Recent Advances in the Electrochemical Sensing of Venlafaxine: An Antidepressant Drug and Environmental Contaminant
Abstract
1. Introduction
2. Electrodes
3. Electrochemical Detection of Venlafaxine
3.1. CPEs
3.2. GCE
3.3. SPE
3.4. Mercury-Based Electrodes
3.5. Various Electrodes (Ion-Selective Electrode, PGE, etc.)
4. Future Outlook
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, L.D.; Li, J.; Li, J.J.; Liu, M.; Yan, D.Z.; Shi, W.Y.; Xu, G. Occurrence and source analysis of selected anti-depressants and their metabolites in municipal wastewater and receiving surface water. Environ. Sci. Process. Impacts 2018, 20, 1020–1029. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Gagnon, C.; Sauvé, S. Determination of basic anti-depressants and their N-desmethyl metabolites in raw sewage and wastewater using solid-phase extraction and liquid chromatography−tandem mass spectrometry. Anal. Chem. 2008, 80, 5325–5333. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Jahani, S.; Tajik, S.; Ganjali, M.; Faridbod, F.; Alizadeh, T. Voltammetric determination of venlafaxine as an antidepressant drug employing Gd2O3 nanoparticles graphite screen printed electrode. J. Rare Earths 2019, 37, 322–328. [Google Scholar] [CrossRef]
- Madrakian, T.; Haryani, R.; Ahmadi, M.; Afkhami, A. Spectrofluorometric determination of venlafaxine in biological samples after selective extraction on the superparamagnetic surface molecularly imprinted nanoparticles. Anal. Methods 2015, 7, 428–435. [Google Scholar] [CrossRef]
- Muth, E.A.; Haskins, J.T.; Moyer, J.A.; Husbands, G.E.; Nielsen, S.T.; Sigg, E.B. Antidepressant biochemical profile of the novel bicyclic compound Wy-45,030, an ethyl cyclohexanol derivative. Biochem. Pharmacol. 1986, 35, 4493–4497. [Google Scholar] [CrossRef]
- Otton, S.V.; Ball, S.E.; Cheung, S.W.; Inaba, T.; Rudolph, R.L.; Sellers, E.M. Venlafaxine oxidation in vitro is catalysed by CYP2D6. Br. J. Clin. Pharmacol. 1996, 41, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Cravey, R.H. A Review ofDisposition of Toxic Drugs and Chemicals in Man. J. Forensic Sci. 1983, 28, 540. [Google Scholar] [CrossRef]
- Kingbäck, M.; Josefsson, M.; Karlsson, L.; Ahlner, J.; Bengtsson, F.; Kugelberg, F.C.; Carlsson, B. Stereoselective determination of venlafaxine and its three demethylated metabolites in human plasma and whole blood by liquid chromatography with electrospray tandem mass spectrometric detection and solid phase extraction. J. Pharm. Biomed. Anal. 2010, 53, 583–590. [Google Scholar] [CrossRef]
- Berm, E.J.J.; Brummel-Mulder, E.; Paardekooper, J.; Hak, E.; Wilffert, B.; Maring, J.G. Determination of venlafaxine and O-desmethylvenlafaxine in dried blood spots for TDM purposes, using LC-MS/MS. Anal. Bioanal. Chem. 2014, 406, 2349–2353. [Google Scholar] [CrossRef]
- Madrakian, T.; Haryani, R.; Ahmadi, M.; Afkhami, A. A sensitive electrochemical sensor for rapid and selective determination of venlafaxine in biological fluids using carbon paste electrode modified with molecularly imprinted polymer-coated magnetite nanoparticles. J. Iran. Chem. Soc. 2015, 13, 243–251. [Google Scholar] [CrossRef]
- Beitollahi, H.; Karimi-Maleh, H.; Khabazzadeh, H. Nanomolar and Selective Determination of Epinephrine in the Presence of Norepinephrine Using Carbon Paste Electrode Modified with Carbon Nanotubes and Novel 2-(4-Oxo-3-phenyl-3,4-dihydro-quinazolinyl)-N′-phenyl-hydrazinecarbothioamide. Anal. Chem. 2008, 80, 9848–9851. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Khalilzadeh, M.A.; Tajik, S.; Safaei, M.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent Advances in Applications of Voltammetric Sensors Modified with Ferrocene and Its Derivatives. ACS Omega 2020, 5, 2049–2059. [Google Scholar] [CrossRef]
- Maddah, B.; Cheraghveisi, M.; Najafi, M. Developing a modified electrode based on La3+/Co3O4 Nanocubes and its usage to electrochemical detection of venlafaxine. Int. J. Environ. Anal. Chem. 2020, 100, 121–133. [Google Scholar] [CrossRef]
- Prakash, P.A.; Yogeswaran, U.; Chena, S.-M. A Review on Direct Electrochemistry of Catalase for Electrochemical Sensors. Sensors 2009, 9, 1821–1844. [Google Scholar] [CrossRef]
- Bratov, A.; Abramova, N.; Ipatov, A. Recent trends in potentiometric sensor arrays—A review. Anal. Chim. Acta 2010, 678, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Stradiotto, N.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Negulescu, G.P. Methods for Total Antioxidant Activity Determination: A Review. Biochem. Anal. Biochem. 2012, 1, 106. [Google Scholar] [CrossRef]
- Helm, I.; Jalukse, L.; Leito, I. Measurement Uncertainty Estimation in Amperometric Sensors: A Tutorial Review. Sensors 2010, 10, 4430–4455. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Y.; Chang, L.; Zhou, L.; Tang, F.; Wu, X. β-cyclodextrin non-covalently modified ionic liquid-based carbon paste electrode as a novel voltammetric sensor for specific detection of bisphenol A. Sensors Actuators B Chem. 2013, 186, 648–656. [Google Scholar] [CrossRef]
- Zaidi, S.A. Graphene: A comprehensive review on its utilization in carbon paste electrodes for improved sensor performances. Int. J. Electrochem. Sci. 2013, 8, 11337–11355. [Google Scholar]
- Yi, Y.; Weinberg, G.; Prenzel, M.; Greiner, M.; Heumann, S.; Becker, S.; Schlögl, R. Electrochemical corrosion of a glassy carbon electrode. Catal. Today 2017, 295, 32–40. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Hossain, M.M.; Safavieh, M.; Wong, Y.L.; Rahman, I.A.; Zourob, M.; Tamiya, E. Toward the development of smart and low cost point-of-care biosensors based on screen printed electrodes. Crit. Rev. Biotechnol. 2015, 36, 1–11. [Google Scholar] [CrossRef]
- Thiyagarajan, N.; Chang, J.-L.; Senthilkumar, K.; Zen, J.-M. Disposable electrochemical sensors: A mini review. Electrochem. Commun. 2014, 38, 86–90. [Google Scholar] [CrossRef]
- Beitollahi, H.; Moghaddam, H.M.; Tajik, S. Voltammetric Determination of Bisphenol A in Water and Juice Using a Lanthanum (III)-Doped Cobalt (II, III) Nanocube Modified Carbon Screen-Printed Electrode. Anal. Lett. 2018, 52, 1432–1444. [Google Scholar] [CrossRef]
- Golas, J.; Galus, Z.; Osteryoung, J. Iridium-based small mercury electrodes. Anal. Chem. 1987, 59, 389–392. [Google Scholar] [CrossRef]
- Kounaves, S.; Buffle, J. An iridium-based mercury-film electrode. J. Electroanal. Chem. Interfacial Electrochem. 1987, 216, 53–69. [Google Scholar] [CrossRef]
- Lai, C.; Fierke, M.A.; Stein, A.; Bühlmann, P. Ion-Selective Electrodes with Three-Dimensionally Ordered Macroporous Carbon as the Solid Contact. Anal. Chem. 2007, 79, 4621–4626. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, Y.; Gu, Y.; Li, T.; Luo, H.; Li, L.-H.; Bai, Y.; Li, L.; Liu, L.; Cao, Y.; et al. Wearable Sweatband Sensor Platform Based on Gold Nanodendrite Array as Efficient Solid Contact of Ion-Selective Electrode. Anal. Chem. 2017, 89, 10224–10231. [Google Scholar] [CrossRef] [PubMed]
- Bolat, G.; Abaci, S.; Vural, T.; Bozdogan, B.; Denkbas, E.B. Sensitive electrochemical detection of fenitrothion pesticide based on self-assembled peptide-nanotubes modified disposable pencil graphite electrode. J. Electroanal. Chem. 2018, 809, 88–95. [Google Scholar] [CrossRef]
- Eslami, E.; Farjami, F. Voltammetric determination of venlafaxine by using multiwalled carbon nanotube-ionic liquid composite electrode. J. Appl. Chem. Res. 2018, 12, 42–52. [Google Scholar]
- Rizk, M.; Taha, E.A.; Alamin, M.; Hendawy, H.A.M.; Sayed, Y. Highly Sensitive Carbon Based Sensors Using Zinc Oxide Nanoparticles Immobilized Multiwalled Carbon Nanotubes for Simultaneous Determination of Desvenlafaxine Succinate and Clonazepam. J. Electrochem. Soc. 2018, 165, H333–H341. [Google Scholar] [CrossRef]
- Sanghavi, B.J.; Srivastava, A. Adsorptive stripping differential pulse voltammetric determination of venlafaxine and desvenlafaxine employing Nafion–carbon nanotube composite glassy carbon electrode. Electrochimica Acta 2011, 56, 4188–4196. [Google Scholar] [CrossRef]
- Ding, L.; Li, L.; You, W.; Gao, Z.-N.; Yang, T.-L. Electrocatalytic Oxidation of Venlafaxine at a Multiwall Carbon Nanotubes-Ionic Liquid Gel Modified Glassy Carbon Electrode and Its Electrochemical Determination. Croat. Chem. Acta 2015, 88, 81–87. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Tajik, S.; Beitollahi, H.; Venditti, R.A. Green Synthesis of Magnetic Nanocomposite with Iron Oxide Deposited on Cellulose Nanocrystals with Copper (Fe3O4@CNC/Cu): Investigation of Catalytic Activity for the Development of a Venlafaxine Electrochemical Sensor. Ind. Eng. Chem. Res. 2020, 59, 4219–4228. [Google Scholar] [CrossRef]
- Lima, J.L.F.C.; Loo, D.V.; Delerue-Matos, C.; Da Silva, A.S.R. Electrochemical behaviour of Venlafaxine and its determination in pharmaceutical products using square wave voltammetry. Il Farm. 1999, 54, 145–148. [Google Scholar] [CrossRef]
- Morais, S.; Ryckaert, C.P.M.C.A.; Delerue-Matos, C. Adsorptive Stripping Voltammetric Determination of Venlafaxine in Urine with a Mercury Film Microelectrode. Anal. Lett. 2003, 36, 2515–2526. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Faridfar, R.; Allafchian, A. A Novel Selective Coated-Wire Potentiometric Sensor for Venlafaxine Determination in Pharmaceutical Compounds, Plasma and Urine. Sens. Lett. 2011, 9, 479–484. [Google Scholar] [CrossRef]
- Mansour, M.F.; El-Moety, M.M.A.; El Kady, E.F.; El Guindi, N.M.; Van Schepdael, A.; Aly, S.M.E.-M. Venlafaxine Determination in Pharmaceutical Formulation and Serum by Ion-Selective Electrodes. Curr. Pharm. Des. 2018, 24, 2625–2630. [Google Scholar] [CrossRef]
- Ali, M.F.B.; El-Zahry, M.R. A comparative study of different electrodeposited NiCo2O4 microspheres anchored on a reduced graphene oxide platform: Electrochemical sensor for anti-depressant drug venlafaxine. RSC Adv. 2019, 9, 31609–31620. [Google Scholar] [CrossRef]
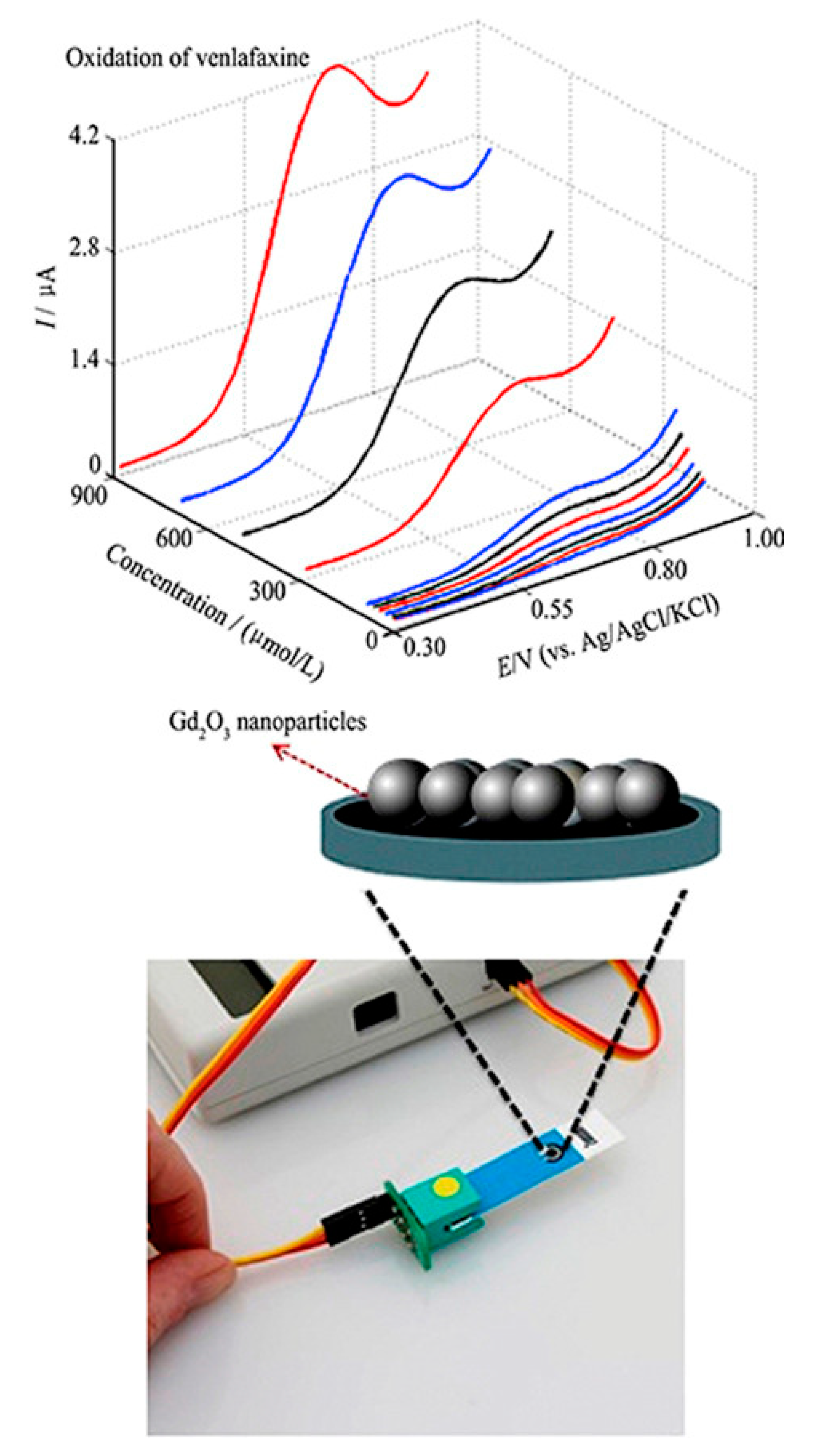
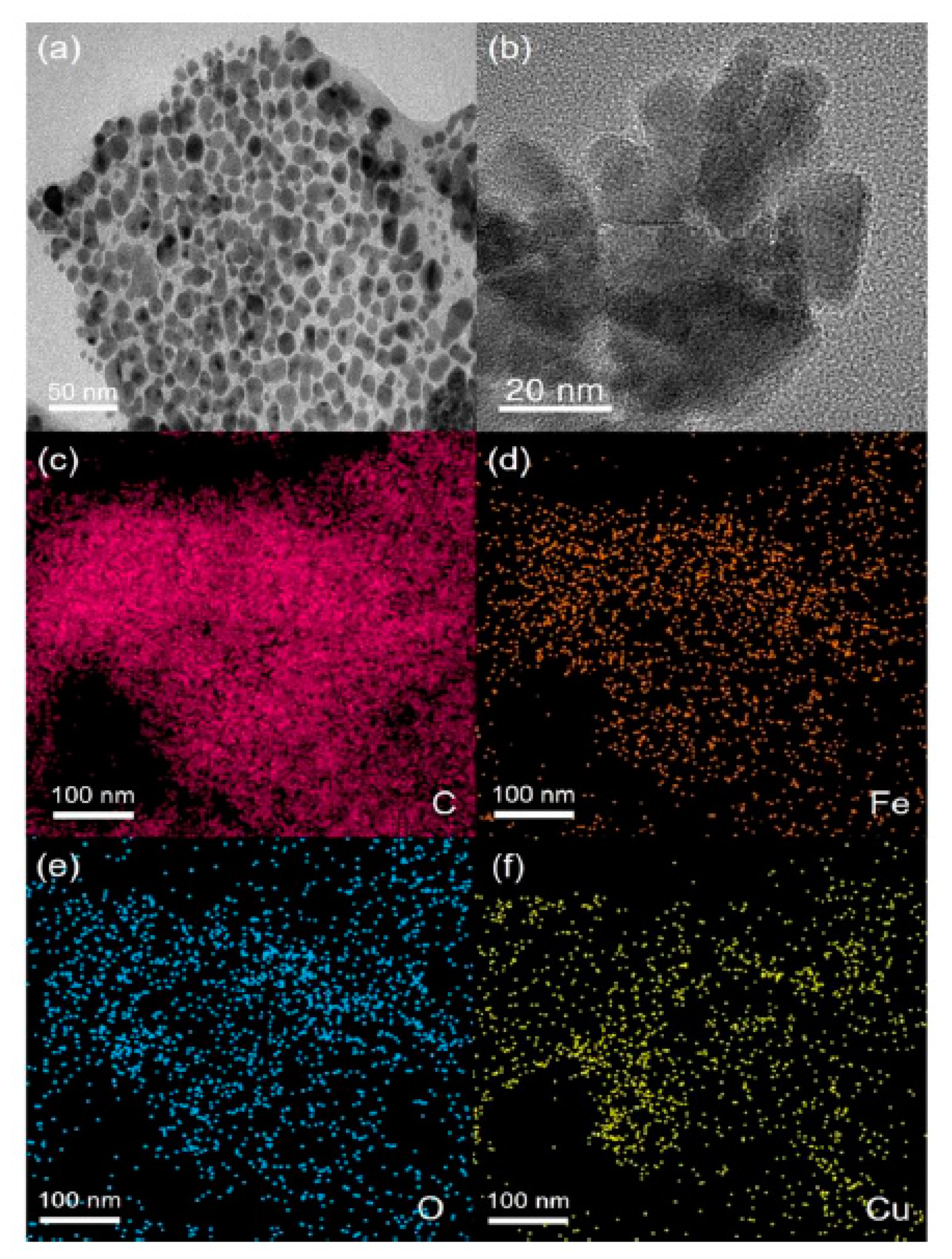
| Sensor | Method | Limit of Detection | Linear Dynamic Range | Ref |
|---|---|---|---|---|
| VENMNPs/CPE | DPV | 6.0 nM | 0.01–10.0 μM | [10] |
| MWCNT/CILE/CPE | CV | 0.47 μM | 10.0–500.0 μM | [30] |
| MWCNTs/ZnONPs/CPE | DPV | 0.28 μg/mL | 0.66–8.42 μg/mL | [31] |
| Sensor | Method | Limit of Detection | Linear Dynamic Range | Ref |
|---|---|---|---|---|
| NAF-CNT-GCE | AdSDPV | 1.24 × 10−8 M | 3.81 × 10−8–6.22 × 10−5 | [32] |
| MWCNTs-RTIL/GCE | SWV | 1.69 × 10−6 M | 2.0 × 10−6–2.0 × 10−3 M | [33] |
| Sensor | Method | Limit of Detection | Linear Dynamic Range | Ref. |
|---|---|---|---|---|
| Gd2O3/SPE | DPV | 0.21 μM | 5.0 × 10−6–9.0 × 10−4 M | [3] |
| La3+/Co3O4/SPE | DPV | 0.5 μM | 1.0–500.0 μM | [13] |
| Fe3O4@CNC/Cu/GSPE | DPV | 0.01 μM | 0.05–600.0 μM | [34] |
| Sensor | Method | Limit of Detection | Linear Dynamic Range | Ref. |
|---|---|---|---|---|
| HMDE | SWV | 0.124 mg/L | 0.25–1.23 mg/L | [35] |
| MFM | DPV | 0.693 × 10−6 M | - | [36] |
| Sensor | Method | Limit of Detection | Linear Dynamic Range | Ref. |
|---|---|---|---|---|
| Ion pair:DBP:PVC | Potentiometry | 5.0 × 10−6 M | 1.0 × 10−2–8.0 × 10−6 M | [37] |
| Ion-selective electrode | Potentiometry | - | 5 × 10−5–1 × 10−2 M | [38] |
| NiCo2O4@rGO Modified PGE electrode | SWV | 3.4 nM | 5.0–500.0 nmol L−1 | [39] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajik, S.; Beitollahi, H.; Dourandish, Z.; Zhang, K.; Le, Q.V.; Nguyen, T.P.; Kim, S.Y.; Shokouhimehr, M. Recent Advances in the Electrochemical Sensing of Venlafaxine: An Antidepressant Drug and Environmental Contaminant. Sensors 2020, 20, 3675. https://doi.org/10.3390/s20133675
Tajik S, Beitollahi H, Dourandish Z, Zhang K, Le QV, Nguyen TP, Kim SY, Shokouhimehr M. Recent Advances in the Electrochemical Sensing of Venlafaxine: An Antidepressant Drug and Environmental Contaminant. Sensors. 2020; 20(13):3675. https://doi.org/10.3390/s20133675
Chicago/Turabian StyleTajik, Somayeh, Hadi Beitollahi, Zahra Dourandish, Kaiqiang Zhang, Quyet Van Le, Thang Phan Nguyen, Soo Young Kim, and Mohammadreza Shokouhimehr. 2020. "Recent Advances in the Electrochemical Sensing of Venlafaxine: An Antidepressant Drug and Environmental Contaminant" Sensors 20, no. 13: 3675. https://doi.org/10.3390/s20133675
APA StyleTajik, S., Beitollahi, H., Dourandish, Z., Zhang, K., Le, Q. V., Nguyen, T. P., Kim, S. Y., & Shokouhimehr, M. (2020). Recent Advances in the Electrochemical Sensing of Venlafaxine: An Antidepressant Drug and Environmental Contaminant. Sensors, 20(13), 3675. https://doi.org/10.3390/s20133675









