Nonporous Inorganic Nanoparticle-Based Humidity Sensor: Evaluation of Humidity Hysteresis and Response Time
Abstract
1. Introduction
2. Materials and Methods
3. Results
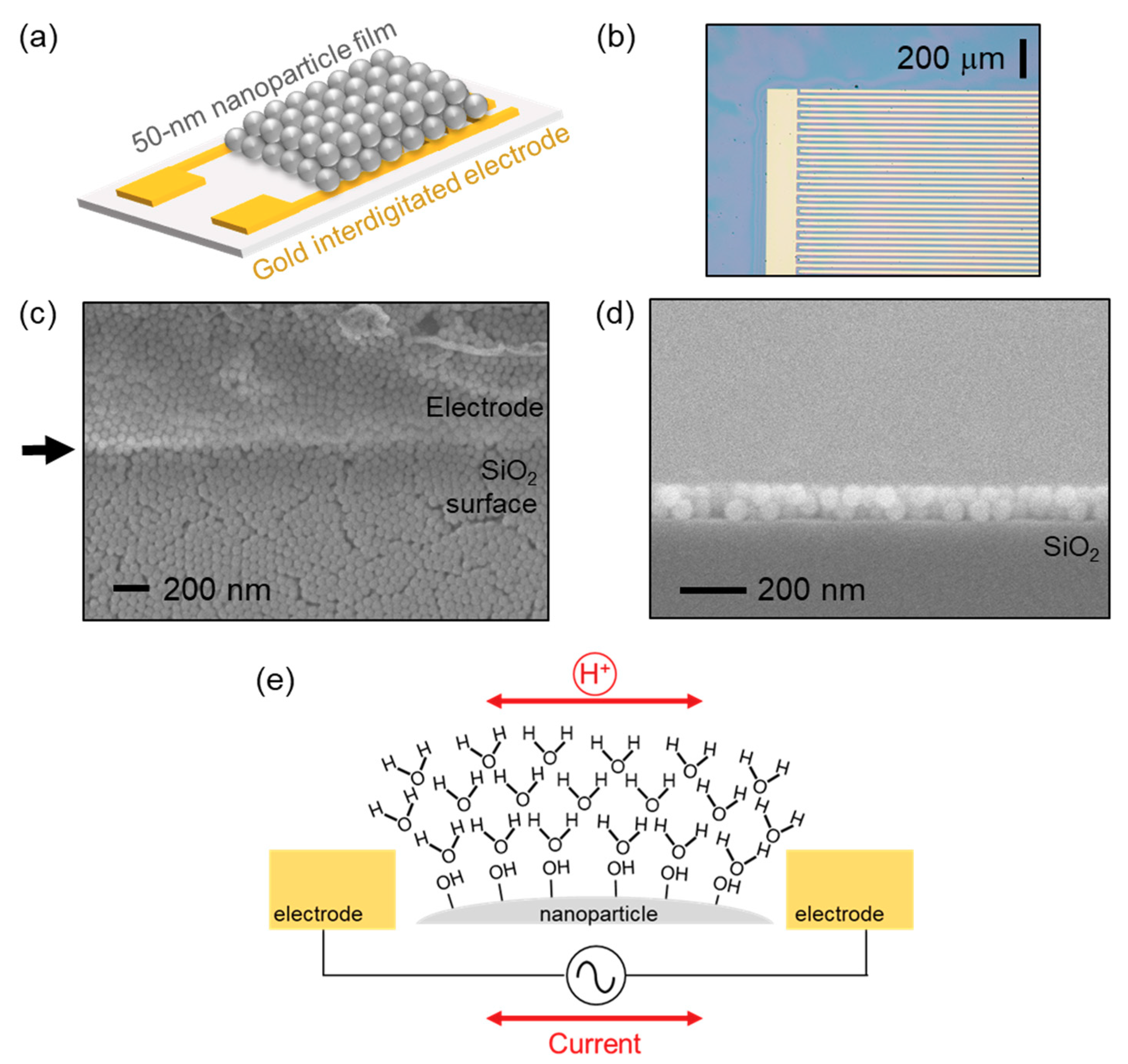
3.1. Sensor Structure
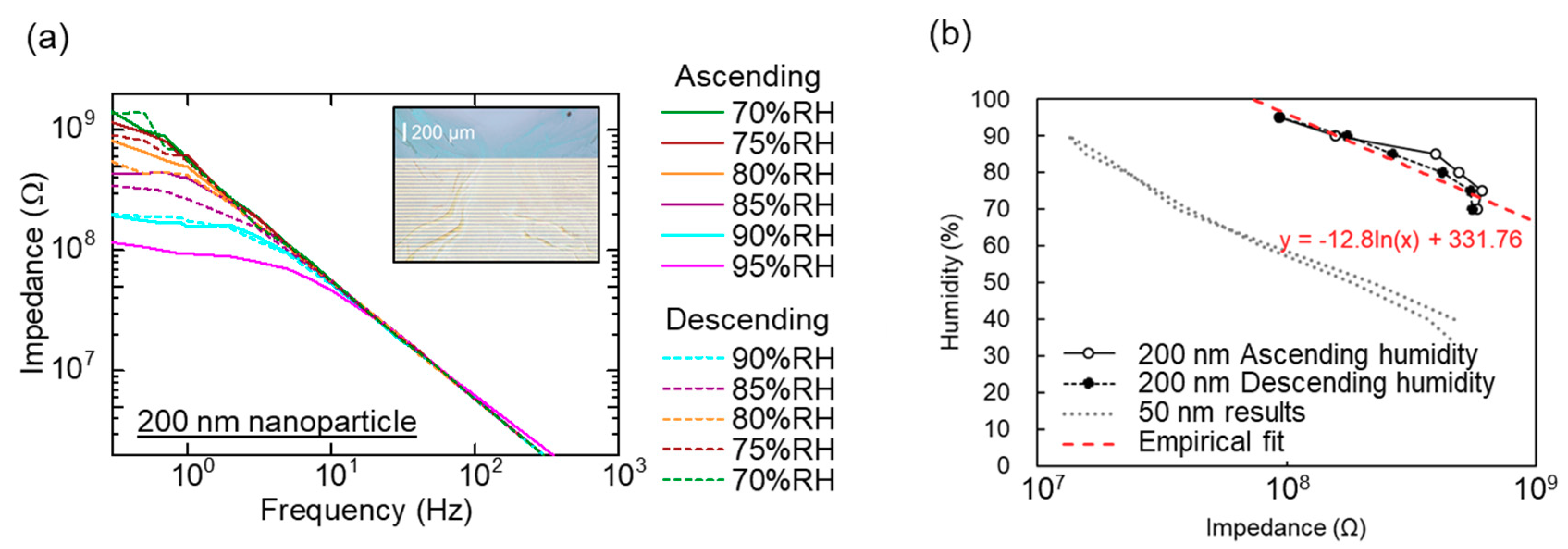
3.2. Sensor Characteristics: Hysteresis Error
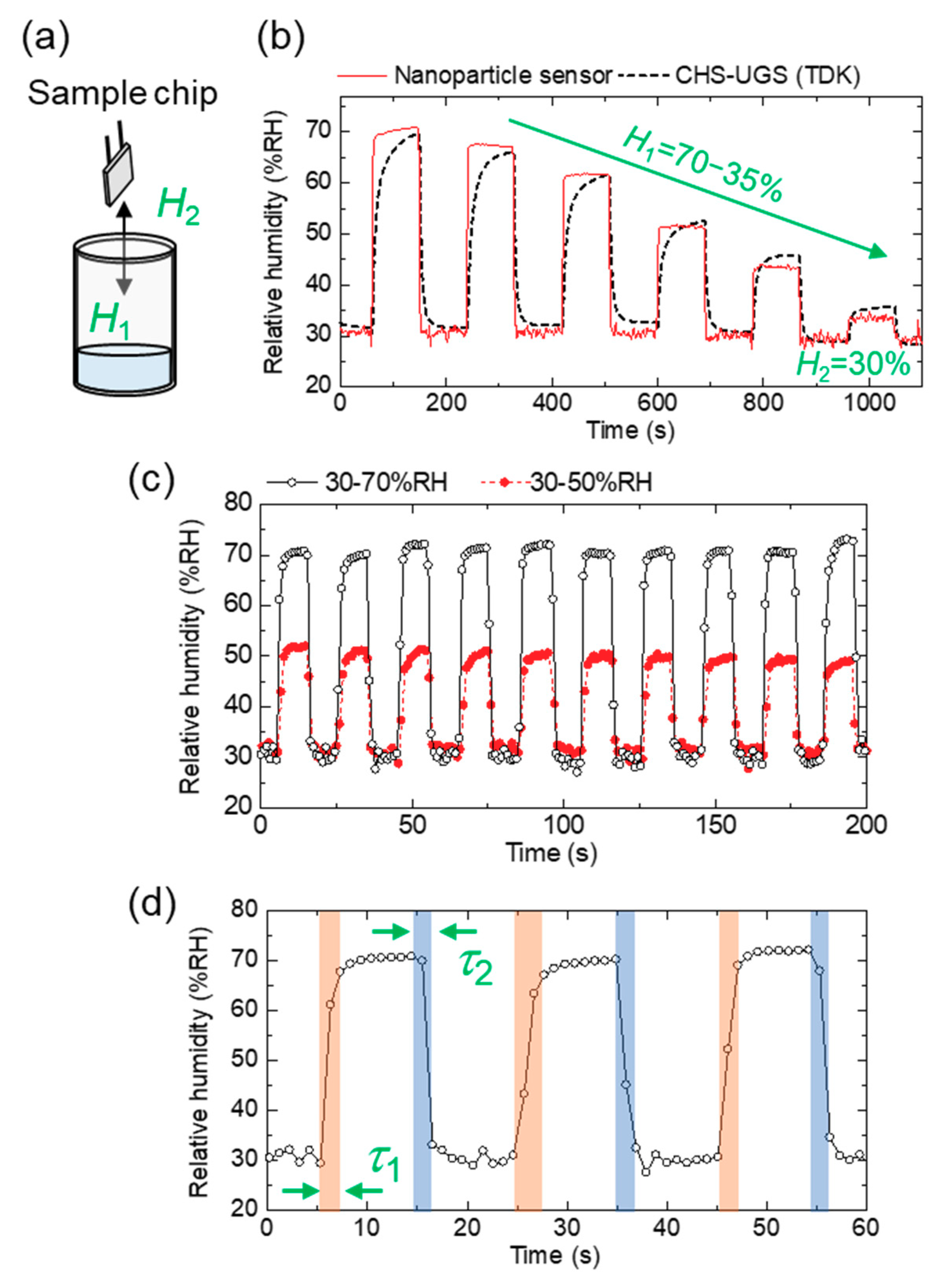
3.3. Sensor Characteristics: Response/Recovery Time
4. Discussions
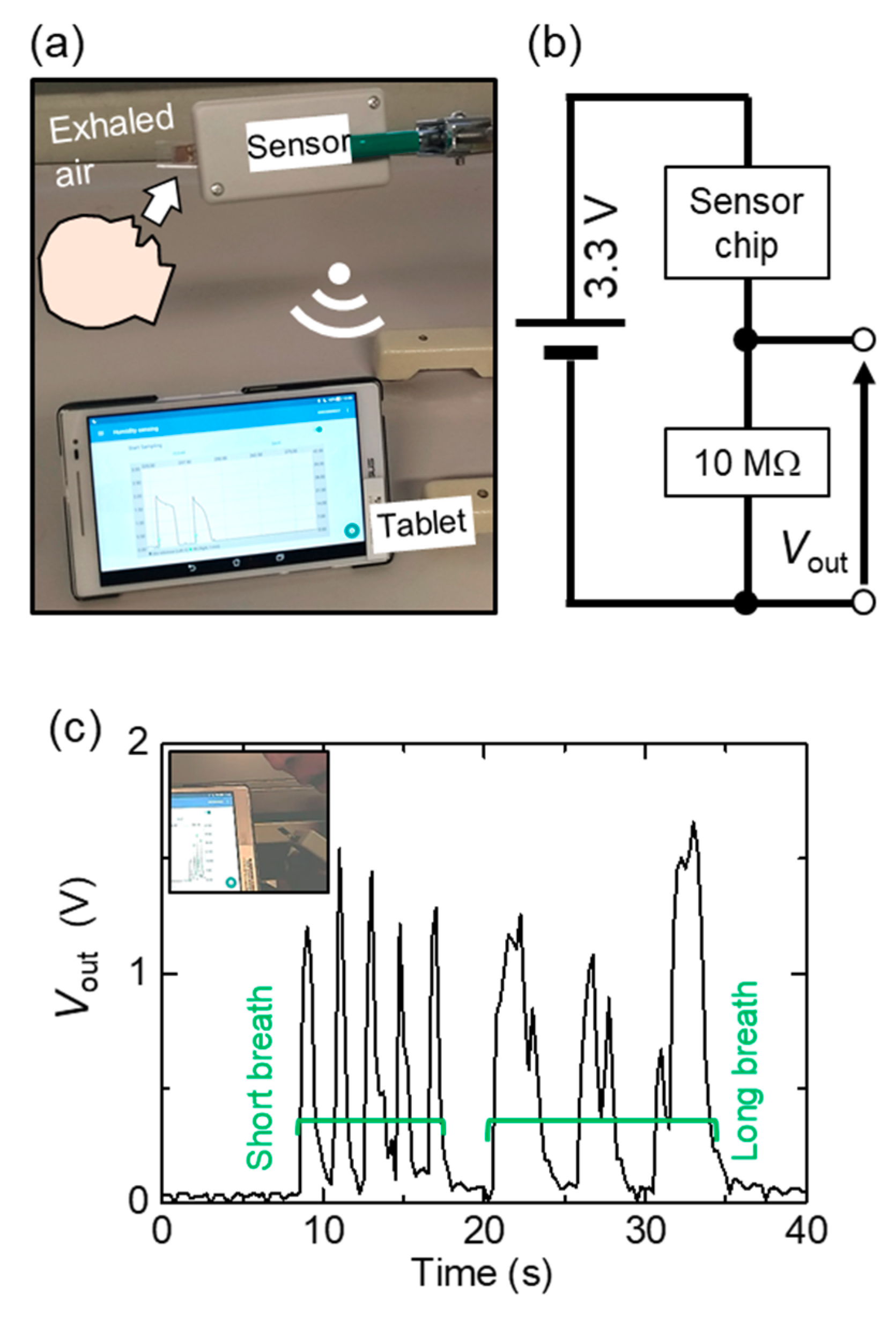
4.1. Intrinsic and Extrinsic Response Time
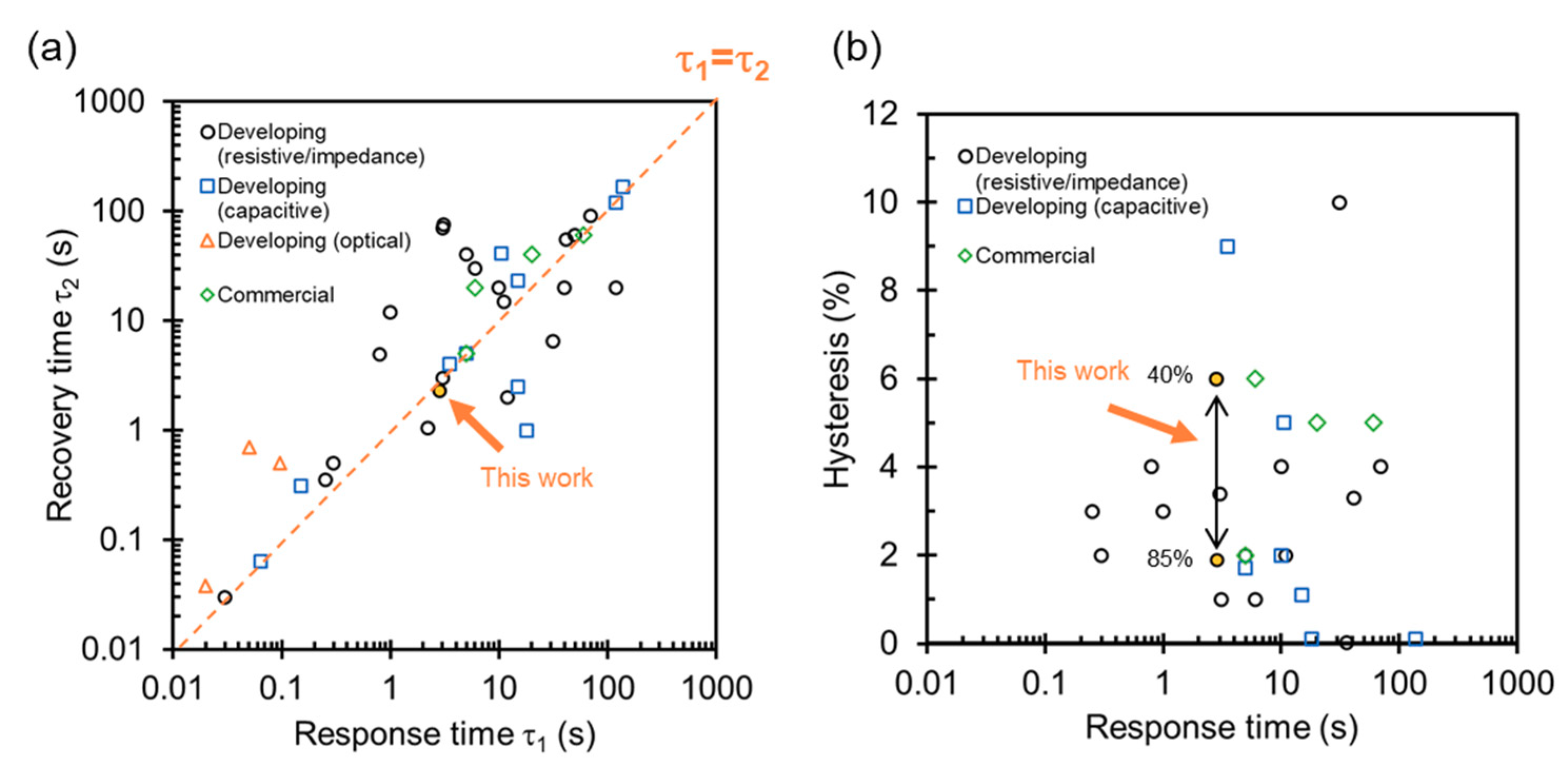
4.2. Specification with State-of-the-Art Humidity Sensors
4.3. Repeatability
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Li, B.; Tian, Q.; Su, H.; Wang, X.; Wang, T.; Zhang, D. High sensitivity protable capacitive humidity sensor based on In2O3 nanocubes-decorated GO nanosheets and its wearable application in respiration detection. Sens. Actuators B Chem. 2019, 299, 126973. [Google Scholar] [CrossRef]
- Seiyama, T.; Yamazoe, N.; Arai, H. Ceramic humidity sensors. Sens. Actuators 1983, 4, 85–96. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimizu, Y. Humidity sensors: Principles and applications. Sens. Actuators 1986, 10, 379–398. [Google Scholar] [CrossRef]
- Przyłuski, J.; Wieczorek, W. Proton polymeric electrolytes-a review. Synth. Met. 1991, 45, 323–333. [Google Scholar] [CrossRef]
- Grange, H.; Bieth, C.; Boucher, H.; Delapiere, G. A capacitive humidity sensor with very fast response time and very low hysteresis. Sens. Actuators 1987, 12, 291–296. [Google Scholar] [CrossRef]
- Borini, S.; White, R.; Wei, D.; Astley, M.; Haque, S.; Spigone, E.; Harris, N.; Kivioja, J.; Ryhänen, T. Ultrafast graphene oxide humidity sensors. ACS Nano 2013, 7, 11166–11173. [Google Scholar] [CrossRef]
- Wang, C.-T.; Wu, C.-L.; Chen, I.-C.; Huang, Y.-H. Humidity sensors based on silica nanoparticle aerogel thin films. Sens. Actuators B Chem. 2005, 107, 402–410. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, T.; Qi, R.; Dai, J.; Liu, S.; Fei, T.; Lu, G. Humidity sensor based on solution processible microporous silica nanoparticles. Sens. Actuators B Chem. 2018, 266, 131–138. [Google Scholar] [CrossRef]
- Mohd-Noor, S.; Jang, H.; Baek, K.; Pei, Y.R.; Alam, A.M.; Kim, Y.H.; Kim, I.S.; Choy, J.H.; Hyun, J.K. Ultrafast humidity-responsive structural colors from disordered nanoporous titania microspheres. J. Mater. Chem. A 2019, 7, 10561–10571. [Google Scholar] [CrossRef]
- Jalkanen, T.; Mäkilä, E.; Määttänen, A.; Tuura, J.; Kaasalainen, M.; Lehto, V.P.; Ihalainen, P.; Peltonen, J.; Salonen, J. Porous silicon micro- and nanoparticles for printed humidity sensors. Appl. Phys. Lett. 2012, 101, 263110. [Google Scholar] [CrossRef]
- Gawli, Y.; Badadhe, S.; Basu, A.; Guin, D.; Shelke, M.V.; Ogale, S. Evaluation of n-type ternary metal oxide NiMn2O4 nanomaterial for humidity sensing. Sens. Actuators B Chem. 2014, 191, 837–843. [Google Scholar] [CrossRef]
- Kano, S.; Kim, K.; Fujii, M. Fast-response and flexible nanocrystal-based humidity sensor for monitoring human respiration and water evaporation on skin. ACS Sens. 2017, 2, 828–833. [Google Scholar] [CrossRef]
- Kano, S.; Dobashi, Y.; Fujii, M. Silica nanoparticle-based portable respiration sensor for analysis of respiration rate, pattern, and phase during exercise. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Kano, S.; Fujii, M. All-painting process to produce respiration sensor using humidity-sensitive nanoparticle film and graphite trace. ACS Sustain. Chem. Eng. 2018, 6, 12217–12223. [Google Scholar] [CrossRef]
- Kano, S.; Yamamoto, A.; Ishikawa, A.; Fujii, M. Respiratory rate on exercise measured by nanoparticle-based humidity sensor. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Berlin, Germany, 23–27 July 2019; pp. 3567–3570. [Google Scholar]
- Adamson, A.W. Physical Chemistry of Surfaces, 4th ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1990; pp. 661–662. [Google Scholar]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef]
- Chen, L.; He, X.; Liu, H.; Qian, L.; Kim, S.H. Water adsorption on hydrophilic and hydrophobic surfaces of silicon. J. Phys. Chem. C 2018, 122, 11385–11391. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Asay, D.B.; Kim, S.H. Evolution of the adsorbed water layer structure on silicon oxide at room temperature. J. Phys. Chem. B 2005, 109, 16760–16763. [Google Scholar] [CrossRef]
- Seo, M.-H.; Yang, H.-H.; Choi, K.-W.; Lee, J.-S.; Yoon, J.-B. A simple breathing rate-sensing method exploiting a temporarily condensed water layer formed on an oxidized surface. Appl. Phys. Lett. 2015, 106, 053701. [Google Scholar] [CrossRef]
- Mansour, E.; Vishinkin, R.; Rihet, S.; Saliba, W.; Fish, F.; Sarfati, P.; Haick, H. Measurement of temperature and relative humidity in exhaled breath. Sens. Actuators B Chem. 2020, 304, 127371. [Google Scholar] [CrossRef]
- Steele, J.J.; Taschuk, M.T.; Brett, M.J. Response time of nanostructured relative humidity sensors. Sens. Actuators B Chem. 2009, 140, 610–615. [Google Scholar] [CrossRef]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81A, 89. [Google Scholar] [CrossRef]
- Ali, S.; Hassan, A.; Hassan, G.; Bae, J.; Lee, C.H. All-printed humidity sensor based on graphene/methyl-red composite with high sensitivity. Carbon N. Y. 2016, 105, 23–32. [Google Scholar] [CrossRef]
- Yang, J.; Shi, R.; Lou, Z.; Chai, R.; Jiang, K.; Shen, G. Flexible smart noncontact control systems with ultrasensitive humidity sensors. Small 2019, 15, 1902801. [Google Scholar] [CrossRef]
- Mathew, J.; Semenova, Y.; Farrell, G. A fiber bend based humidity sensor with a wide linear range and fast measurement speed. Sens. Actuators A Phys. 2012, 174, 47–51. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, C.; Yuan, Q.; Fang, L.; Li, Y.; Yin, J.; Ma, X.; Zhao, J. High performance graphene oxide-based humidity sensor integrated on a photonic crystal cavity. Appl. Phys. Lett. 2017, 110. [Google Scholar] [CrossRef]
- Zhang, D.; Zong, X.; Wu, Z.; Zhang, Y. Hierarchical self-assembled SnS2 nanoflower/Zn2SnO4 hollow sphere nanohybrid for humidity-sensing applications. ACS Appl. Mater. Interfaces 2018, 10, 32631–32639. [Google Scholar] [CrossRef]
- Burman, D.; Choudhary, D.S.; Guha, P.K. ZnO/MoS 2—Based enhanced humidity sensor prototype with android app interface for mobile platform. IEEE Sens. J. 2019, 19, 3993–3999. [Google Scholar] [CrossRef]
- Nakagawa, S.; Tsuchida, A. Humidity Meter. U.S. Patent 5,317,274, 31 May 1994. [Google Scholar]
- Tan, Z.; Chae, Y.; Daamen, R.; Humbert, A.; Ponomarev, Y.V.; Pertijs, M.A.P. A 1.2V 8.3nJ energy-efficient CMOS humidity sensor for RFID applications. IEEE J. Solid-State Circuits 2013, 2469–2477. [Google Scholar] [CrossRef]
- Su, P.G.; Huang, L.N. Humidity sensors based on TiO2 nanoparticles/polypyrrole composite thin films. Sens. Actuators B Chem. 2007, 123, 501–507. [Google Scholar] [CrossRef]
- Duan, Z.; Xu, M.; Li, T.; Zhang, Y.; Zou, H. Super-fast response humidity sensor based on La0.7Sr0.3MnO3 nanocrystals prepared by PVP-assisted sol-gel method. Sens. Actuators B Chem. 2018, 258, 527–534. [Google Scholar] [CrossRef]
- Vogel, N.; Retsch, M.; Fustin, C.A.; Del Campo, A.; Jonas, U. Advances in colloidal assembly: The design of structure and hierarchy in two and three dimensions. Chem. Rev. 2015, 115, 6265–6311. [Google Scholar] [CrossRef] [PubMed]

| Materials | Porous/Nonporous Particles | Particle Diameter (nm) | Response Time/Recovery Time (s) | Method (Chamber/Salt) | Hysteresis (%) | Reference |
|---|---|---|---|---|---|---|
| SnS2 nanoflower/Zn2SoO4 hollow sphere | Porous | 400 | 18/2 | Salt | 0.1 | [30] |
| Porous silica nanoparticle aerogel | Porous | 30 | 41/55 | Chamber | 3.3 | [8] |
| Microporous silica nanoparticle | Porous | 105 | 5/40 | Salt | 2 | [9] |
| Porous silicon nanoparticle | Porous | 900 | 36/NA | Chamber | 0.02 | [11] |
| TiO2 nanoparticles/polypyrrole composite | Nonporous | 7 | 40/20 | Chamber | NA | [34] |
| La0.7Sr0.3MnO3 nanocrystal | Nonporous | 20 to 40 | 0.8/4.9 | Salt | 4 | [35] |
| In2O3 nanocube/graphene oxide nanosheet | Nonporous | 20 to 40 | 15/2.5 | Salt | NA | [2] |
| Silica nanoparticle | Nonporous | 50 | 2.8/2.3 | Salt | 2–6 | This work |
| HTU21 (TE connectivity, commercial sensor) | 5/5 * | Chamber | 2 | Datasheet |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kano, S.; Mekaru, H. Nonporous Inorganic Nanoparticle-Based Humidity Sensor: Evaluation of Humidity Hysteresis and Response Time. Sensors 2020, 20, 3858. https://doi.org/10.3390/s20143858
Kano S, Mekaru H. Nonporous Inorganic Nanoparticle-Based Humidity Sensor: Evaluation of Humidity Hysteresis and Response Time. Sensors. 2020; 20(14):3858. https://doi.org/10.3390/s20143858
Chicago/Turabian StyleKano, Shinya, and Harutaka Mekaru. 2020. "Nonporous Inorganic Nanoparticle-Based Humidity Sensor: Evaluation of Humidity Hysteresis and Response Time" Sensors 20, no. 14: 3858. https://doi.org/10.3390/s20143858
APA StyleKano, S., & Mekaru, H. (2020). Nonporous Inorganic Nanoparticle-Based Humidity Sensor: Evaluation of Humidity Hysteresis and Response Time. Sensors, 20(14), 3858. https://doi.org/10.3390/s20143858





