A Combined Deep-Learning and Lattice Boltzmann Model for Segmentation of the Hippocampus in MRI
Abstract
1. Introduction
2. Method
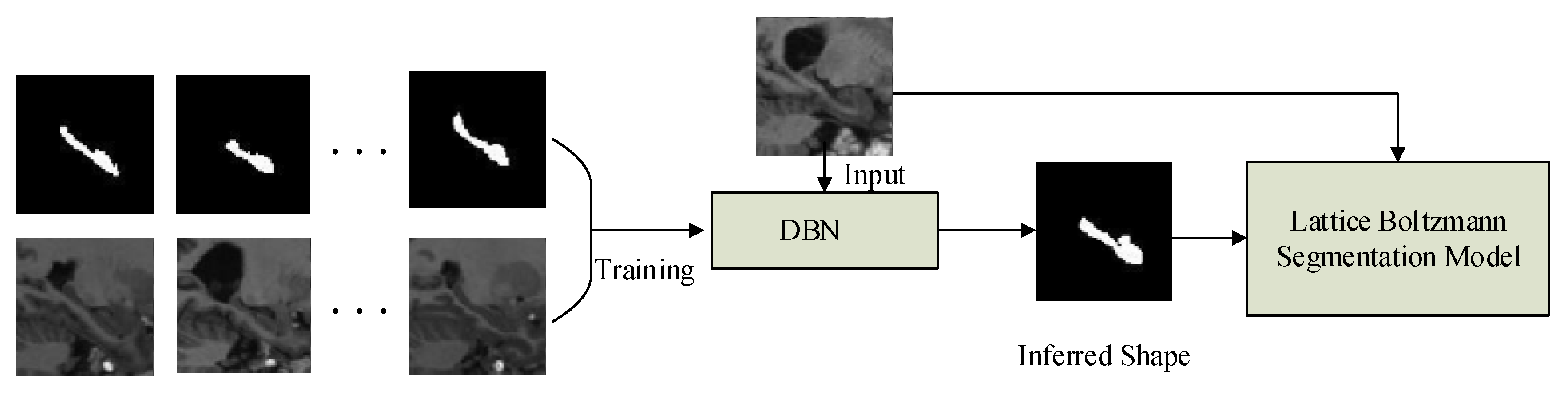
2.1. Method Overview
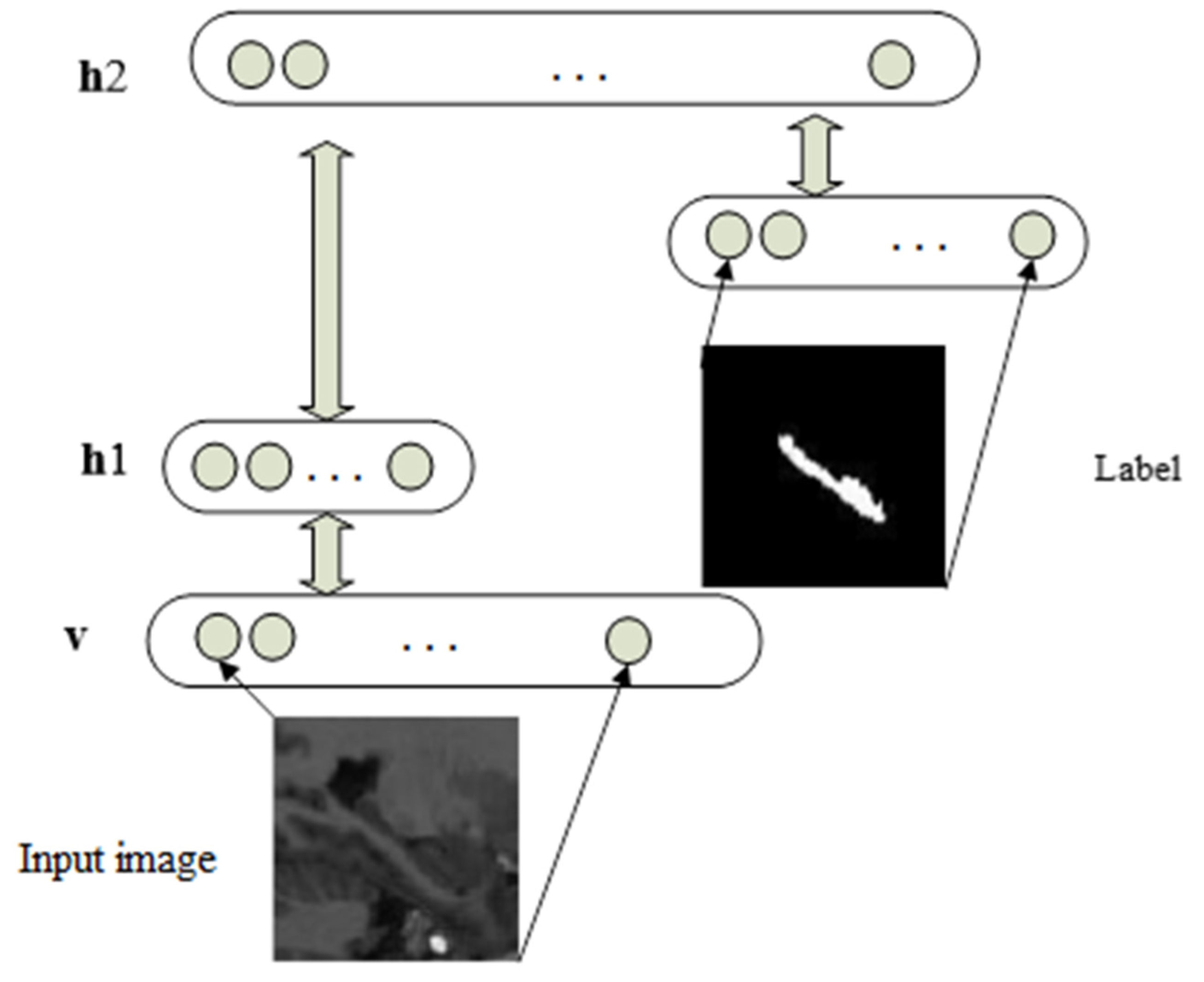
2.2. Shape Inferring
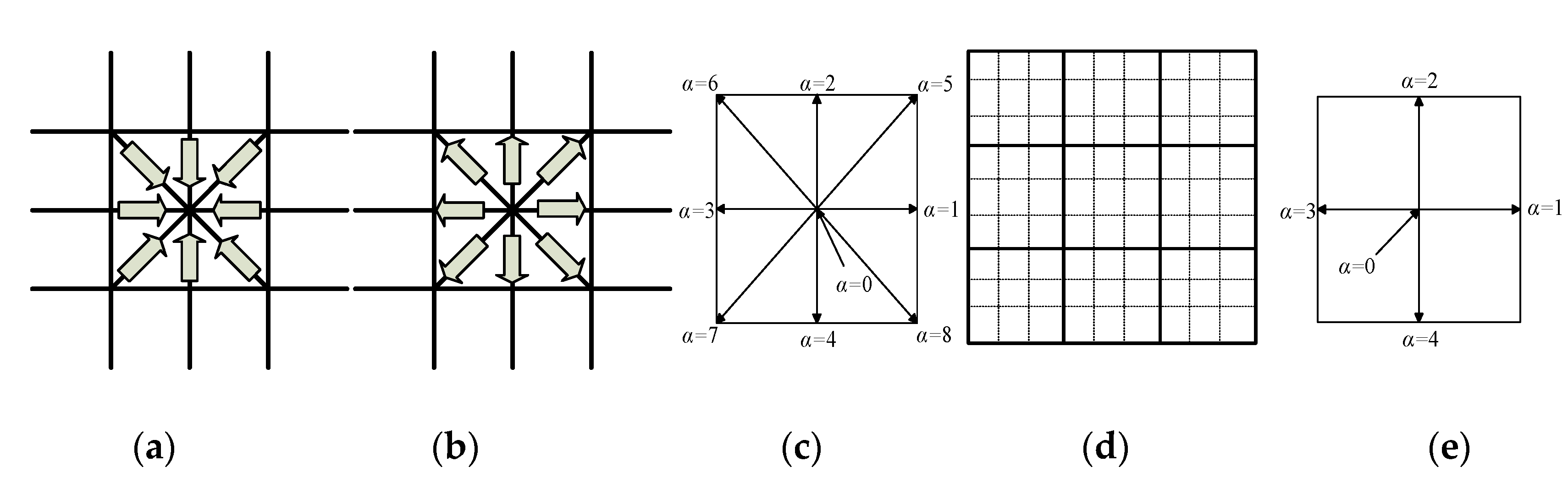
2.3. Explanation of Lattice Boltzmann Method
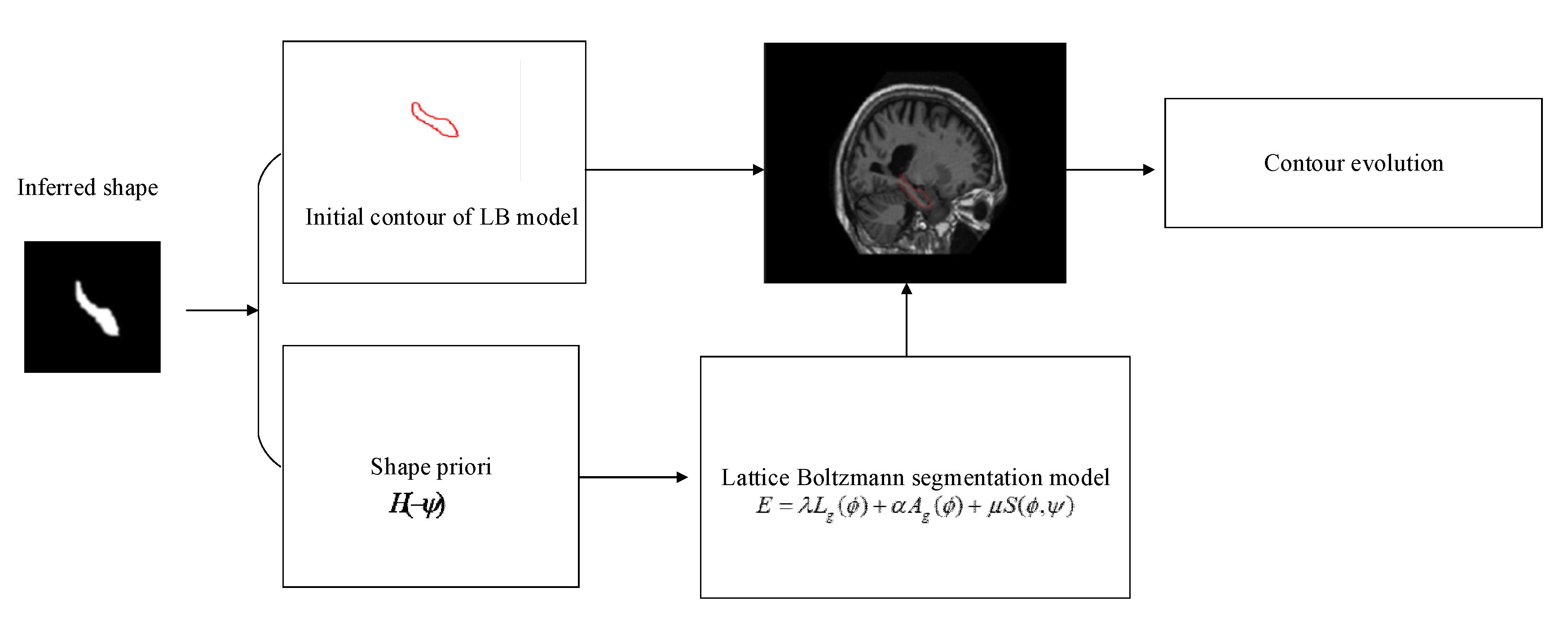
2.4. Lattice Boltzmann Model Driven by DBN
2.5. Algorithm
| Algorithm 1. DBN Driven LB Method for Image Segmentation |
| 1: Setting the initial position of evolving curve C and defining level set function as a signed distance function, such as: where is the position of one pixel in the image, is a constant, and denote the inside and outside region of the evolving curve , respectively |
| 2: Initialize local equilibrium distribution function , compute relaxation parameter with Equation (19) |
| 3: Compute the external force term and discretize it with Equation (20) |
| 4: Updating the evolving curve and after collision and streaming described in Equations (6) and (7), separately |
| 5: If the segmentation is not done, jump back to step (2) |
| 6: Output the segmentation result |
3. Experiments
3.1. Data Preparation and Experimental Setup
3.2. Validation Framework and Evaluation Measures
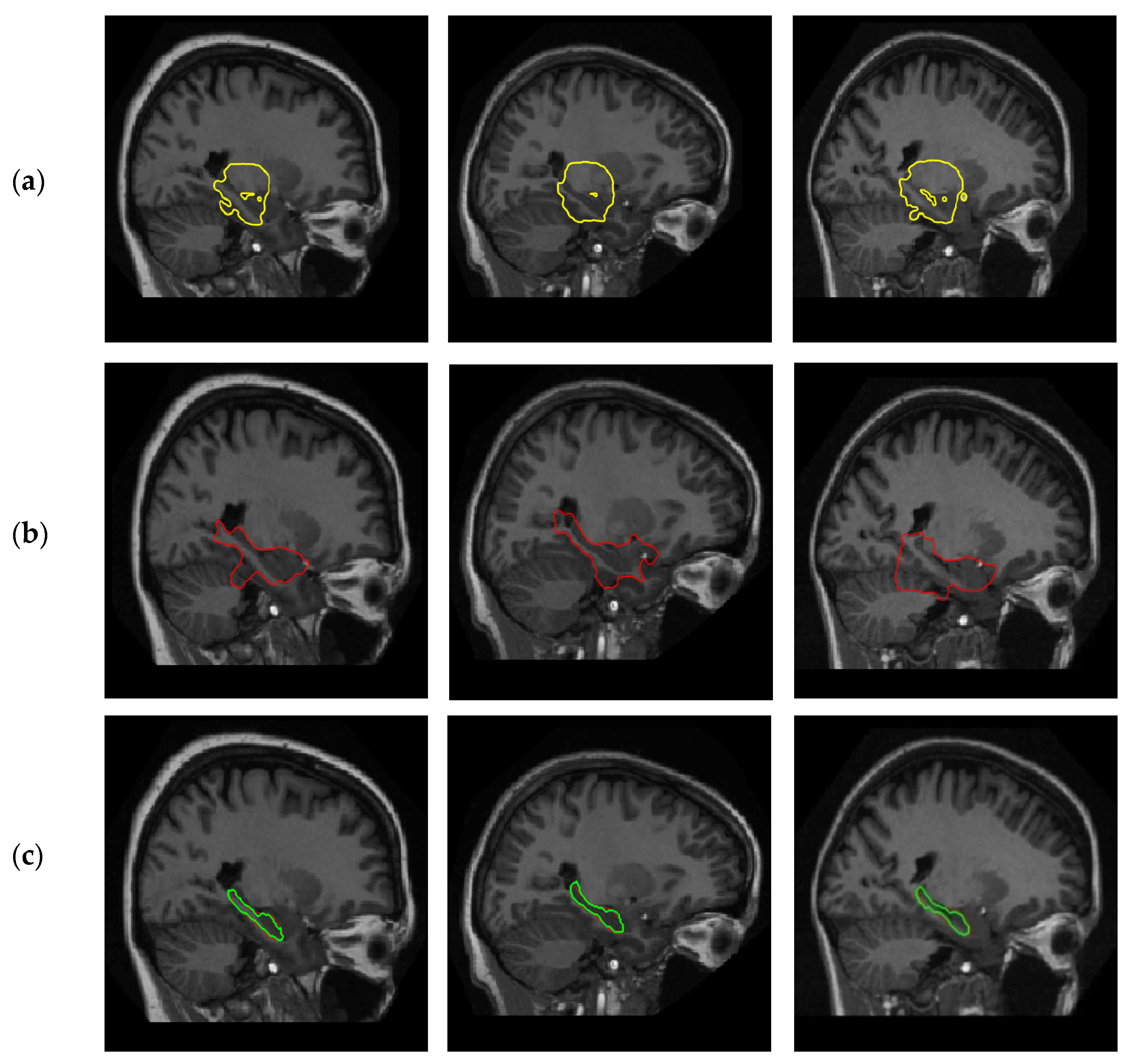
4. Results
4.1. Positive Effect of Shape Prior
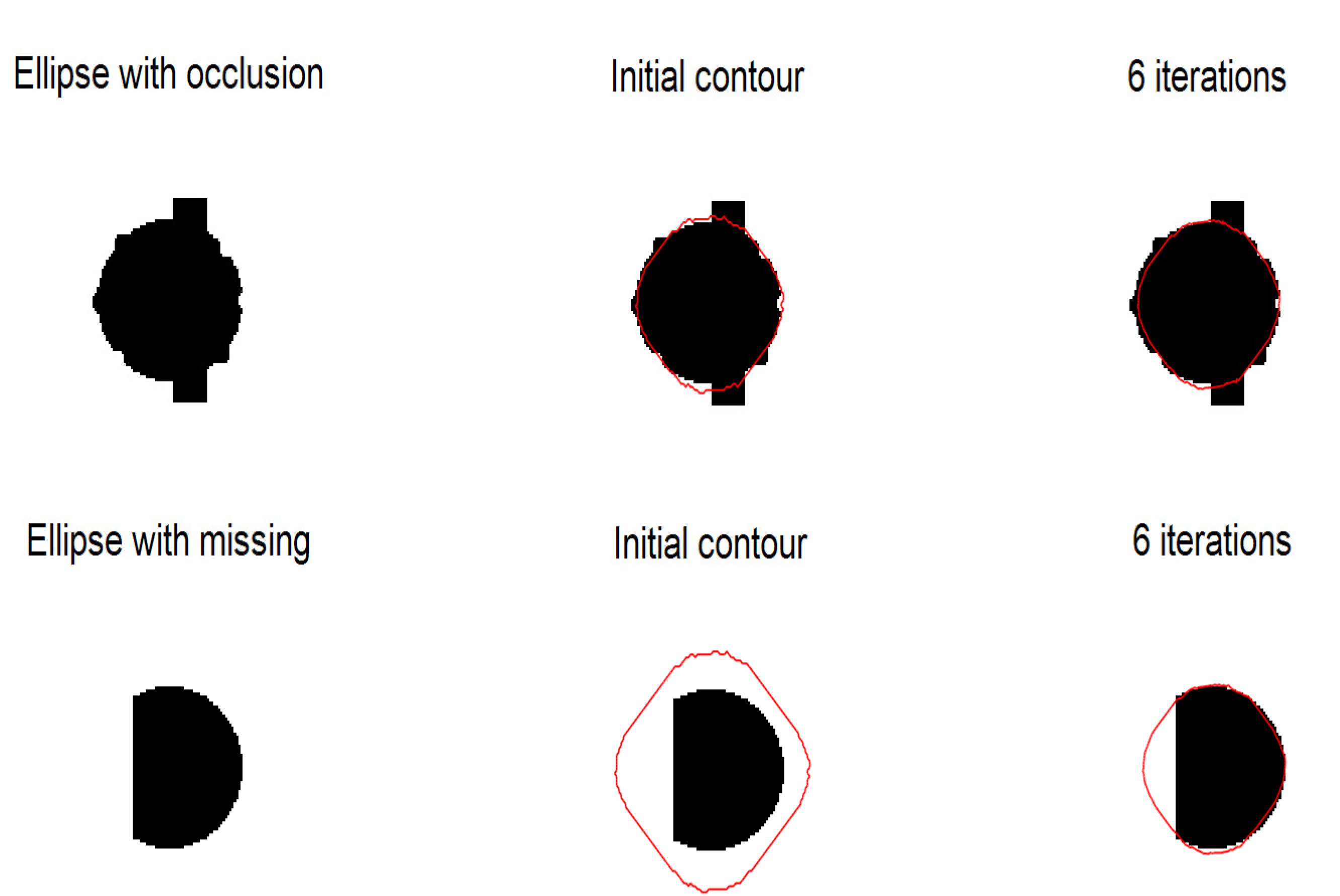
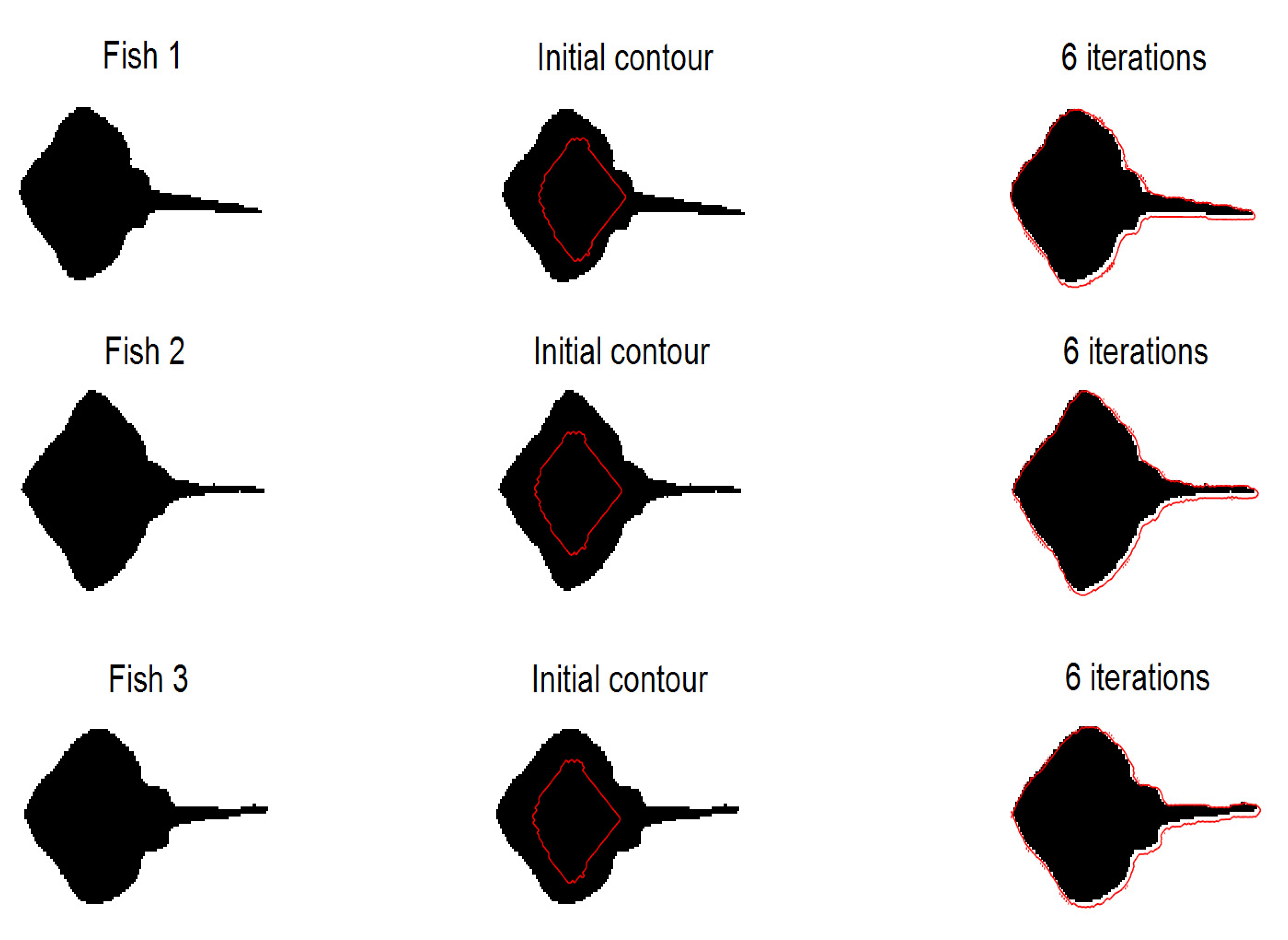
4.2. Sensitivity to Shape and Size Change
4.2.1. Experiments on Synthetic Images
4.2.2. Experiments on Hippocampus Images
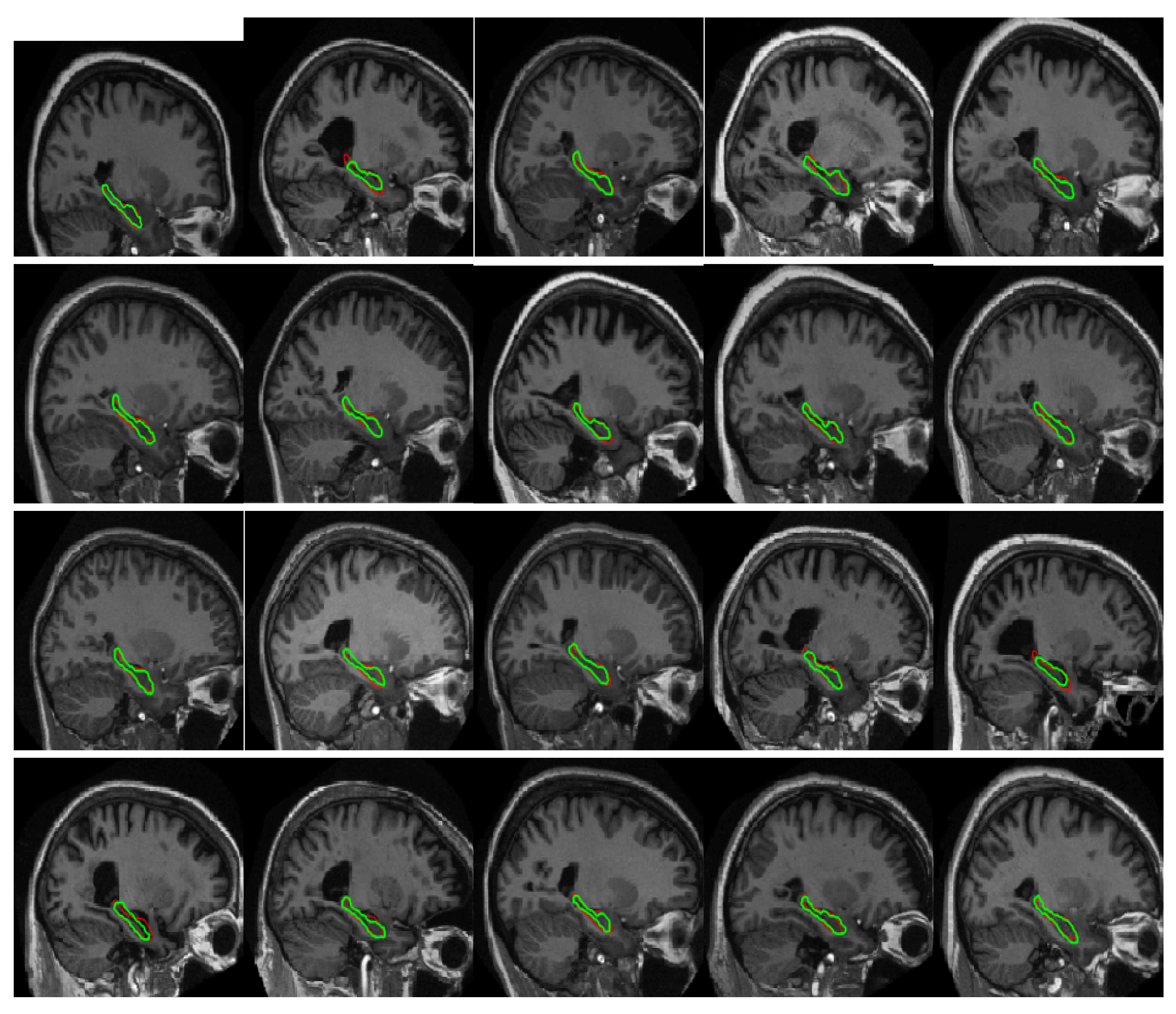
4.3. Comparison with Other Methods
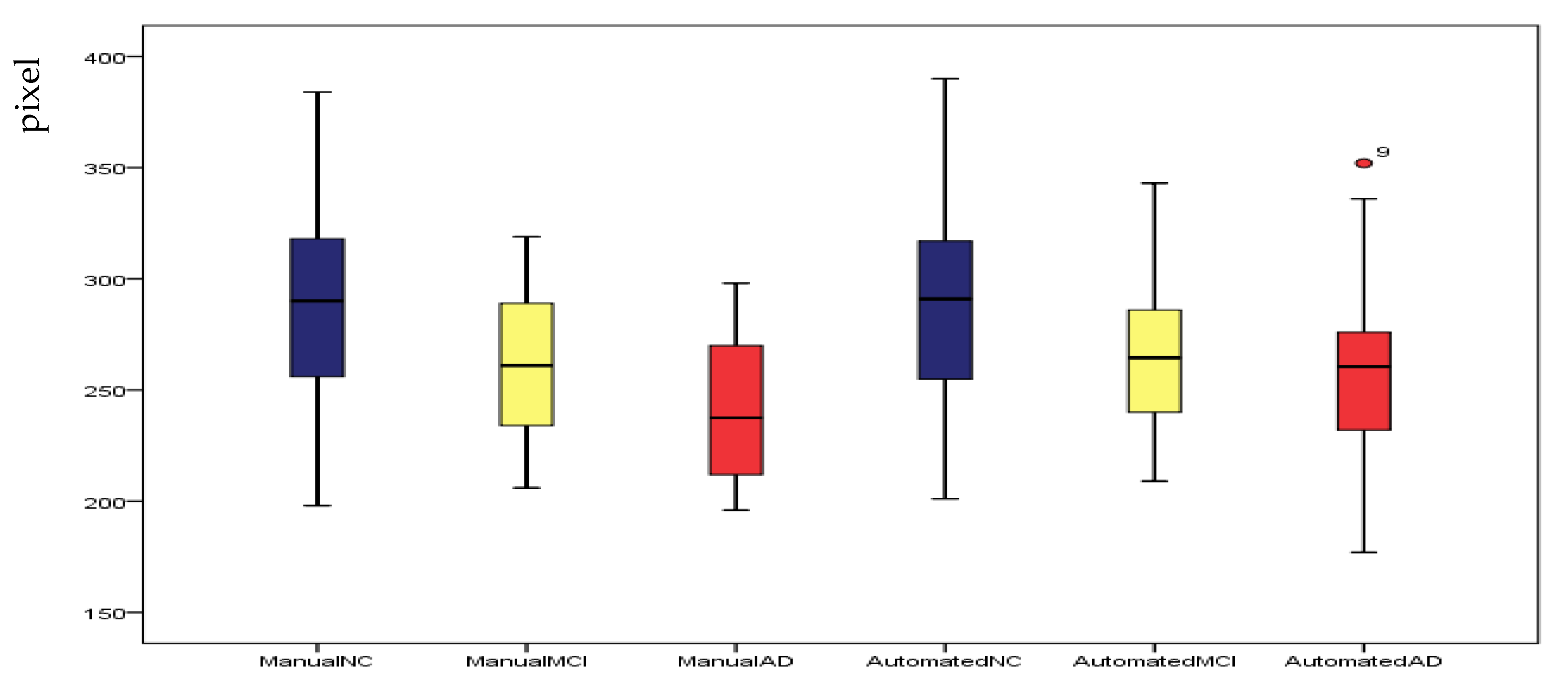
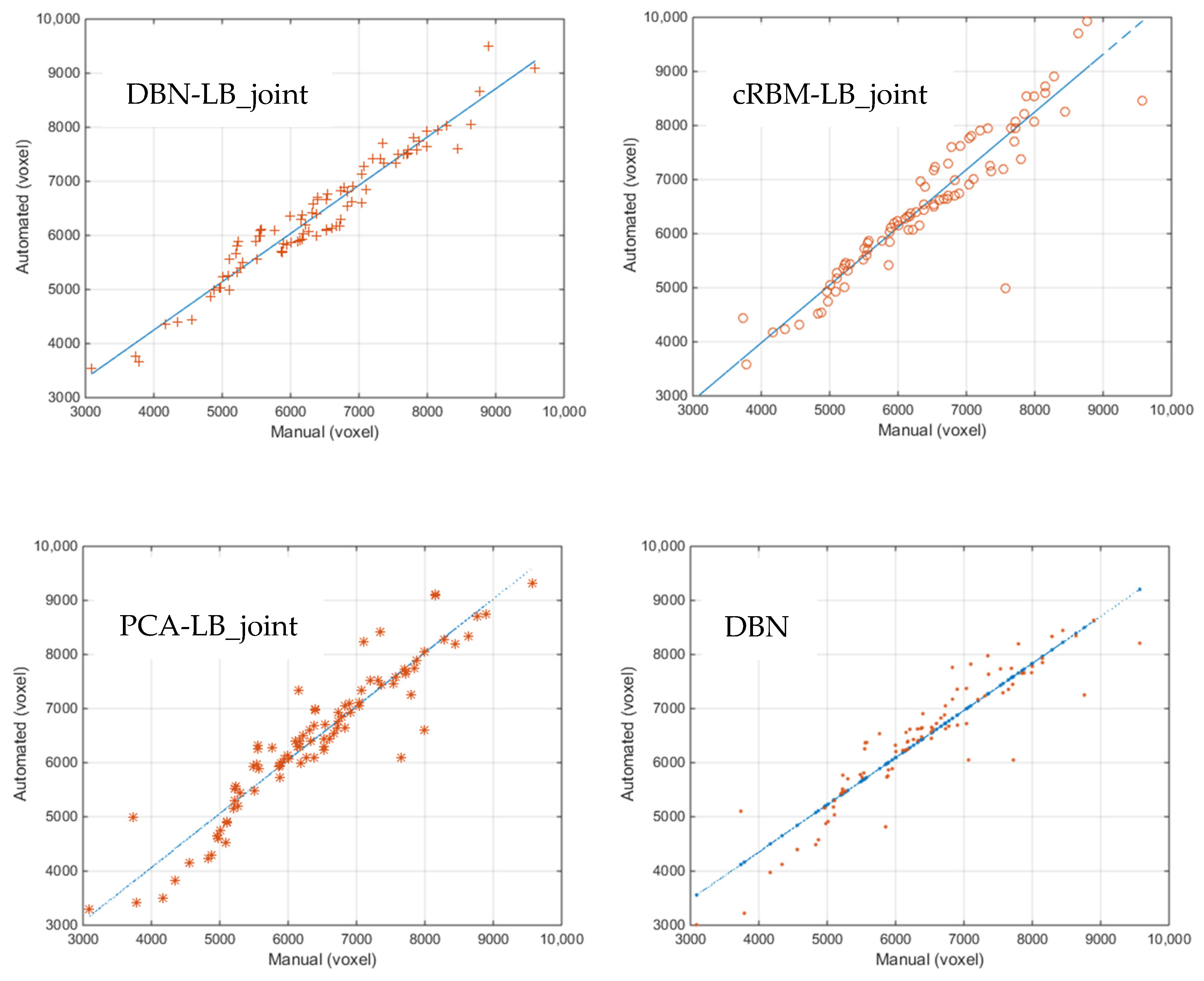
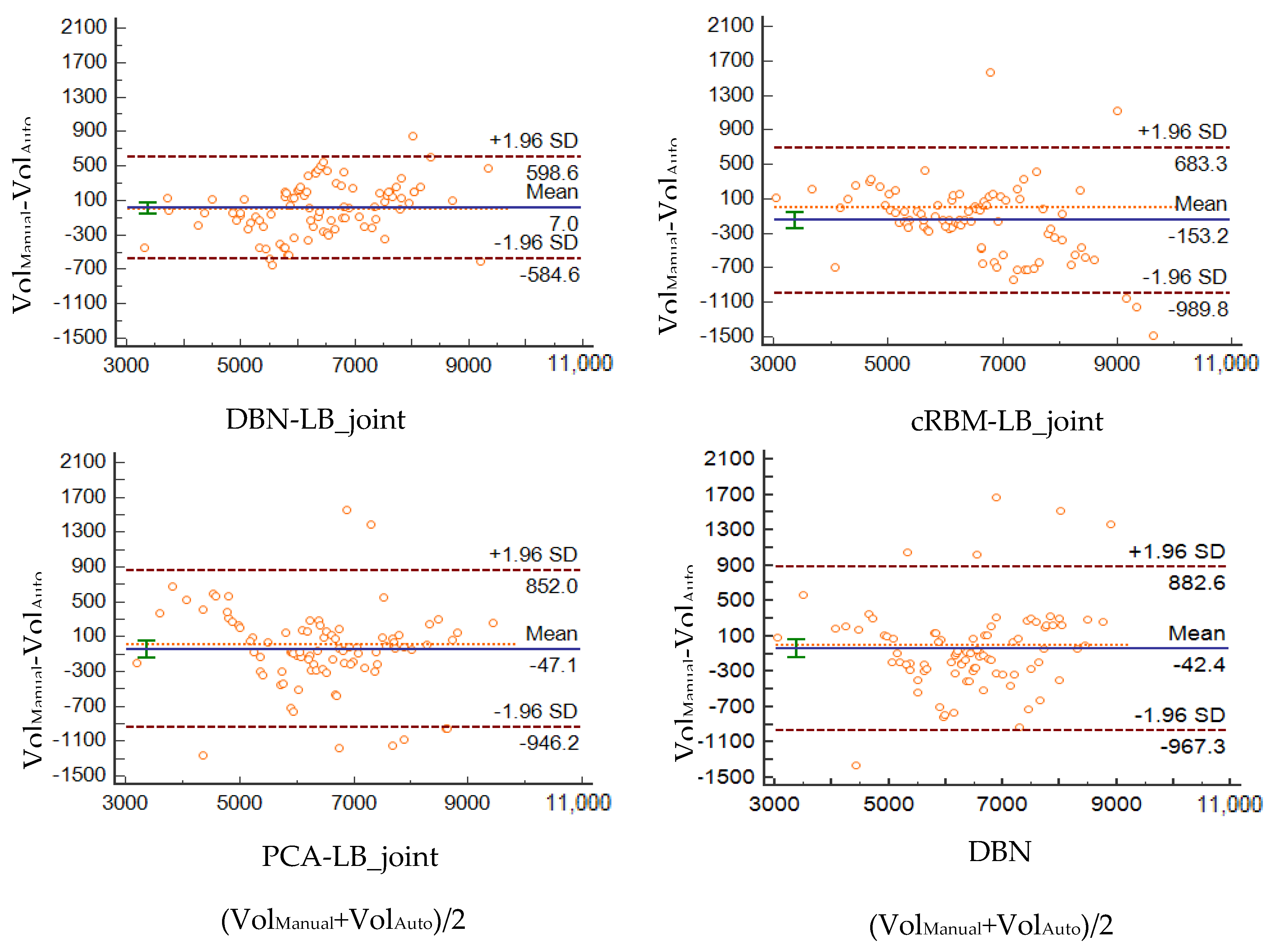
4.4. Correlation and Consistency
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dill, V.; Franco, A.R.; Pinho, M.S. Automated methods for hippocampus segmentation: The evolution and a review of the state of the art. Neuroinformatics 2015, 13, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Bron, E.E.; Smits, M.; van der Flier, W.M.; Vrenken, H.; Barkhof, F.; Scheltens, P.; Papma, J.M.; Steketee, R.M.E.; Orellana, C.M.; Meijboom, R.; et al. Standardized evaluation of algorithms for computer-aided diagnosis of dementia based on structural MRI: The CADDementia challenge. NeuroImage 2015, 111, 562–579. [Google Scholar] [CrossRef] [PubMed]
- Frost, R.; Wighton, P.; Karahanoğlu, F.I.; Robertson, R.L.; Grant, P.E.; Fischl, B.; Tisdall, M.D.; van der Kouwe, A. Markerless high-frequency prospective motion correction for neuroanatomical MRI. Magn. Reson. Med. 2019, 82, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Zarpalas, D.; Gkontra, P.; Daras, P.; Maglaveras, N. Gradient-based reliability maps for ACM-based segmentation of hippocampus. IEEE Trans. Bio-Med. Eng. 2014, 61, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Y.; Coupé, P.; Pruessner, J.C.; Collins, D.L. Appearance-based modeling for segmentation of Hippocampus and Amygdala using multi-contrast MR imaging. Neuroimage 2011, 58, 549–559. [Google Scholar] [CrossRef]
- Zhu, H.C.; Cheng, H.W.; Yang, X.S.; Fan, Y. Alzheimer’s Disease Neuroimaging Initiative. Metric learning for multi-atlas based segmentation of hippocampus. Neuroinformatics 2017, 15, 41–50. [Google Scholar] [CrossRef]
- Chen, Y.N.; Shi, B.B.; Wang, Z.W.; Zhang, P.; Smith, C.D.; Liu, J.D. Hippocampus segmentation through multi-view ensemble ConvNets. In Proceedings of the 14th International Symposium on Biomedical Imaging, Melbourne, Australia, 18–21 April 2017; IEEE: New York, NY, USA, 2017; pp. 192–196. [Google Scholar]
- Thyreau, B.; Sato, K.; Fukuda, H.; Taki, Y. Segmentation of the hippocampus by transferring algorithmic knowledge for large cohort processing. Med. Image Anal. 2018, 43, 214–228. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Eltanboly, A.; Ghazal, M.; Hajjdiab, H.; Shalaby, A.; Switala, A.; Mahmoud, A.; Sahoo, P.; EL-Azab, M.; El-Baz, A. Level sets-based image segmentation approach using statistical shape priors. Appl. Math. Comput. 2019, 340, 164–179. [Google Scholar] [CrossRef]
- Zarpalas, D.; Gkontra, P.; Daras, P.; Maglaveras, N. Accurate and fully automatic hippocampus segmentation using subject-specific 3D optimal local maps into a hybrid active contour model. IEEE J. Transl. Eng. Health Med.-JTEHM. 2014, 2, 1800116. [Google Scholar] [CrossRef]
- Hinton, G.E.; Salakhutdinov, R.R. Reducing the dimensionality of data with neural networks. Science 2006, 313, 504–507. [Google Scholar] [CrossRef]
- Agn, M.; Puonti, O.; Rosensch#xF6;ld, P.M.; Law, I.; Van Leemput, K. Brain tumor segmentation using a generative model with an RBM prior on tumor shape. In Brainlesion: Glioma, Multiple Sclerosis, Stroke and Traumatic Brain Injuries; Crimi, A., Menze, B., Maier, O., Reyes, M., Handels, H., Eds.; Springer: Cham, Switzerland, 2016; Volume 9556, pp. 168–180. [Google Scholar]
- Zhang, H.; Zhang, S.T.; Li, K.; Metaxas, D.N. Robust shape prior modeling based on Gaussian-Bernoulli restricted Boltzmann Machine. In Proceedings of the 11th IEEE International Symposium on Biomedical Imaging (ISBI), Beijing, China, 29 April–2 May 2014; IEEE: New York, NY, USA, 2010; pp. 270–273. [Google Scholar]
- Ngo, T.A.; Lu, Z.; Carneiro, G. Combining deep learning and level set for the automated segmentation of the left ventricle of the heart from cardiac cine magnetic resonance. Med. Image Anal. 2017, 35, 159–171. [Google Scholar] [CrossRef]
- Avendi, M.R.; Kheradvar, A.; Jafarkhani, H. A combined deep-learning and deformable-model approach to fully automatic segmentation of the left ventricle in cardiac MRI. Med. Image Anal. 2016, 30, 108–119. [Google Scholar] [CrossRef]
- Carneiro, G.; Nascimento, J.C. Combining multiple dynamic models and deep learning architectures for tracking the left ventricle endocardium in ultrasound data. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 2592–2607. [Google Scholar] [CrossRef] [PubMed]
- Fasel, I.; Berry, J. Deep belief networks for real-time extraction of tongue contours from ultrasound during speech. In Proceedings of the 20th International Conference on Pattern Recognition, Istanbul, Turkey, 23–26 August 2010; IEEE: New York, NY, USA, 2010; pp. 1493–1496. [Google Scholar]
- Yan, Z.Z.; Sun, Y.B.; Jiang, J.H.; Wen, J.L.; Lin, X. Novel explanation, modeling and realization of lattice Boltzmann methods for image processing. Multidimens. Syst. Signal Process. 2015, 26, 645–663. [Google Scholar] [CrossRef]
- Chen, J.H.; Chai, Z.H.; Shi, B.C.; Zhang, W.H. Lattice Boltzmann method for filtering and contour detection of the natural images. Comput. Math. Appl. 2014, 68, 257–268. [Google Scholar] [CrossRef]
- Liji, R.F.; Sasikumar, M.; Sreejaya, P.; Seelan, K.J. A comparative study and analysis of lattice Boltzmann method and exemplar method for still color image inpainting technique. In Proceedings of the 2nd International Conference on Intelligent Computing, Instrumentation and Control Technologies, Kannur, India, 5–6 July 2019; IEEE: New York, NY, USA, 2019; pp. 45–48. [Google Scholar]
- Li, C.; Balla-Arabé, S.; Ginhac, D.; Yang, F. Embedded implementation of VHR satellite image segmentation. Sensors 2016, 16, 771. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.W. A fast hybrid level set model for image segmentation using lattice Boltzmann method and sparse field constraint. Int. J. Pattern Recognit. Artif. Intell. 2018, 32, 1854015. [Google Scholar] [CrossRef]
- Nguyen, K.L.; Tekitek, M.M.; Delachartre, P.; Berthier, M. Multiple relaxation time lattice Boltzmann models for multigrid phase-field segmentation of tumors in 3D ultrasound images. SIAM J. Imaging Sci. 2019, 12, 1324–1346. [Google Scholar] [CrossRef]
- Chen, Y.; Navarro, L.; Wang, Y.; Courbebaisse, G. Segmentation of the thrombus of giant intracranial aneurysms from CT angiography scans with lattice Boltzmann method. Med. Image Anal. 2014, 18, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Navarro, L.; Zhang, Y.; Kao, E.; Zhu, Y.M.; Courbebaisse, G. Intracranial aneurysm phantom segmentation using a 4D lattice Boltzmann method. Comput. Sci. Eng. 2017, 19, 56–67. [Google Scholar] [CrossRef]
- Wen, J.L.; Jiang, J.H.; Yan, Z.Z. A new lattice Boltzmann algorithm for assembling local statistical information with MR brain imaging segmentation applications. Multidimens. Syst. Signal Process. 2017, 28, 1611–1627. [Google Scholar] [CrossRef]
- Wen, J.L. Hippocampus MRI Segmentation: A Method Based on Lattice Boltzmann Model. Ph.D. Thesis, Shanghai University, Shanghai, China, 2016. [Google Scholar]
- Agn, M.; Law, I.; Af Rosenschöld, P.M.; Van Leemput, K. A generative model for segmentation of tumor and organs-at-risk for radiation therapy planning of glioblastoma patients. In Medical Imaging 2016: Image Processing, Proceedings of the Conference on Medical Imaging-Image Processing, San Diego, CA, USA, 1–3 Mar 2016; SPIE International Society for Optical Engineering: Bellingham, WA, USA, 2016; p. 97841D. [Google Scholar]
- Hippocampus Segmentation Masks from Brain MRIs, Segmentation Masks of the Hippocampus from 23 Randomly Selected Images from the OASIS Dataset. Available online: http://vcl.iti.gr/hippocampus-segmentation/ (accessed on 27 December 2019).
- Wang, Z.Q.; Yan, Z.Z.; Chen, G. Lattice Boltzmann method of active contour for image segmentation. In Proceedings of the 6th International Conference on Image and Graphics, Hefei, China, 12–15 August 2011; IEEE: New York, NY, USA, 2011; pp. 338–343. [Google Scholar]
- Thivya Roopini, I.; Vasanthi, M.; Rajinikanth, V.; Rekha, M.; Sangeetha, M. Segmentation of tumor from brain MRI using fuzzy entropy and distance regularised level set. In Computational Signal Processing and Analysis, Proceedings of the International Conference on NextGen Electronic Technologies: Solicon to Software, VIT University, Chennai, India, 23–25 March 2017; Nandi, A., Sujatha, N., Menaka, R., Alex, J., Eds.; Springer: Singapore, 2018; pp. 297–304. [Google Scholar]
- Inglese, P.; Amoroso, N.; Boccardi, M.; Bocchetta, M.; Bruno, S.; Chincarini, A.; Errico, R.; Frisoni, G.B.; Maglietta, R.; Redolfi, A.; et al. Multiple RF classifier for the hippocampus segmentation: Method and validation on EADC-ADNI Harmonized Hippocampal Protocol. Phys. Medica 2015, 31, 1085–1091. [Google Scholar] [CrossRef]
- Zheng, Q.; Fan, Y. Integrating semi-supervised label propagation and random forests for multi-atlas based hippocampus segmentation. In Proceedings of the 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018; IEEE: New York, NY, USA, 2018; pp. 154–157. [Google Scholar]
- van der Lijn, F.; den Heijer, T.; Breteler, M.M.; Niessen, W.J. Hippocampus segmentation in MR images using atlas registration, voxel classification, and graph cuts. NeuroImage 2008, 43, 708–720. [Google Scholar] [CrossRef] [PubMed]
| Method | Method Description | |
|---|---|---|
| DBN | 0.84 ± 0.07 | DBN separately |
| PCA-LB_joint | 0.84 ± 0.06 | PCA driven LB |
| cRBM-LB_joint | 0.85 ± 0.06 | RBM driven LB |
| DBN-LB_jonit | 0.87 ± 0.05 | DBN driven LB |
| multiple Random Forest classifier [33] | 0.87 ± 0.03 | multiple Random Forest classifier |
| multi-atlas [34] | 0.88 ± 0.02 | integrating label propagation and random forests method |
| Deep learning [8] | 0.85 | deep convolutional neural networks |
| Method | Time (s/slice) | The Number of Iterations |
|---|---|---|
| DBN | 0.878 | |
| PCA-LB_joint | 6.035 | 11 |
| cRBM-LB_joint | 3.587 | 9 |
| DBN-LB_jonit | 2.220 | 6 |
| DRLS | 21.984 | 210 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Yan, Z. A Combined Deep-Learning and Lattice Boltzmann Model for Segmentation of the Hippocampus in MRI. Sensors 2020, 20, 3628. https://doi.org/10.3390/s20133628
Liu Y, Yan Z. A Combined Deep-Learning and Lattice Boltzmann Model for Segmentation of the Hippocampus in MRI. Sensors. 2020; 20(13):3628. https://doi.org/10.3390/s20133628
Chicago/Turabian StyleLiu, Yingqian, and Zhuangzhi Yan. 2020. "A Combined Deep-Learning and Lattice Boltzmann Model for Segmentation of the Hippocampus in MRI" Sensors 20, no. 13: 3628. https://doi.org/10.3390/s20133628
APA StyleLiu, Y., & Yan, Z. (2020). A Combined Deep-Learning and Lattice Boltzmann Model for Segmentation of the Hippocampus in MRI. Sensors, 20(13), 3628. https://doi.org/10.3390/s20133628





