Ultrasound Measurement of Skeletal Muscle Contractile Parameters Using Flexible and Wearable Single-Element Ultrasonic Sensor
Abstract
:1. Introduction
2. Methodology
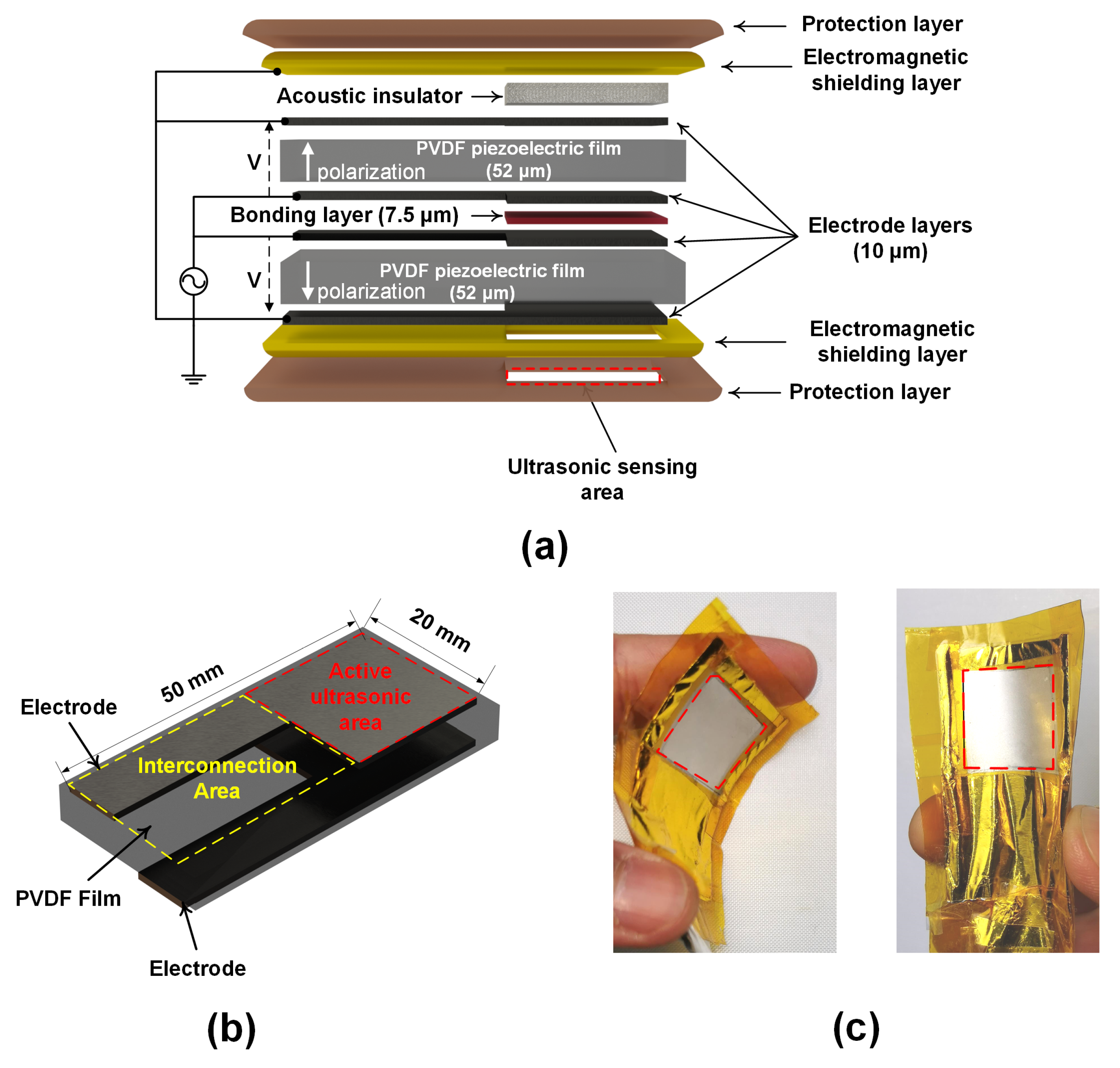
2.1. Wearable Ultrasonic Sensor
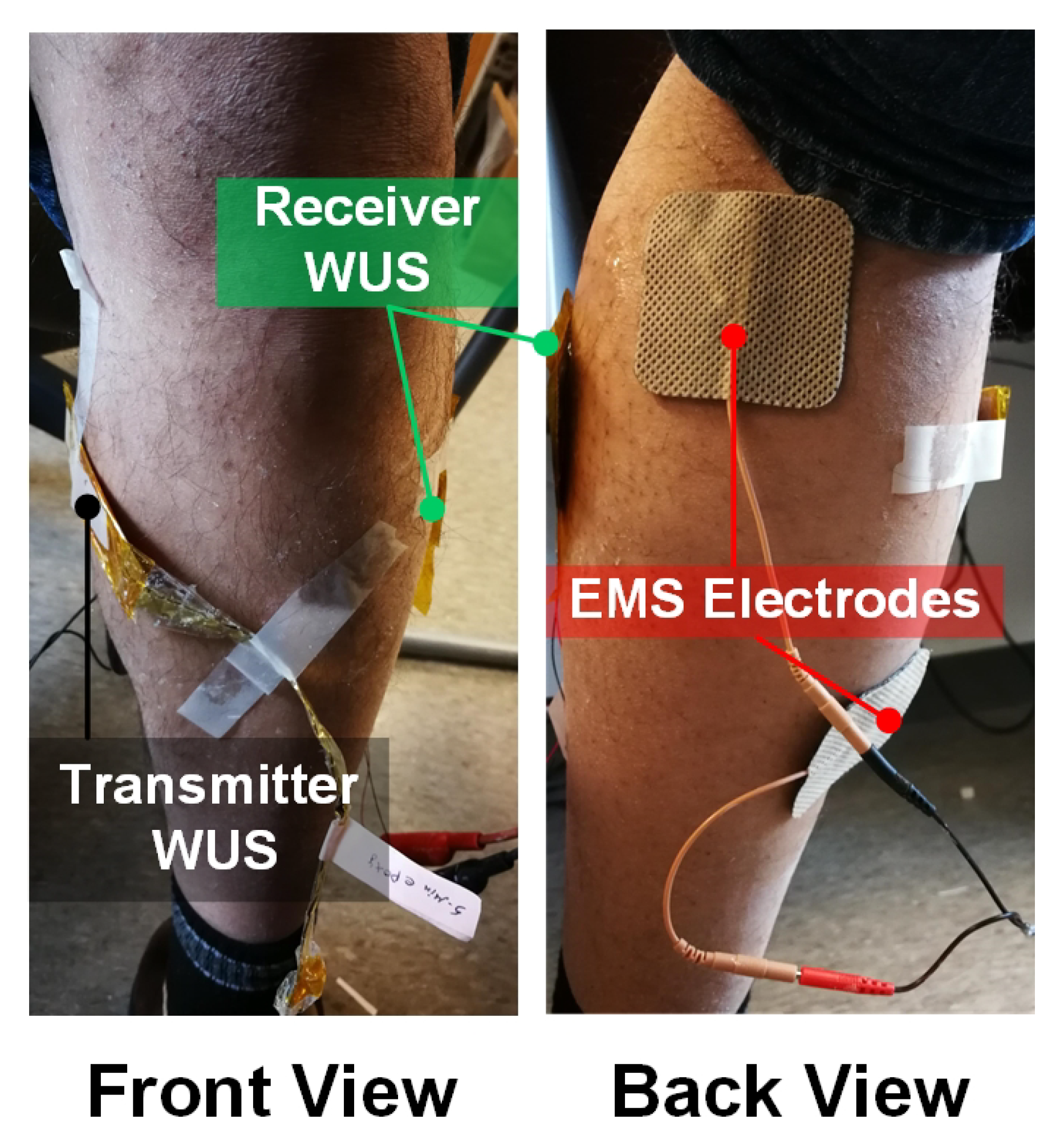
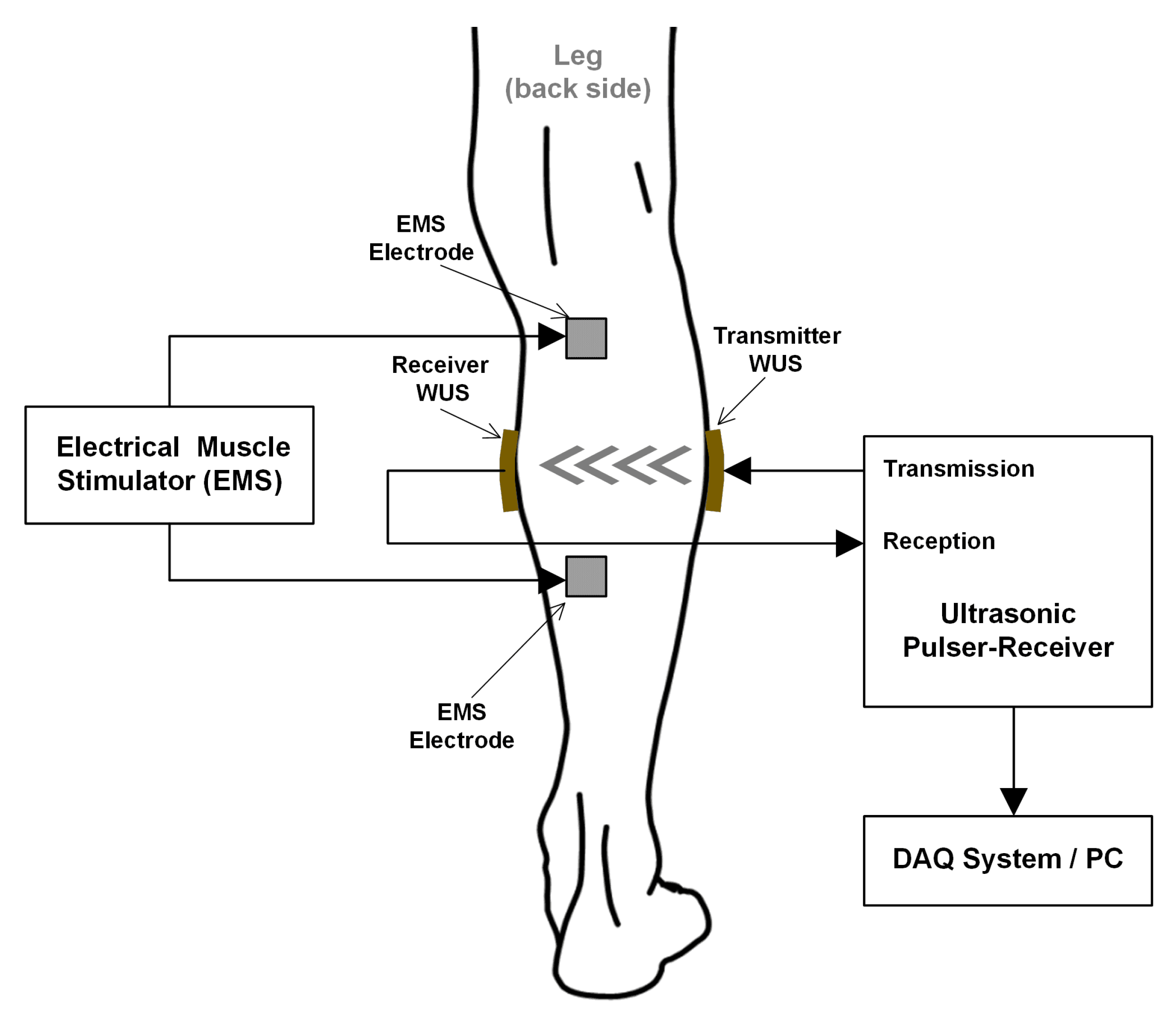
2.2. Experimental Configuration
3. Results and Discussions
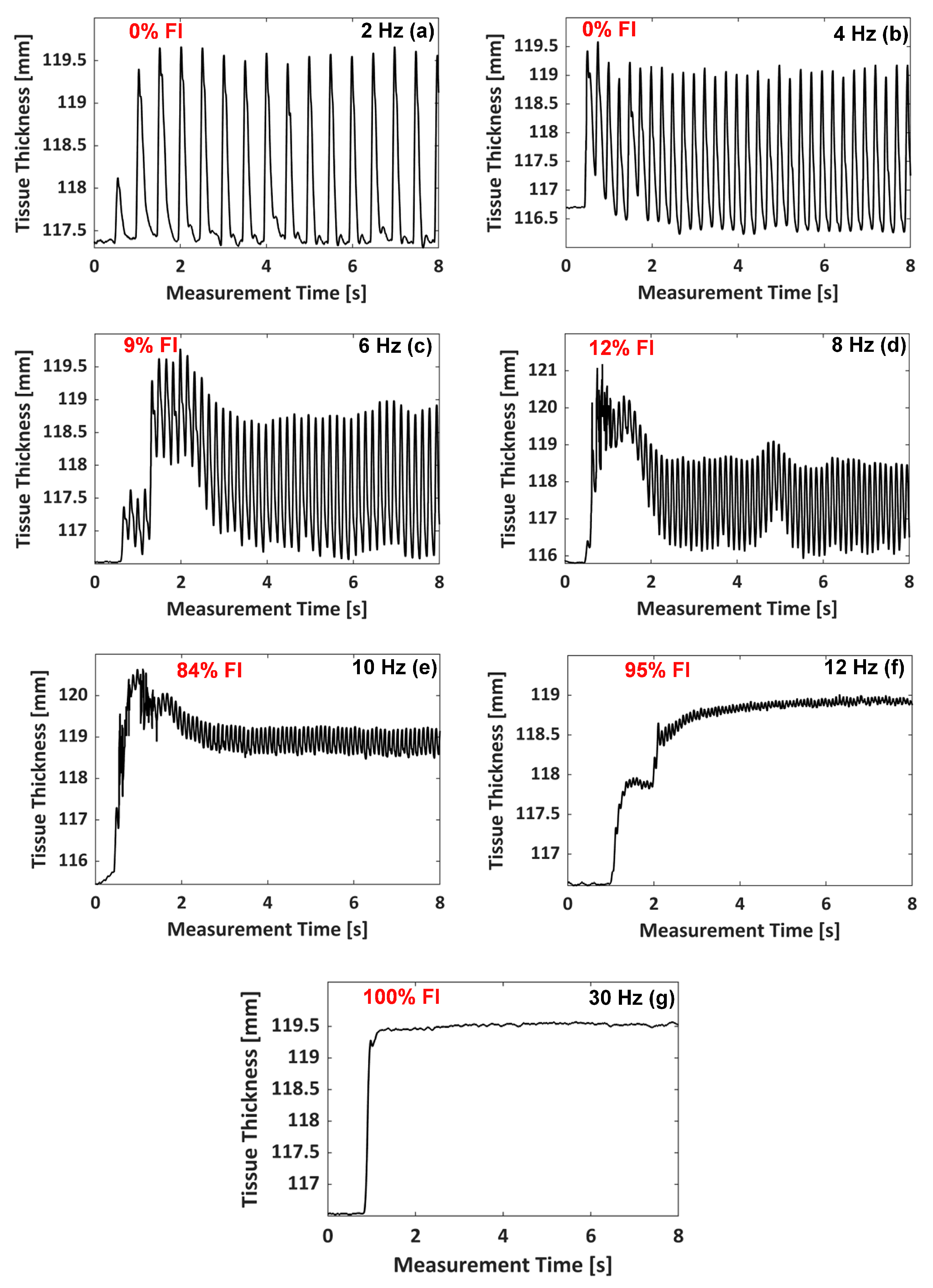
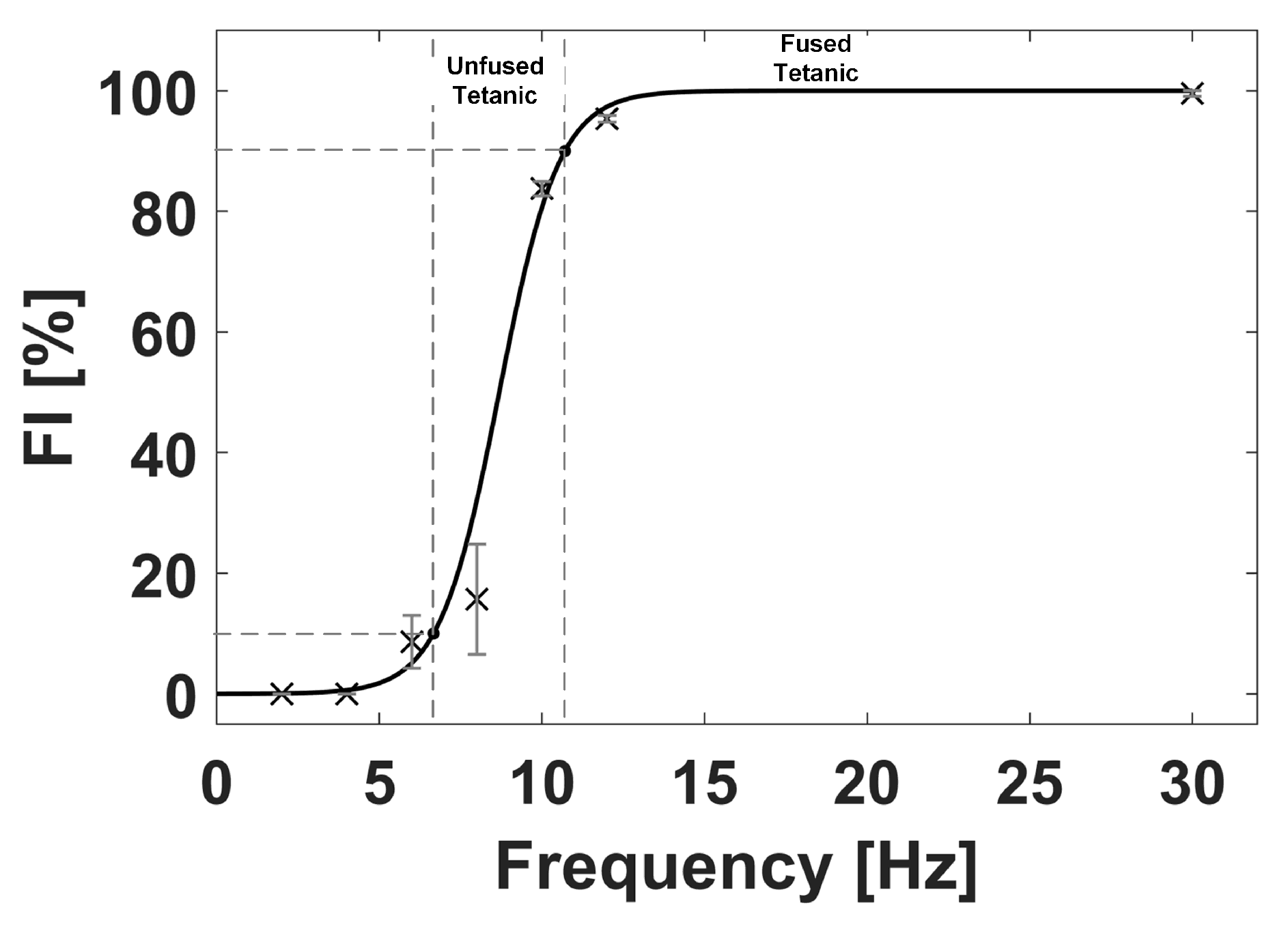
3.1. Monitoring of Muscle Tetanic Contractions
3.2. Muscle Contractile Parameters
4. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoshitake, Y.; Ue, H.; Miyazaki, M.; Moritani, T. Assessment of lower-back muscle fatigue using electromyography, mechanomyography, and near-infrared spectroscopy. Eur. J. Appl. Physiol. 2001, 84, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.Y.; Pourmajidian, M.; Hamzaid, N.A. Mechanomyography sensors for detection of muscle activities and fatigue during Fes-evoked contraction. In Proceedings of the IEEE International Functional Electrical Stimulation Society Annual Conference (IFESS), Kuala Lumpur, Malaysia, 17–19 September 2014; pp. 1–3. [Google Scholar] [CrossRef]
- Akataki, K.; Mita, K.; Itoh, K.; Suzuki, N.; Watakabe, M. Acoustic and electrical activities during voluntary isometric contraction of biceps brachii muscles in patients with spastic cerebral palsy. Muscle Nerve 1996, 19, 1252–1257. [Google Scholar] [CrossRef]
- Pedersen, S.W.; Bäckman, E.; Öberg, B. Characteristics of tetanic muscle contraction in Parkinson patients. Acta Neurol. Scand. 1991, 84, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Gea, J. Respiratory and Limb Muscle Dysfunction in COPD. J. Chronic Obstr. Pulm. Dis. (COPD) 2015, 12, 413–426. [Google Scholar] [CrossRef]
- Dias, P.S.; Fort, J.S.; Marinho, D.A.; Santos, A.; Marques, M.C. Tensiomyography in Physical Rehabilitation of High Level Athletes. Open Sports Sci. J. 2010, 3, 47–48. [Google Scholar] [CrossRef]
- Alvarez-Diaz, P.; Alentorn-Geli, E.; Ramon, S.; Marin, M.; Steinbacher, G.; Rius, M.; Seijas, R.; Ballester, J.; Cugat, R. Effects of anterior cruciate ligament reconstruction on neuromuscular tensiomyographic characteristics of the lower extremity in competitive male soccer players. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 3407–3413. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Alvarez-Diaz, P.; Ramon, S.; Marin, M.; Steinbacher, G.; Boffa, J.J.; Cuscó, X.; Ballester, J.; Cugat, R. Assessment of neuromuscular risk factors for anterior cruciate ligament injury through tensiomyography in male soccer players. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2508–2513. [Google Scholar] [CrossRef]
- Macgregor, L.J.; Hunter, A.M.; Orizio, C.; Fairweather, M.M.; Ditroilo, M. Assessment of Skeletal Muscle Contractile Properties by Radial Displacement: The Case for Tensiomyography. Sports Med. 2018, 48, 1607–1620. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; He, Q.; Zeng, L.; Zhou, Y.; Shen, M.; Dan, G. Motion intent recognition of individual fingers based on mechanomyogram. Pattern Recognit. Lett. 2017, 88, 41–48. [Google Scholar] [CrossRef]
- Lyons, K.R.; Joshi, S.S.; Joshi, S.S.; Lyons, K.R. Upper Limb Prosthesis Control for High-Level Amputees via Myoelectric Recognition of Leg Gestures. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1056–1066. [Google Scholar] [CrossRef]
- Lobo-Prat, J.; Kooren, P.N.; Stienen, A.H.A.; Herder, J.L.; Koopman, B.F.J.M.; Veltink, P.H. Non-invasive control interfaces for intention detection in active movement-assistive devices. J. Neuro Eng. Rehabil. 2014, 11, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, D.; Riener, R. A survey of sensor fusion methods in wearable robotics. Robot. Auton. Syst. 2015, 73, 155–170. [Google Scholar] [CrossRef]
- Baskin, R.; Paolini, P. Volume change and pressure development in muscle during contraction. Am. J. Physiol. Leg. Content 1967, 213, 1025–1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenčič, V.; Knez, N. Measuring of Skeletal Muscles’ Dynamic Properties. Artif. Organs 1997, 21, 240–242. [Google Scholar] [CrossRef] [PubMed]
- Pišot, R.; Narici, M.V.; Šimunič, B.; De Boer, M.; Seynnes, O.; Jurdana, M.; Biolo, G.; Mekjavić, I.B. Whole muscle contractile parameters and thickness loss during 35-day bed rest. Eur. J. Appl. Physiol. 2008, 104, 409–414. [Google Scholar] [CrossRef]
- Than, C.; Tosovic, D.; Seidl, L.; Mark Brown, J. The effect of exercise hypertrophy and disuse atrophy on muscle contractile properties: A mechanomyographic analysis. Eur. J. Appl. Physiol. 2016, 116, 2155–2165. [Google Scholar] [CrossRef]
- Dahmane, R.; Valenčič, V.; Knez, N.; Eržen, I. Evaluation of the ability to make non-invasive estimation of muscle contractile properties on the basis of the muscle belly response. Med. Biol. Eng. Comput. 2001, 39, 51–55. [Google Scholar] [CrossRef]
- Dahmane, R.; Djordjevič, S.; Šimunič, B.; Valenčič, V. Spatial fiber type distribution in normal human muscle. J. Biomech. 2005, 38, 2451–2459. [Google Scholar] [CrossRef]
- García-manso, J.M.; Rodríguez-Ruiz, D.; Rodríguez-Matoso, D.; de Saa, Y.; Sarmiento, S.; Quiroga, M. Assessment of muscle fatigue after an ultra-endurance triathlon using tensiomyography (TMG). J. Sports Sci. 2011, 29, 619–625. [Google Scholar] [CrossRef]
- Carrasco, L.; Sañudo, B.; de Hoyo, M.; Pradas, F.; Silva, M.E.D. Effectiveness of low-frequency vibration recovery method on blood lactate removal, muscle contractile properties and on time to exhaustion during cycling at VO2max power output. Eur. J. Appl. Physiol. 2011, 111, 2271–2279. [Google Scholar] [CrossRef] [Green Version]
- García-Manso, J.M.; Rodríguez-Matoso, D.; Sarmiento, S.; de Saa, Y.; Vaamonde, D.; Rodríguez-Ruiz, D.; Silva-Grigoletto, M.E.D. Effect of high-load and high-volume resistance exercise on the tensiomyographic twitch response of biceps brachii. J. Electromyogr. Kinesiol. 2012, 22, 612–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macgregor, L.J.; Ditroilo, M.; Smith, I.J.; Fairweather, M.M.; Hunter, A.M. Reduced Radial Displacement of the Gastrocnemius Medialis Muscle After Electrically Elicited Fatigue. J. Sport Rehabil. 2016, 25, 241–247. [Google Scholar] [CrossRef] [PubMed]
- de Paula Simola, R.Á.; Raeder, C.; Wiewelhove, T.; Kellmann, M.; Meyer, T.; Pfeiffer, M.; Ferrauti, A. Muscle mechanical properties of strength and endurance athletes and changes after one week of intensive training. J. Electromyogr. Kinesiol. 2016, 30, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cè, E.; Longo, S.; Limonta, E.; Coratella, G.; Rampichini, S.; Esposito, F. Peripheral fatigue: New mechanistic insights from recent technologies. Eur. J. Appl. Physiol. 2020, 120, 17–39. [Google Scholar] [CrossRef]
- Hunter, A.M.; Galloway, S.D.; Smith, I.J.; Tallent, J.; Ditroilo, M.; Fairweather, M.M.; Howatson, G. Assessment of eccentric exercise-induced muscle damage of the elbow flexors by tensiomyography. J. Electromyogr. Kinesiol. 2012, 22, 334–341. [Google Scholar] [CrossRef]
- Loturco, I.; Gil, S.; Laurino, C.F.D.S.; Roschel, H.; Kobal, R.; Cal Abad, C.C.; Nakamura, F.Y. Differences in Muscle Mechanical Properties Between Elite Power and Endurance Athletes. J. Strength Cond. Res. 2015, 29, 1723–1728. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Montalvo, Z.; Sánchez-Martínez, G.; Torrontegi, E.; De La Calle-Herrero, J.; Domínguez-castells, R.; Maffiuletti, N.A.; De La Villa, P. Relationship between skeletal muscle contractile properties and power production capacity in female Olympic rugby players. Eur. J. Sport Sci. 2018, 18, 677–684. [Google Scholar] [CrossRef]
- Papagiannis, G.I.; Triantafyllou, A.I.; Roumpelakis, I.M.; Zampeli, F.; Eleni, P.G.; Koulouvaris, P.; Papadopoulos, E.C.; Papagelopoulos, P.J.; Babis, G.C. Methodology of surface electromyography in gait analysis: Review of the literature. J. Med. Eng. Technol. 2019, 43, 59–65. [Google Scholar] [CrossRef]
- Devasahayam, S.R. The Electromyogram. In Signals and Systems in Biomedical Engineering; Springer: Boston, MA, USA, 2013; Chapter 11; pp. 253–279. [Google Scholar] [CrossRef]
- Kuriki, H.U.; de Azevedo, F.M.; Takahashi, L.S.O.; Mello, E.M.; Negrao Filho, R.d.F.; Alves, N. The Relationship Between Electromyography and Muscle Force. In EMG Methods for Evaluating Muscle and Nerve Function; Schwartz, M., Ed.; IntechOpen: London, UK, 2012; Chapter 3; pp. 31–54. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, R.; Reaz, M.; Ali, M.; Bakar, A.; Chellappan, K.; Chang, T. Surface Electromyography Signal Processing and Classification Techniques. Sensors 2013, 13, 12431–12466. [Google Scholar] [CrossRef]
- Ibitoye, M.O.; Hamzaid, N.A.; Zuniga, J.M.; Abdul Wahab, A.K. Mechanomyography and muscle function assessment: A review of current state and prospects. Clin. Biomech. 2014, 29, 691–704. [Google Scholar] [CrossRef]
- Orizio, C.; Liberati, D.; Locatelli, C.; Grandis, D.D.; Veicsteinas, A. Surface mechanomyogram reflects muscle fibres twitches summation. J. Biomech. 1996, 29, 475–481. [Google Scholar] [CrossRef]
- Islam, M.A.; Sundaraj, K.; Ahmad, R.B.; Ahamed, N.U.; Ali, M.A. Mechanomyography sensor development, related signal processing, and applications: A systematic review. IEEE Sens. J. 2013, 13, 2499–2516. [Google Scholar] [CrossRef]
- Orizio, C. Surface Mechanomyogram. In Electromyography, 1st ed.; Merlett, R., Parker, P., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; Chapter 11; pp. 305–322. [Google Scholar] [CrossRef]
- Talib, I.; Sundaraj, K.; Lam, C.K.; Sundaraj, S. A systematic review of muscle activity assessment of the biceps brachii muscle using mechanomyography. J. Musculoskelet. Neuronal Interact. 2018, 18, 446–462. [Google Scholar] [PubMed]
- Watakabe, M.; Mita, K.; Akataki, K.; Itoh, Y. Mechanical behaviour of condenser microphone in mechanomyography. Med Biol. Eng. Comput. 2001, 39, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.W.; Housh, T.J.; Johnson, G.O.; Weir, J.P.; Cramer, J.T.; Coburn, J.W.; Malek, M.H. Comparison of a piezoelectric contact sensor and an accelerometer for examining mechanomyographic amplitude and mean power frequency versus torque relationships during isokinetic and isometric muscle actions of the biceps brachii. J. Electromyogr. Kinesiol. 2006, 16, 324–335. [Google Scholar] [CrossRef]
- Qi, L.; Wakeling, J.M.; Ferguson-Pell, M. Spectral properties of electromyographic and mechanomyographic signals during dynamic concentric and eccentric contractions of the human biceps brachii muscle. J. Electromyogr. Kinesiol. 2011, 21, 1056–1063. [Google Scholar] [CrossRef]
- Jaskólska, A.; Madeleine, P.; Jaskólski, A.; Kisiel-Sajewicz, K.; Arendt-Nielsen, L. A comparison between mechanomyographic condenser microphone and accelerometer measurements during submaximal isometric, concentric and eccentric contractions. J. Electromyogr. Kinesiol. 2007, 17, 336–347. [Google Scholar] [CrossRef]
- Stock, M.S.; Beck, T.W.; DeFreitas, J.M.; Dillon, M.A. Linearity and reliability of the mechanomyographic amplitude versus dynamic constant external resistance relationships for the biceps brachii. Physiol. Meas. 2010, 31, 1487–1498. [Google Scholar] [CrossRef]
- Stock, M.S.; Beck, T.W.; DeFreitas, J.M.; Dillon, M.A. Linearity and reliability of the mechanomyographic amplitude versus dynamic torque relationships for the superficial quadriceps femoris muscles. Muscle Nerve 2010, 41, 342–349. [Google Scholar] [CrossRef]
- Hendrix, C.R.; Housh, T.J.; Camic, C.L.; Zuniga, J.M.; Johnson, G.O.; Schmidt, R.J. Comparing electromyographic and mechanomyographic frequency-based fatigue thresholds to critical torque during isometric forearm flexion. J. Neurosci. Methods 2010, 194, 64–72. [Google Scholar] [CrossRef]
- Uchiyama, T.; Shinohara, K. System identification of mechanomyograms detected with an acceleration sensor and a laser displacement meter. In Proceedings of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 7131–7134. [Google Scholar] [CrossRef]
- Tosovic, D.; Than, C.; Brown, J.M.M. The effects of accumulated muscle fatigue on the mechanomyographic waveform: Implications for injury prediction. Eur. J. Appl. Physiol. 2016, 116, 1485–1494. [Google Scholar] [CrossRef]
- Seidl, L.; Tosovic, D.; Brown, J.M. Test-Retest Reliability and Reproducibility of Laser-versus Contact- Displacement Sensors in Mechanomyography: Implications for Musculoskeletal Research. J. Appl. Biomech. 2017, 33, 130–136. [Google Scholar] [CrossRef]
- Than, C.; Seidl, L.; Tosovic, D.; Brown, J.M. Test-retest reliability of laser displacement mechanomyography in paraspinal muscles while in lumbar extension or flexion. J. Electromyogr. Kinesiol. 2018, 41, 60–65. [Google Scholar] [CrossRef]
- Beck, T.W.; DeFreitas, J.M.; Stock, M.S. The linearity and reliability of the mechanomyographic amplitude versus submaximal isometric force relationship. Physiol. Meas. 2009, 30, 1009–1016. [Google Scholar] [CrossRef]
- Posatskiy, A.O.; Chau, T. The effects of motion artifact on mechanomyography: A comparative study of microphones and accelerometers. J. Electromyogr. Kinesiol. 2012, 22, 320–324. [Google Scholar] [CrossRef]
- Ryan, E.D.; Cramer, J.T.; Housh, T.J.; Beck, T.W.; Herda, T.J.; Hartman, M.J. Inter-individual variability in the torque-related patterns of responses for mechanomyographic amplitude and mean power frequency. J. Neurosci. Methods 2007, 161, 212–219. [Google Scholar] [CrossRef]
- Herda, T.J.; Ryan, E.D.; Beck, T.W.; Costa, P.B.; DeFreitas, J.M.; Stout, J.R.; Cramer, J.T. Reliability of mechanomyographic amplitude and mean power frequency during isometric step and ramp muscle actions. J. Neurosci. Methods 2008, 171, 104–109. [Google Scholar] [CrossRef]
- Al-Zahrani, E.; Gunasekaran, C.; Callaghan, M.; Gaydecki, P.; Benitez, D.; Oldham, J. Within-day and between-days reliability of quadriceps isometric muscle fatigue using mechanomyography on healthy subjects. J. Electromyogr. Kinesiol. 2009, 19, 695–703. [Google Scholar] [CrossRef]
- Cè, E.; Rampichini, S.; Limonta, E.; Esposito, F. Reliability of the Electromechanical Delay Components Assessment during the Relaxation Phase. Physiol. J. 2013, 2013, 517838. [Google Scholar] [CrossRef] [Green Version]
- Cè, E.; Rampichini, S.; Esposito, F. Novel insights into skeletal muscle function by mechanomyography: From the laboratory to the field. Sport Sci. Health 2015, 11, 1–28. [Google Scholar] [CrossRef]
- García-García, O.; Cuba-Dorado, A.; Álvarez-Yates, T.; Carballo-López, J.; Iglesias-Caamaño, M. Clinical utility of tensiomyography for muscle function analysis in athletes. Open Access J. Sports Med. 2019, 10, 49–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Križaj, D.; Šimunič, B.; Žagar, T. Short-term repeatability of parameters extracted from radial displacement of muscle belly. J. Electromyogr. Kinesiol. 2008, 18, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Wiewelhove, T.; Raeder, C.; de Paula Simola, R.A.; Schneider, C.; Döweling, A.; Ferrauti, A. Tensiomyographic Markers Are Not Sensitive for Monitoring Muscle Fatigue in Elite Youth Athletes: A Pilot Study. Front. Physiol. 2017, 8, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saúl Martín-Rodríguez and Irineu Loturco and Angus M. Hunter and David Rodríguez-Ruiz and Diego Munguia-Izquierdo. Reliability and Measurement Error of Tensiomyography to Assess Mechanical Muscle Function. J. Strength Cond. Res. 2017, 31, 3524–3536. [Google Scholar] [CrossRef] [PubMed]
- Nazarian, L.N. The Top 10 Reasons Musculoskeletal Sonography Is an Important Complementary or Alternative Technique to MRI. Am. J. Roentgenol. 2008, 190, 1621–1626. [Google Scholar] [CrossRef] [Green Version]
- Hodges, P.; Pengel, L.; Herbert, R.; Gandevia, S. Measurement of muscle contraction with ultrasound imaging. Muscle Nerve 2003, 27, 682–692. [Google Scholar] [CrossRef]
- Kwah, L.K.; Pinto, R.Z.; Diong, J.; Herbert, R.D. Reliability and validity of ultrasound measurements of muscle fascicle length and pennation in humans: A systematic review. J. Appl. Physiol. 2013, 114, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Zheng, Y.; Chen, X.; Xie, H. Modeling the relationship between wrist angle and muscle thickness during wrist flexion-extension based on the bone-muscle lever system: A comparison study. Med. Eng. Phys. 2009, 31, 1255–1260. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, Y.P.; Guo, J.Y.; Shi, J. Sonomyography (smg) control for powered prosthetic hand: A Study with normal subjects. Ultrasound Med. Biol. 2010, 36, 1076–1088. [Google Scholar] [CrossRef]
- Koo, T.K.; Wong, C.; Zheng, Y. Reliability of Sonomyography for Pectoralis Major Thickness Measurement. J. Manip. Physiol. Ther. 2010, 33, 386–394. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Stokes, M. Ultrasound Imaging and Muscle Function. J. Orthop. Sports Phys. Therapy 2011, 41, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zheng, Y.P.; Guo, J.Y.; Zhu, Z.; Chan, S.C.; Zhang, Z. Sonomyographic responses during voluntary isometric ramp contraction of the human rectus femoris muscle. Eur. J. Appl. Physiol. 2012, 112, 2603–2614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.; Zheng, Y.P. Human motion analysis with ultrasound and sonomyography. In Proceedings of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 6479–6482. [Google Scholar] [CrossRef]
- Dieterich, A.V.; Pickard, C.M.; Strauss, G.R.; Deshon, L.E.; Gibson, W.; McKay, J. Muscle thickness measurements to estimate gluteus medius and minimus activity levels. Manual Ther. 2014, 19, 453–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Zhou, Y.; Lu, Y.; Zhou, G.; Wang, L.; Zheng, Y.P. The sensitive and efficient detection of quadriceps muscle thickness changes in cross-sectional plane using ultrasonography: A feasibility investigation. IEEE J. Biomed. Health Inform. 2014, 18, 628–635. [Google Scholar] [CrossRef]
- Qiu, S.; Feng, J.; Xu, J.; Xu, R.; Zhao, X.; Zhou, P.; Qi, H.; Zhang, L.; Ming, D. Sonomyography Analysis on Thickness of Skeletal Muscle during Dynamic Contraction Induced by Neuromuscular Electrical Stimulation: A Pilot Study. IEEE Trans. Neural Syst. Rehabilit. Eng. 2017, 25, 59–67. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, X.; Shi, W.; Wang, J.; Xiang, Y.; Zhou, Y.; Yang, W.Z. Ultrasonic Measurement of Dynamic Muscle Behavior for Poststroke Hemiparetic Gait. BioMed Res. Int. 2017, 2017, 8208764. [Google Scholar] [CrossRef]
- Zhou, G.Q.; Zheng, Y.P.; Zhou, P. Measurement of Gender Differences of Gastrocnemius Muscle and Tendon Using Sonomyography during Calf Raises: A Pilot Study. BioMed Res. Int. 2017, 2017, 6783824. [Google Scholar] [CrossRef] [Green Version]
- Jahanandish, M.H.; Fey, N.P.; Hoyt, K. Lower Limb Motion Estimation Using Ultrasound Imaging: A Framework for Assistive Device Control. IEEE J. Biomed. Health Inform. 2019, 23, 2505–2514. [Google Scholar] [CrossRef]
- Yang, X.; Yan, J.; Liu, H. Comparative Analysis of Wearable A-mode Ultrasound and sEMG for Muscle-Computer Interface. IEEE Trans. Biomed. Eng. 2019. [Google Scholar] [CrossRef]
- Macedo Fraiz, G.; Herminia Gallo, L.; Iraci Rabito, E.; Silveira Gomes, A.R.; Madalozzo Schieferdecker, M.E. Relationship between muscle thickness and calf circumference in healthy older women. Arch. Gerontol. Geriatr. 2020, 86, 103942. [Google Scholar] [CrossRef]
- Abraham, A.; Drory, V.E.; Fainmesser, Y.; Lovblom, L.E.; Bril, V. Quantitative sonographic evaluation of muscle thickness and fasciculation prevalence in healthy subjects. Muscle Nerve 2020, 61, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Ono, Y.; Adler, A. An ultrasonic technique for imaging of tissue motion due to muscle contraction. In Proceedings of the IEEE International Ultrasonics Symposium, Rome, Italy, 20–23 September 2009; pp. 2441–2444. [Google Scholar] [CrossRef]
- Abraham, A.; Drory, V.E.; Fainmesser, Y.; Algom, A.A.; Lovblom, L.E.; Bril, V. Muscle thickness measured by ultrasound is reduced in neuromuscular disorders and correlates with clinical and electrophysiological findings. Muscle Nerve 2019, 60, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Zheng, Y.P.; Huang, Q.H.; Chen, X. Dynamic monitoring of forearm muscles using one-dimensional sonomyography system. J. Rehabil. Res. Dev. 2008, 45, 187–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuehne, T.E.; Yitzchaki, N.; Jessee, M.B.; Graves, B.S.; Buckner, S.L. A comparison of acute changes in muscle thickness between A-mode and B-mode ultrasound. Physiol. Meas. 2019, 40, 115004. [Google Scholar] [CrossRef]
- Wagner, D.R.; Thompson, B.J.; Anderson, D.A.; Schwartz, S. A-mode and B-mode ultrasound measurement of fat thickness: A cadaver validation study. Eur. J. Clin. Nutr. 2019, 73, 518–523. [Google Scholar] [CrossRef]
- Guo, J.Y.; Zheng, Y.P.; Huang, Q.H.; Chen, X.; He, J.F.; Chan, H.L. Performances of One-Dimensional Sonomyography and Surface Electromyography in Tracking Guided Patterns of Wrist Extension. Ultrasound Med. Biol. 2009, 35, 894–902. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Li, Y.; Liu, H. Muscle fatigue assessment using one-channel single-element ultrasound transducer. In Proceedings of the International IEEE/EMBS Conference on Neural Engineering (NER), Shanghai, China, 25–28 May 2017; pp. 122–125. [Google Scholar] [CrossRef]
- Hettiarachchi, N.; Ju, Z.; Liu, H. A New Wearable Ultrasound Muscle Activity Sensing System for Dexterous Prosthetic Control. In Proceedings of the IEEE International Conference on Systems, Man, and Cybernetics, Kowloon, Hongkong, China, 9–12 October 2015; pp. 1415–1420. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Zhou, D.; Li, Y.; Liu, H. Towards Wearable A-Mode Ultrasound Sensing for Real-Time Finger Motion Recognition. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1199–1208. [Google Scholar] [CrossRef] [Green Version]
- Shahshahani, A.; Laverdiere, C.; Bhadra, S.; Zilic, Z. Ultrasound Sensors for Diaphragm Motion Tracking: An Application in Non-Invasive Respiratory Monitoring. Sensors 2018, 18, 2617. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Tian, H.; Yan, B.; Sun, H.; Wu, C.; Shu, Y.; Wang, L.G.; Ren, T.L. A flexible piezoelectric micromachined ultrasound transducer. RSC Adv. 2013, 3, 24900. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, Q.T.; Chen, Y.Q.; Shu, Y.; Tian, H.; Yang, Y.; Xie, D.; Luo, J.W.; Ren, T.L. A Flexible Ultrasound Transducer Array with Micro-Machined Bulk PZT. Sensors 2015, 15, 2538–2547. [Google Scholar] [CrossRef] [Green Version]
- Mastronardi, V.M.; Guido, F.; Amato, M.; De Vittorio, M.; Petroni, S. Piezoelectric ultrasonic transducer based on flexible AlN. Microelectron. Eng. 2014, 121, 59–63. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, M.; Gao, C.; Liu, B.; Pang, W. Flexible Piezoelectric Micromachined Ultrasonic Transducers Towards New Applications. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Kobe, Japan, 22–25 October 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Singh, R.S.; Culjat, M.; Lee, M.; Bennett, D.; Natarjan, S.; Cox, B.; Brown, E.; Grundfest, W.; Lee, H. Conformal Ultrasound Imaging System. In Acoustical Imaging; Springer: Dordrecht, The Netherlands, 2011; Volume 30, pp. 211–222. [Google Scholar] [CrossRef]
- Sadeghpour, S.; Lips, B.; Kraft, M.; Puers, R. Flexible Soi-Based Piezoelectric Micromachined Ultrasound Transducer (PMUT) Arrays. In Proceedings of the Solid-State Sensors, Actuators Microsystems Eurosensors XXXIII (Transducers Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019; pp. 250–253. [Google Scholar] [CrossRef]
- Hu, H.; Zhu, X.; Wang, C.; Zhang, L.; Li, X.; Lee, S.; Huang, Z.; Chen, R.; Chen, Z.; Wang, C.; et al. Stretchable ultrasonic transducer arrays for three-dimensional imaging on complex surfaces. Sci. Adv. 2018, 4, eaar3979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.H.; Chao, C.; Shi, X.; Leung, W.W. A flexible capacitive micromachined ultrasonic transducer (CMUT) array with increased effective capacitance from concave bottom electrodes for ultrasonic imaging applications. In Proceedings of the IEEE Ultrasonics Symposium, Rome, Italy, 20–23 September 2009; pp. 996–999. [Google Scholar] [CrossRef]
- Chong, P.F.; Shi, X.; Cheng, C.H. A novel flexible capacitive micromachined ultrasonic transducer (CMUT) array with isolated metallic islands riveted to a polymer film. In Proceedings of the IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Suzhou, China, 7–10 April 2013; Volume 1, pp. 923–926. [Google Scholar] [CrossRef]
- Gerardo, C.D.; Cretu, E.; Rohling, R. Fabrication and testing of polymer-based capacitive micromachined ultrasound transducers for medical imaging. Microsyst. Nanoeng. 2018, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lanata, A.; Scilingo, E.P.; De Rossi, D. A Multimodal Transducer for Cardiopulmonary Activity Monitoring in Emergency. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 817–825. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- AlMohimeed, I. Development of Wearable Ultrasonic Sensor s for Monitoring Muscle Contraction. Master’s Thesis, Carleton University, Ottawa, ON, Canada, 2013. [Google Scholar]
- AlMohimeed, I.; Agarwal, M.; Ono, Y. Wearable Ultrasonic Sensor Using Double-Layer PVDF Films for Monitoring Tissue Motion. In Proceedings of the IEEE Canadian Conference on Electrical & Computer Engineering (CCECE), Quebec City, QC, Canada, 13–16 May 2018; pp. 1–4. [Google Scholar] [CrossRef]
- Swartz, R.G.; Plummer, J.D. On the Generation of High-Frequency Acoustic Energy with Polyvinylidene Fluoride. IEEE Trans. Sonics Ultrason. 1980, 27, 295–302. [Google Scholar] [CrossRef]
- Zhang, Q.; Lewin, P.; Bloomfield, P. PVDF transducers-a performance comparison of single-layer and multilayer structures. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1997, 44, 1148–1156. [Google Scholar] [CrossRef]
- Turkistani, H.; AlMohimeed, I.; Ono, Y. Continuous Monitoring of Muscle Thickness Changes During Isometric Contraction Using a Wearable Ultrasonic Sensor. In Proceedings of the Canadian Medical and Biological Engineering Society (CMBES), Ottawa, ON, Canada, 21–24 May 2013; Volume 36. [Google Scholar]
- Trindade, B.M.; Ono, Y.; Lemaire, E.D.; AlMohimeed, I. Development of a wearable ultrasonic sensor and method for continuous monitoring of mechanical properties of plantar soft tissue for diabetic patients. In Proceedings of the IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 2112–2115. [Google Scholar] [CrossRef]
- Huang, A.; Ono, Y.; Liu, K. Ultrasonic monitoring of skeletal muscle response to electrical stimulation of peripheral nerve. In Proceedings of the IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; pp. 1053–1056. [Google Scholar] [CrossRef]
- Huang, A.; Ono, Y. Estimation of wrist flexion angle from muscle thickness changes measured by a flexible ultrasonic sensor. In Proceedings of the IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Las Vegas, NV, USA, 24–27 February 2016; pp. 188–191. [Google Scholar] [CrossRef]
- Huang, A.; Yoshida, M.; Ono, Y.; Rajan, S. Continuous measurement of arterial diameter using wearable and flexible ultrasonic sensor. In Proceedings of the IEEE Ultrasonics Symposium, Washington, DC, USA, 6–9 September 2017; pp. 1–4. [Google Scholar] [CrossRef]
- Marc Lethiecq and Franck Levassort and Dominique Certon and Louis Pascal Tran-Huu-Hue. Piezoelectric Transducer Design for Medical Diagnosis and NDE. In Piezoelectric and Acoustic Materials for Transducer Applications; Springer: Boston, MA, USA, 2008; Chapter 10; pp. 191–215. [Google Scholar] [CrossRef]
- Orizio, C.; Gobbo, M.; Diemont, B. Changes of the force–frequency relationship in human tibialis anterior at fatigue. J. Electromyogr. Kinesiol. 2004, 14, 523–530. [Google Scholar] [CrossRef]
- Celichowski, J.; Grottel, K. The relationship between fusion index and stimulation frequency in tetani of motor units in rat medial gastrocnemius. Arch. Ital. Biol. 1995, 133, 81–87. [Google Scholar]
- Watanabe, S.; Kitawaki, T.; Oka, H. Mathematical equation of fusion index of tetanic contraction of skeletal muscles. J. Electromyogr. Kinesiol. 2010, 20, 284–289. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Fukuhara, S.; Fujinaga, T.; Oka, H. Estimating the minimum stimulation frequency necessary to evoke tetanic progression based on muscle twitch parameters. Physiol. Meas. 2017, 38, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Ditroilo, M.; Smith, I.J.; Fairweather, M.M.; Hunter, A.M. Long-term stability of tensiomyography measured under different muscle conditions. J. Electromyogr. Kinesiol. 2013, 23, 558–563. [Google Scholar] [CrossRef] [PubMed]
- De Paula Simola, R.Á.; Harms, N.; Raeder, C.; Kellmann, M.; Meyer, T.; Pfeiffer, M.; Ferrauti, A. Tensiomyography reliability and prediction of changes in muscle force following heavy eccentric strength exercise using muscle mechanical properties. Sports Technol. 2015, 8, 58–66. [Google Scholar] [CrossRef]
- Alvarez-Diaz, P.; Alentorn-Geli, E.; Ramon, S.; Marin, M.; Steinbacher, G.; Boffa, J.J.; Cuscó, X.; Ares, O.; Ballester, J.; Cugat, R. Effects of anterior cruciate ligament injury on neuromuscular tensiomyographic characteristics of the lower extremity in competitive male soccer players. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2264–2270. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Alvarez-Diaz, P.; Ramon, S.; Marin, M.; Steinbacher, G.; Rius, M.; Seijas, R.; Ares, O.; Cugat, R. Assessment of gastrocnemius tensiomyographic neuromuscular characteristics as risk factors for anterior cruciate ligament injury in male soccer players. Knee Surg. Sports Traumatol. Arthrosc. 2014, 23, 2502–2507. [Google Scholar] [CrossRef]
- Zubac, D.; Paravlić, A.; Koren, K.; Felicita, U.; Šimunič, B. Plyometric exercise improves jumping performance and skeletal muscle contractile properties in seniors. J. Musculoskelet. Neuronal Interact. 2019, 19, 38–49. [Google Scholar]
- Jones, A.D.; Hind, K.; Wilson, H.; Johnson, M.I.; Francis, P. A standardised protocol for the assessment of lower limb muscle contractile properties in football players using Tensiomyography. Adv. Skelet. Muscle Funct. Assess. 2017, 1, 13–16. [Google Scholar]
- Wilson, H.V.; Jones, A.; Johnson, M.I.; Francis, P. The effect of inter-electrode distance on radial muscle displacement and contraction time of the biceps femoris, gastrocnemius medialis and biceps brachii, using tensiomyography in healthy participants. Physiol. Meas. 2019, 40, 075007. [Google Scholar] [CrossRef]
- Piqueras-Sanchiz, F.; Martín-Rodríguez, S.; Pareja-Blanco, F.; Baraja-Vegas, L.; Blázquez-Fernández, J.; Bautista, I.J.; García-García, Ó. Mechanomyographic Measures of Muscle Contractile Properties are Influenced by Electrode Size and Stimulation Pulse Duration. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Wiewelhove, T.; Raeder, C.; Meyer, T.; Kellmann, M.; Pfeiffer, M.; Ferrauti, A. Markers for Routine Assessment of Fatigue and Recovery in Male and Female Team Sport Athletes during High-Intensity Interval Training. PLoS ONE 2015, 10, e0139801. [Google Scholar] [CrossRef]
- Rusu, L.D.; Cosma, G.G.H.; Cernaianu, S.M.; Marin, M.N.; Rusu, P.F.A.; Ciocanescu, D.P.; Neferu, F.N. Tensiomyography method used for neuromuscular assessment of muscle training. J. Neuro Eng. Rehabil. 2013, 10, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AlMohimeed, I.; Turkistani, H.; Ono, Y. Development of wearable and flexible ultrasonic sensor for skeletal muscle monitoring. In Proceedings of the IEEE International Ultrasonics Symposium (IUS), Prague, Czech Republic, 21–25 July 2013; pp. 1137–1140. [Google Scholar] [CrossRef]
- Yeung, E.; AlMohimeed, I.; Ono, Y. Estimation of Tissue Thickness Changes Due to Electrical Muscle Stimulation Using Wearable Ultrasonic Sensor in Pulse Echo Mode. In Proceedings of the IEEE Sensors, Montreal, QC, Canada, 27–30 October 2019; pp. 1–4. [Google Scholar] [CrossRef]


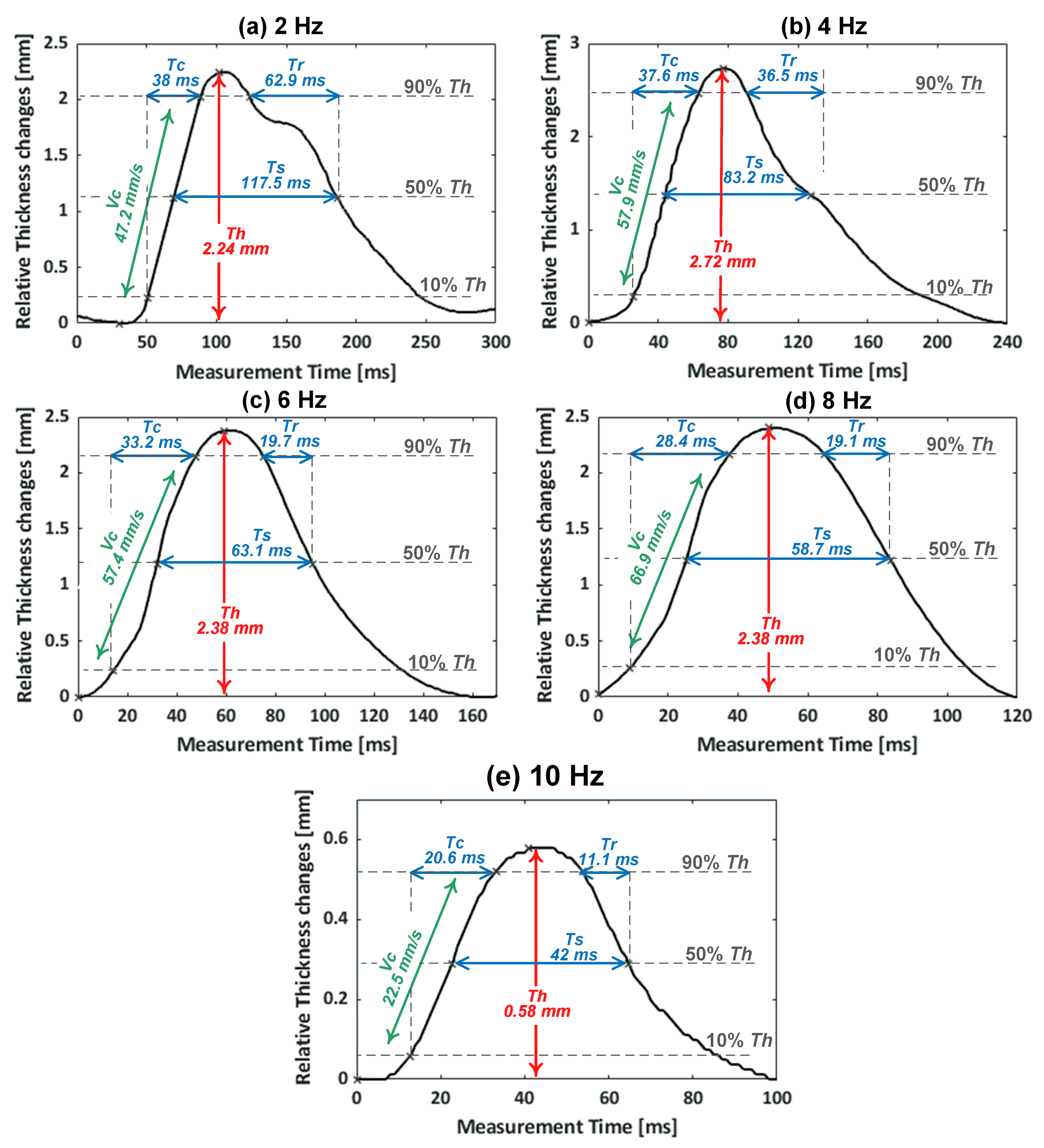
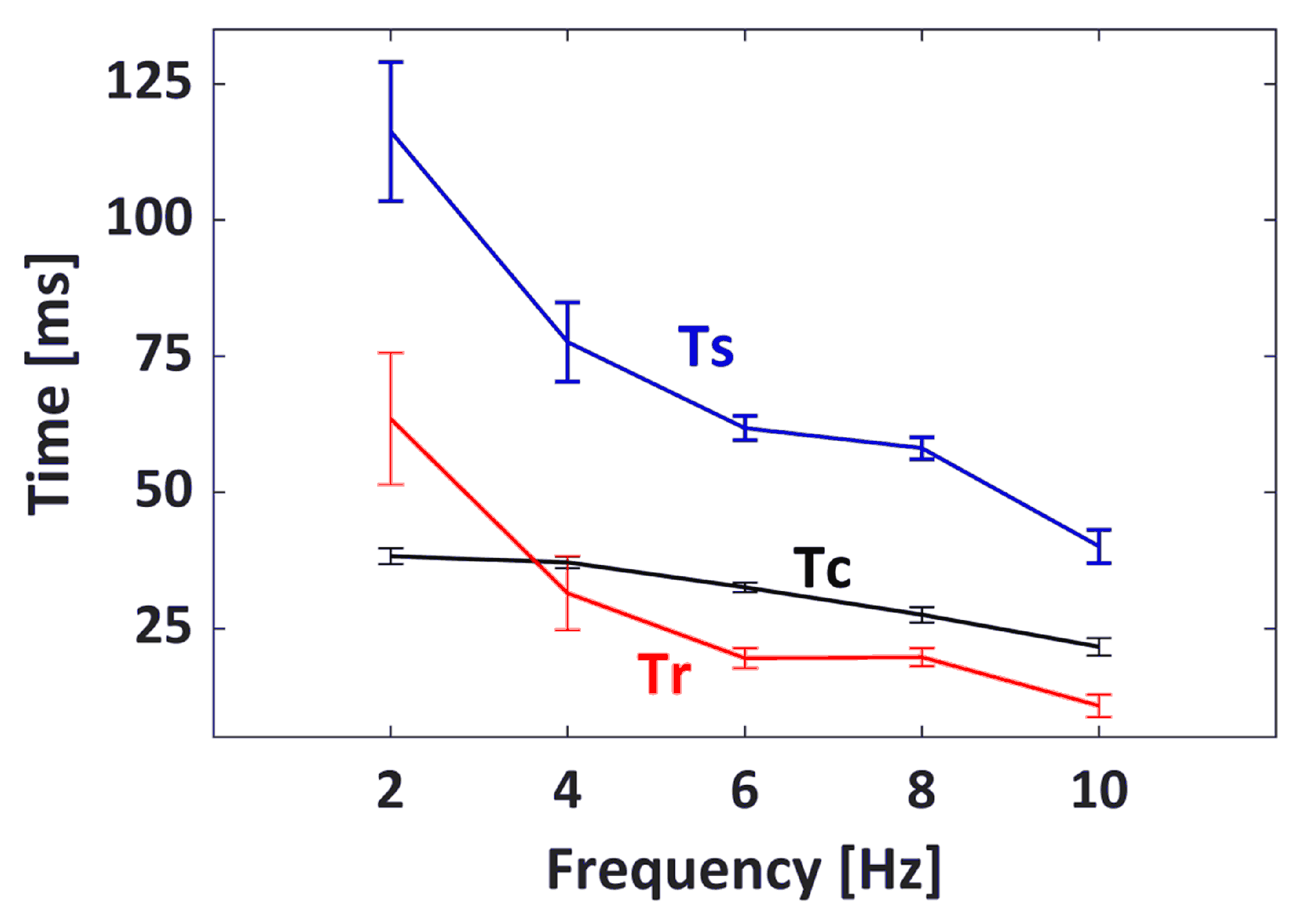
| EMS | Extracted Parameters Mean ± SD (CV) | ||||
|---|---|---|---|---|---|
| (mm) | (mm/s) | (ms) | (ms) | (ms) | |
| 2 Hz | 2.20 ± 0.08 (3.6%) | 46.13 ± 2.76 (6%) | 38.25 ± 1.50 (3.9%) | 116.28 ± 12.75 (11%) | 63.49 ± 12.12 (19.1%) |
| 4 Hz | 2.77 ± 0.09 (3.2%) | 59.68 ± 2.05 (3.4%) | 37.12 ± 1.05 (2.8%) | 77.57 ± 7.29 (9.4%) | 31.46 ± 6.76 (21.5%) |
| 6 Hz | 2.04 ± 0.15 (7.4%) | 50.22 ± 3.68 (7.3%) | 32.54 ± 0.88 (2.7%) | 61.77 ± 2.23 (3.6%) | 19.54 ± 1.81 (9.3%) |
| 8 Hz | 2.36 ± 0.19 (8.1%) | 68.56 ± 5.47 (8.0%) | 27.51 ± 1.41 (5.1%) | 58.10 ± 2.00 (3.4%) | 19.72 ± 1.64 (8.3%) |
| 10 Hz | 0.59 ± 0.03 (5.1%) | 22.03 ± 1.58 (7.2%) | 21.62 ± 1.58 (7.3%) | 40.07 ± 3.07 (7.7%) | 10.80 ± 2.08 (19.3%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlMohimeed, I.; Ono, Y. Ultrasound Measurement of Skeletal Muscle Contractile Parameters Using Flexible and Wearable Single-Element Ultrasonic Sensor. Sensors 2020, 20, 3616. https://doi.org/10.3390/s20133616
AlMohimeed I, Ono Y. Ultrasound Measurement of Skeletal Muscle Contractile Parameters Using Flexible and Wearable Single-Element Ultrasonic Sensor. Sensors. 2020; 20(13):3616. https://doi.org/10.3390/s20133616
Chicago/Turabian StyleAlMohimeed, Ibrahim, and Yuu Ono. 2020. "Ultrasound Measurement of Skeletal Muscle Contractile Parameters Using Flexible and Wearable Single-Element Ultrasonic Sensor" Sensors 20, no. 13: 3616. https://doi.org/10.3390/s20133616





