 21.4 eV.

Figure 4.
Top and side views of (a) ZGNR-O, (b) ZGNR-O-NO, (c) ZGNR-O-NO2, and (d) ZGNR-O-NH3.

Table 2.
Adsorption parameters of the optimized NO, NO2, and NH3 gases adsorbed on the ZGNR-O system.
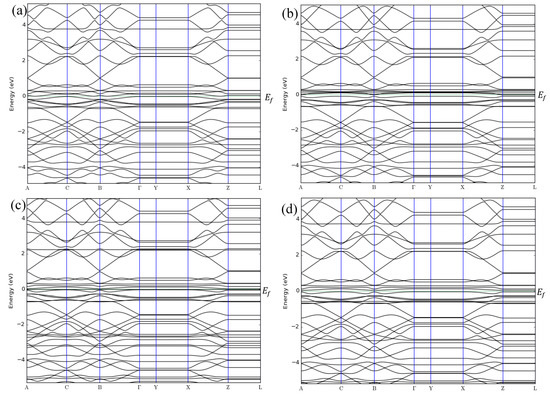
Figure 5.
Band structures of (a) ZGNR-O, (b) ZGNR-O-NO, (c) ZGNR-O-NO2, and (d) ZGNR-O-NH3.
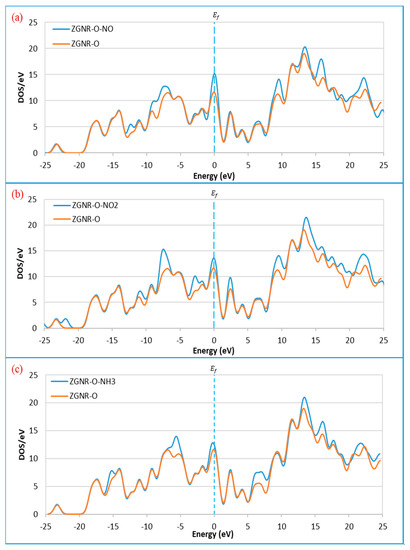
Figure 6.
Density of states of the ZGNR-O system before and after the adsorption of (a) NO, (b) NO2, and (c) NH3.
3.3. ZGNR-OH System
In this part, the surface of ZGNR was functionalized with one -OH group and then used as a gas sensor to detect NO, NO2, and NH3 gases, as shown in Figure 7. The results show that the C-C bond length of ZGNR increased a little to 1.5 Å around the -OH group, while the C-O and O-H bonds were 1.49 Å and 0.98 Å, respectively. Table 3 shows the adsorption parameters of the optimized NO, NO2, and NH3 gases adsorbed on ZGNR-OH. On one hand, the adsorption distances between NO, NO2, and NH3 and ZGNR-OH decreased to 2.24, 1.74, and 2.18 Å, respectively. On the other hand, a significant improvement in the adsorption energy was observed with −0.641, −0.618, and −0.244 eV for the cases of NO, NO2, and NH3 gases, respectively, as compared with the ZGNR and ZGNR-O systems. Moreover, the Mulliken charge analysis showed that 0.146 and 0.137 e transfer from NO and NH3 to ZGNR-OH (negative ∆Q) during the adsorption process, while 0.074 e transfer from ZGNR-OH to NO2. Therefore, the sensing signal is expected to be negative for the former and positive of the latter.
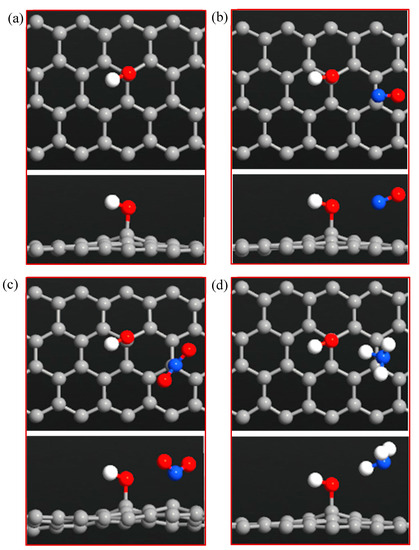
Figure 7.
Top and side views of (a) ZGNR-OH, (b) ZGNR-OH-NO, (c) ZGNR-OH-NO2, and (d) ZGNR-OH-NH3.

Table 3.
Adsorption parameters of the optimized NO, NO2, and NH3 gases adsorbed on the ZGNR-OH system.
The band structures of ZGNR-OH before and after adsorption of NO, NO2, and NH3 gases are shown in Figure 8a–d, respectively. The results show no significant changes in the band gap after the adsorption of NO, NO2, and NH3 gases; however, some changes were detected below and above the Fermi level as a result of the gas adsorption.

Figure 8.
Band structures of (a) ZGNR-OH, (b) ZGNR-OH-NO, (c) ZGNR-OH-NO2, and (d) ZGNR-OH-NH3.
The DOS results in Figure 9 show that the DOS at the Fermi level increases after the adsorption of NO and NH3, reflecting an improvement of ZGNR-OH conductivity upon gas adsorption. The results reveal that the DOS around −12.2, −8.9, −7.4, and 9.8 eV increased after the adsorption of NO gas, as shown in Figure 9a, while two new peaks were observed around 21.1 and 22.2 eV. In addition, the peak around 19.9 eV shifted to 19.0 eV. Although no significant change was detected at the Fermi level after the adsorption on NO2 (Figure 9b), a considerable increase in the DOS around −8.9, −7.4, −2.1, 9.8, and 15.7 eV was detected. Moreover, the peak around −21.0 eV shifted to −21.8 eV, the peak around 19.9 eV shifted to 19.0 eV, and two new peaks around 21.1, and 22.2 eV were observed. Figure 9c shows that the DOS at Femi level increases slightly after the adsorption of NH3 gas. A significant increase in the DOS around −5.2 and 6.5 eV was also observed.
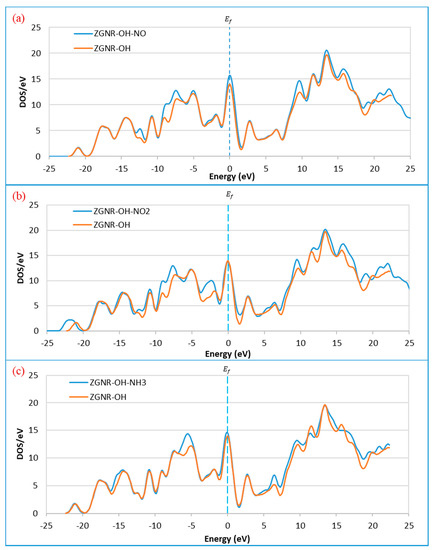
Figure 9.
Density of states of the ZGNR-OH system before and after the adsorption of (a) NO, (b) NO2, and (c) NH3.
3.4. ZGNR-O-OH System
To improve the adsorption capacity, the surface of ZGNR was functionalized with both -O- and -OH groups, as shown in Figure 10, and then used to detect NO, NO2, and NH3 gases. The adsorption energies, adsorption distances, and charge transfer of the optimized NO, NO2, and NH3 gases adsorbed on ZGNR-O-OH system are listed in Table 4. The results show that modifying the surface of ZGNR with -O- and -OH groups significantly enhanced its adsorption capacity. For instance, the adsorption distances decreased to 1.98, 1.68, and 2.45 Å, while the adsorption energies increased significantly to −0.625, −0.953, and −0.219 eV for the cases of the NO, NO2, and NH3 gases, respectively. On the other hand, the Mulliken charge analysis demonstrated that 0.118 and 0.141 e transfer from NO and NH3 to ZGNR-O-OH during the adsorption process, while 0.092 e transfer from ZGNR-O-OH to NO2 gas.
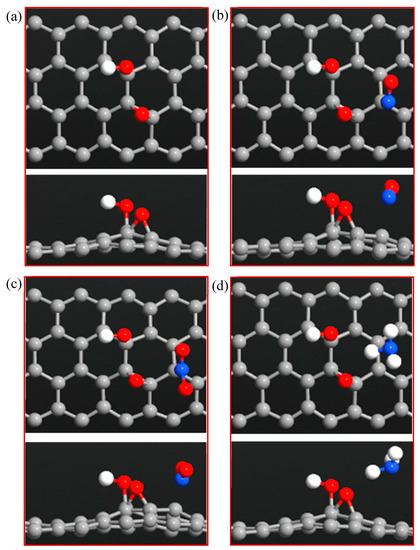
Figure 10.
Top and side views of (a) ZGNR-O-OH, (b) ZGNR-O-OH-NO, (c) ZGNR-O-OH-NO2, and (d) ZGNR-O-OH-NH3.

Table 4.
Adsorption parameters of the optimized NO, NO2, and NH3 gases adsorbed on the ZGNR-O-OH system.
The band structures of the ZGNR-O-OH system before and after the adsorption of NO, NO2, and NH3 gases are shown in Figure 11a–d, respectively. The band structure results show that the band gap of ZGNR increased slightly to 0.043 eV after the functionalization with -O- and -OH groups. The band gap then decreased to 0 eV after the adsorption of the gas molecules, reflecting an improvement in its conductivity and the differentiation between the two cases before and after gas adsorption. Moreover, some other changes were observed below and above the Fermi level due to the adsorption of NO, NO2, and NH3 gases.

Figure 11.
Band structures of (a) ZGNR-O-OH, (b) ZGNR-O-OH-NO, (c) ZGNR-O-OH-NO2, and (d) ZGNR-O-OH-NH3.
These changes are confirmed by the DOS results in Figure 12. The DOS results show that the DOS at Fermi level increased after the adsorption of NO and NH3. Figure 12a shows a remarkable increase in the DOS around −12.3, −7.5, 9.6, 13.5, 16.0, and 22.2 eV upon the adsorption of NO gas. The peaks around 18.3 and 19.7 eV shifted to around 17.6 and 18.9 eV and a new peak around 24.3 eV was observed, confirming the adsorption of NO gas on the surface of ZGNR-O-OH. For the case of NO2 gas in Figure 12b, a significant increase in the DOS around −7.5, −2.5, 16.0, 17.6, and 21.1 eV was detected. Moreover, the peak around −21.0 eV shifted to −21.8 eV and a new peak around 24.0 eV was observed. Figure 12c shows a significant increase in the DOS around −5.2, 6.5, 9.6, 18.2, 19.5, and 22.2 eV after the adsorption of NH3 gas. In addition, a new peak around 23.7 eV was also observed.
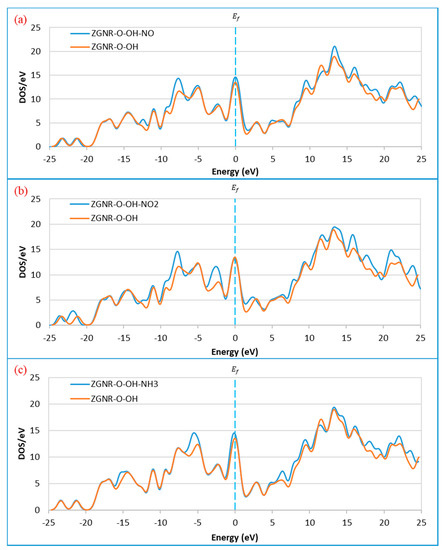
Figure 12.
Density of states of the ZGNR-O-OH system before and after the adsorption of (a) NO, (b) NO2, and (c) NH3.
4. Discussion
To improve the adsorption capacity of ZGNR, which is goal in this study, the surface of ZGNR was functionalized with -O- and -OH groups, creating three different systems (ZGNR-O, ZGNR-OH, and ZGNR-O-OH). Interestingly, the adsorption parameters of ZGNR reflect a significant improvement upon surface functionalization. This improvement is attributed mainly to the presence of the functional groups, which have been reported to play a significant role in facilitating the adsorption of the gas molecules during the sensing process [53,54,55].
The Mullekin charge analysis showed that the charge transfer is negative for the cases of NO and NH3 gases, which is an indication that the charge transfers from the gas molecules to the ZGNR systems (negative sensing signal), which is in good agreement with the reported data stating that NO and NH3 gases behave as donors [5,56]. On the other hand, for the case of NO2 the charge transfer was positive, which means that the charge transfers from the ZGNR systems to the gas molecules (positive sensing signal), which have been reported to behave as an acceptor [5,56].
In addition to the adsorption energy, adsorption distance, and charge transfer, the adsorption of NO, NO2, and NH3 gases on the surface of the ZGNR systems was also confirmed by the band structure and density of states results. The band structure results show some changes in the bands before and after the Fermi level as a result of the gas adsorption. These changes confirm the successful adsorption of the gas molecules on the surface of ZGNR systems. Moreover, the DOS results demonstrated a significant increase of the density at Fermi level and the appearance of some new peaks. The significant increase in the density of states is related to the improvement of the ZGNR system conductivity upon gas adsorption, which is in good agreement with the reported data that relate the change of adsorbent’s conductivity after adsorption of gas molecules [57,58,59].
The sensing of nitrogen oxides can be assigned to their easy and rapid diffusion on the surface of ZGNR. This leads to formation of hydrogen bonds among the nitrogen oxides and ZGNR, which significantly contributes to increasing their binding energy. The interaction among nitrogen oxides with ZGNR with the epoxy group and hydroxyl groups can be attributed to the development of hydrogen bonds OH…O(N) between NOx and -OH and the formation of new weak covalent bonds (C…N as well as C…O), as well as H elimination to produce nitrous acid- and nitric acid-like moieties [60,61].
5. Conclusions
The main goal of this study was to enhance the sensing performance of zigzag graphene nanoribbon (ZGNR) towards the detection of NO, NO2, and NH3 gases. First, primary calculations using density functional theory (DFT), based on Atomistic ToolKit Virtual NanoLab (ATK-VNL), were used to study the adsorption of the target gases on the surface of the ZGNR. The best sensing performance was found for the case of NO gas, with −0.273 eV adsorption energy and −0.104 e charge transfer during the adsorption process. In order to improve the sensing performance of ZGNR, three different functionalized systems were built: ZGNR-O, ZGNR-OH, and ZGNR-O-OH. The sensing performance towards the three gases was improved significantly after the functionalization, especially for the cases of the ZGNR-OH and ZGNR-O-OH systems. Nevertheless, the best sensing performance from the four systems based on the adsorption energy, adsorption distance, charge transfer, and conductivity improvement was identified for the case of NO gas. Among the four systems, ZGNR-OH with adsorption energy of −0.641 eV and −0.146 e charge transfer was the best. Thus, it is the most selective to NO gas, reflecting that ZGNR-OH can be considered as a promising gas sensor to detect NO gas.
Author Contributions
Conceptualization, A.I.A.; Formal analysis, A.I.A.; Investigation, E.S.; Project administration, A.I.A.; Supervision, A.I.A.; Writing–original draft, E.S.; Writing–review & editing, A.I.A. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The publication of this article was funded by the Qatar National Library.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Novoselov, K.; Geim, A.K.; Morozov, S.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, K.I.; Ghahari, F.; Shulman, M.D.; Stormer, H.L.; Kim, P. Observation of the fractional quantum Hall effect in graphene. Nature 2009, 462, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Mueller, T.; Lin, Y.-M.; Valdes-Garcia, A.; Avouris, P. Ultrafast graphene photodetector. Nat. Nanotechnol. 2009, 4, 839–843. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Adsorption of H2O, NH3, CO, NO2, and NO on graphene: A first-principles study. Phys. Rev. B 2008, 77, 125416. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Chen, Y.-B.; Zhou, K.-G.; Liu, C.; Zeng, J.; Zhang, H.-L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef]
- Dai, J.; Yuan, J.; Giannozzi, P. Gas adsorption on graphene doped with B, N, Al, and S: A theoretical study. Appl. Phys. Lett. 2009, 95, 232105. [Google Scholar] [CrossRef]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P. Reduced Graphene Oxide Molecular Sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef]
- Shao, L.; Chen, G.; Ye, H.; Niu, H.; Wu, Y.; Zhu, Y.; Ding, B. Sulfur dioxide molecule sensors based on zigzag graphene nanoribbons with and without Cr dopant. Phys. Lett. A 2014, 378, 667–671. [Google Scholar] [CrossRef]
- Anasthasiya, A.N.A.; Khaneja, M.; Jeyaprakash, B.G. Electronic Structure Calculations of Ammonia Adsorption on Graphene and Graphene Oxide with Epoxide and Hydroxyl Groups. J. Electron. Mater. 2017, 46, 5642–5656. [Google Scholar] [CrossRef]
- Yang, L.; Cohen, M.L.; Louie, S.G. Magnetic Edge-State Excitons in Zigzag Graphene Nanoribbons. Phys. Rev. Lett. 2008, 101, 186401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, Y.-W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Han, M.Y.; Özyilmaz, B.; Zhang, Y.; Kim, P.; Oezyilmaz, B. Energy Band-Gap Engineering of Graphene Nanoribbons. Phys. Rev. Lett. 2007, 98, 206805. [Google Scholar] [CrossRef] [PubMed]
- Reina, G.; Gonzalez-Dominguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416. [Google Scholar] [CrossRef]
- Yan, Q.; Huang, B.; Yu, J.; Zheng, F.; Zang, J.; Wu, J.; Gu, B.-L.; Liu, F.; Duan, W. Intrinsic Current−Voltage Characteristics of Graphene Nanoribbon Transistors and Effect of Edge Doping. Nano Lett. 2007, 7, 1469–1473. [Google Scholar] [CrossRef]
- Huang, B.; Yan, Q.; Zhou, G.; Wu, J.; Gu, B.-L.; Duan, W.; Liu, F. Making a field effect transistor on a single graphene nanoribbon by selective doping. Appl. Phys. Lett. 2007, 91, 253122. [Google Scholar] [CrossRef]
- Cervantes-Sodi, F.; Csányi, G.; Piscanec, S.; Ferrari, A.C. Edge-functionalized and substitutionally doped graphene nanoribbons: Electronic and spin properties. Phys. Rev. B 2008, 77, 165427. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, B.; Hwang, K.-C.; Gao, H. Surface-adsorption-induced bending behaviors of graphene nanoribbons. Appl. Phys. Lett. 2011, 98, 121909. [Google Scholar] [CrossRef]
- Huang, B.; Li, Z.; Liu, Z.; Zhou, G.; Hao, S.; Wu, J.; Gu, B.-L.; Duan, W. Adsorption of Gas Molecules on Graphene Nanoribbons and Its Implication for Nanoscale Molecule Sensor. J. Phys. Chem. C 2008, 112, 13442–13446. [Google Scholar] [CrossRef]
- Saffarzadeh, A. Modeling of gas adsorption on graphene nanoribbons. J. Appl. Phys. 2010, 107, 114309. [Google Scholar] [CrossRef]
- Fiori, G.; Iannaccone, G. Simulation of Graphene Nanoribbon Field-Effect Transistors. IEEE Electron Device Lett. 2007, 28, 760–762. [Google Scholar] [CrossRef]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A Review on Graphene-Based Gas/Vapor Sensors with Unique Properties and Potential Applications. Nano-Micro Lett. 2015, 8, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, H.; Phan, V.; Kim, S.S. A Novel X-ray Radiation Sensor Based on Networked SnO2 Nanowires. Appl. Sci. 2019, 9, 4878. [Google Scholar] [CrossRef]
- Al-Sulaiti, L.A.; Salah, B.; Ayesh, A.I. Investigation of flexible polymer-Tl2O3 nanocomposites for X-ray detector applications. Appl. Surf. Sci. 2019, 489, 351–357. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Salah, B.; Al-Sulaiti, L.A. Production and characterization of flexible semiconducting polymer-nanoparticle composites for X-ray sensors. Radiat. Phys. Chem. 2020, 167, 108233. [Google Scholar] [CrossRef]
- Aghaei, S.M.; Monshi, M.M.; Calizo, I. A theoretical study of gas adsorption on silicene nanoribbons and its application in a highly sensitive molecule sensor. RSC Adv. 2016, 6, 94417–94428. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, W.; Han, Y.; Yang, Z.; Huang, Y. Single-Nanowire Fuse for Ionization Gas Detection. Sensors 2019, 19, 4358. [Google Scholar] [CrossRef]
- Gancarz, M.; Nawrocka, A.; Rusinek, R. Identification of Volatile Organic Compounds and Their Concentrations Using a Novel Method Analysis of MOS Sensors Signal. J. Food Sci. 2019, 84, 2077–2085. [Google Scholar] [CrossRef]
- Rusinek, R.; Siger, A.; Gawrysiak-Witulska, M.; Rokosik, E.; Malaga-Toboła, U.; Gancarz, M. Application of an electronic nose for determination of pre-pressing treatment of rapeseed based on the analysis of volatile compounds contained in pressed oil. Int. J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Ayesh, A.I.; Alyafei, A.A.; Anjum, R.S.; Mohamed, R.M.; Abuharb, M.B.; Salah, B.; El-Muraikhi, M. Production of sensitive gas sensors using CuO/SnO2 nanoparticles. Appl. Phys. A 2019, 125, 550. [Google Scholar] [CrossRef]
- Lee, C.-T.; Lee, H.-Y.; Chiu, Y.-S. Performance improvement of nitrogen oxide gas sensors using Au catalytic metal on SnO2/WO3 complex nanoparticle sensing layer. IEEE Sens. J. 2016, 16, 7581–7585. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting metal oxides as sensors for environmentally hazardous gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Marquis, B.T.; Vetelino, J.F. A semiconducting metal oxide sensor array for the detection of NOx and NH3. Sens. Actuators B Chem. 2001, 77, 100–110. [Google Scholar] [CrossRef]
- Xia, H.; Kong, F.; Wang, S.; Zhu, B.; Guo, X.; Zhang, J.; Wang, Y.; Wu, S. Au-doped WO3-based sensor for NO2 detection at low operating temperature. Sens. Actuators B Chem. 2008, 134, 133–139. [Google Scholar] [CrossRef]
- Yang, L.; Marikutsa, A.V.; Rumyantseva, M.; Konstantinova, E.; Khmelevsky, N.; Gaskov, A. Quasi Similar Routes of NO2 and NO Sensing by Nanocrystalline WO3: Evidence by In Situ DRIFT Spectroscopy. Sensors 2019, 19, 3405. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, J.; Xu, J.-L.; Li, X.; Xie, D.; Xiang, L.; Komarneni, S. Confined Formation of Ultrathin ZnO Nanorods/Reduced Graphene Oxide Mesoporous Nanocomposites for High-Performance Room-Temperature NO2 Sensors. ACS Appl. Mater. Interfaces 2016, 8, 35454–35463. [Google Scholar] [CrossRef]
- Jalil, A.R.; Chang, H.; Bandari, V.K.; Robaschik, P.; Zhang, J.; Siles, P.F.; Li, G.; Bürger, D.; Grimm, D.; Liu, X.; et al. Fully Integrated Organic Nanocrystal Diode as High Performance Room Temperature NO2 Sensor. Adv. Mater. 2016, 28, 2971–2977. [Google Scholar] [CrossRef]
- Kanzawa, K.; Kitano, J. A semiconductor device manufacturer’s efforts for controlling and evaluating atmospheric pollution. In Proceedings of the SEMI Advanced Semiconductor Manufacturing Conference and Workshop, Cambridge, MA, USA, 13–15 November 1995; pp. 190–193. [Google Scholar]
- Chang, Y.-C.; Bai, H.; Li, S.-N.; Kuo, C.-N. Bromocresol Green/Mesoporous Silica Adsorbent for Ammonia Gas Sensing via an Optical Sensing Instrument. Sensors 2011, 11, 4060–4072. [Google Scholar] [CrossRef]
- Toxicological Profile for Ammonia, U.S. Department of Health and Human Services. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp126.pdf (accessed on September 2004).
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xiao, P.; Henkelman, G.; Liechti, K.; Huang, R. Interfacial adhesion between graphene and silicon dioxide by density functional theory with van der Waals corrections. J. Phys. D Appl. Phys. 2014, 47, 255301. [Google Scholar] [CrossRef]
- Liu, D.; Gui, Y.; Ji, C.; Tang, C.; Zhou, Q.; Li, J.; Zhang, X. Adsorption of SF6 decomposition components over Pd (1 1 1): A density functional theory study. Appl. Surf. Sci. 2019, 465, 172–179. [Google Scholar] [CrossRef]
- Neugebauer, J.; Scheffler, M. Adsorbate-substrate and adsorbate-adsorbate interactions of Na and K adlayers on Al(111). Phys. Rev. B 1992, 46, 16067–16080. [Google Scholar] [CrossRef]
- Manna, B.; Raha, H.; Chakrabarti, I.; Guha, P.K. Selective Reduction of Oxygen Functional Groups to Improve the Response Characteristics of Graphene Oxide-Based Formaldehyde Sensor Device: A First Principle Study. IEEE Trans. Electron Devices 2018, 65, 1–8. [Google Scholar] [CrossRef]
- Bo, Z.; Guo, X.; Wei, X.; Yang, H.; Yan, J.; Cen, K. Density functional theory calculations of NO2 and H2S adsorption on the group 10 transition metal (Ni, Pd and Pt) decorated graphene. Phys. E Low-Dimens. Syst. Nanostruct. 2019, 109, 156–163. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Son, Y.-W.; Cohen, M.L.; Louie, S.G. Energy Gaps in Graphene Nanoribbons. Phys. Rev. Lett. 2006, 97, 216803. [Google Scholar] [CrossRef]
- Nakada, K.; Fujita, M.; Dresselhaus, G.; Dresselhaus, M.S. Edge state in graphene ribbons: Nanometer size effect and edge shape dependence. Phys. Rev. B 1996, 54, 17954–17961. [Google Scholar] [CrossRef]
- Toda, K.; Furue, R.; Hayami, S. Recent progress in applications of graphene oxide for gas sensing: A review. Anal. Chim. Acta 2015, 878, 43–53. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B Chem. 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J. Ammonia adsorption on graphene and graphene oxide: A first-principles study. Front. Environ. Sci. Eng. 2013, 7, 403–411. [Google Scholar] [CrossRef]
- Hosseingholipourasl, A.; Ahmadi, M.T.; Ismail, R.; Gharaei, N. Analytical modelling and simulation of gas adsorption effects on graphene nanoribbon electrical properties. Mol. Simul. 2017, 44, 551–557. [Google Scholar]
- Ko, G.; Kim, H.-Y.; Ahn, J.; Park, Y.-M.; Lee, K.-Y.; Kim, J. Graphene-based nitrogen dioxide gas sensors. Curr. Appl. Phys. 2010, 10, 1002–1004. [Google Scholar] [CrossRef]
- Ahmadi, M.T.; Ismail, R.; Anwar, S. Handbook of Research on Nanoelectronic Sensor Modeling and Applications; IGI Global: Hershey, PA, USA, 2017. [Google Scholar]
- Akbari, E.; Afroozeh, A.; Tan, M.L.P.; Arora, V.K.; Ghadiry, M.; Yusof, R.; Ahmadi, M.T.; Enzevaee, A.; Rahmani, M. Analytical assessment of carbon allotropes for gas sensor applications. Measurement 2016, 92, 295–302. [Google Scholar] [CrossRef]
- Tang, S.; Cao, Z. Adsorption of nitrogen oxides on graphene and graphene oxides: Insights from density functional calculations. J. Chem. Phys. 2011, 134, 44710. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Hao, Y.-W.; Li, P.-L.; Song, J.-F.; Yang, R.-Z.; Fu, X.-Y.; Xie, S.-Y.; Zhao, J.; Zhang, W. Improved NO2 Gas Sensing Properties of Graphene Oxide Reduced by Two-beam-laser Interference. Sci. Rep. 2018, 8, 4918. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).