An Ultrasonic Rheometer to Measure Gas Absorption in Ionic Liquids: Design, Calibration and Testing
Abstract
1. Introduction
2. Materials and Methods
2.1. Background on Reflection Coefficient Measurement Techniques
2.2. Background on Ultrasonic Viscometry
2.3. Materials
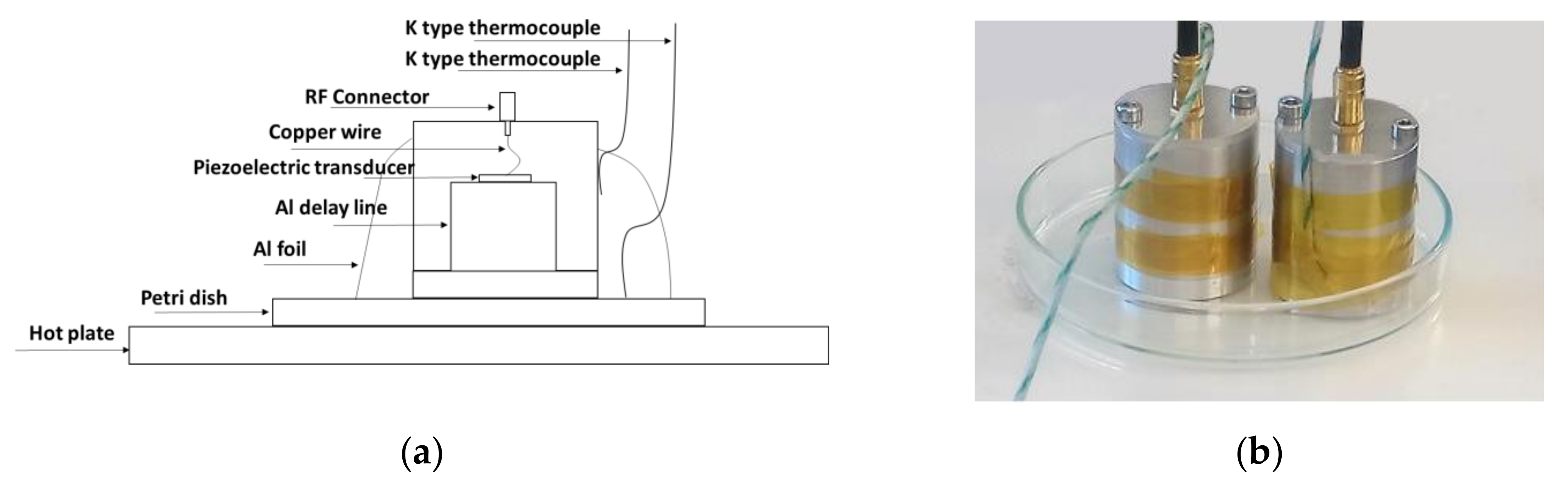
2.3.1. Piezoelectric Materials
2.3.2. Test Samples
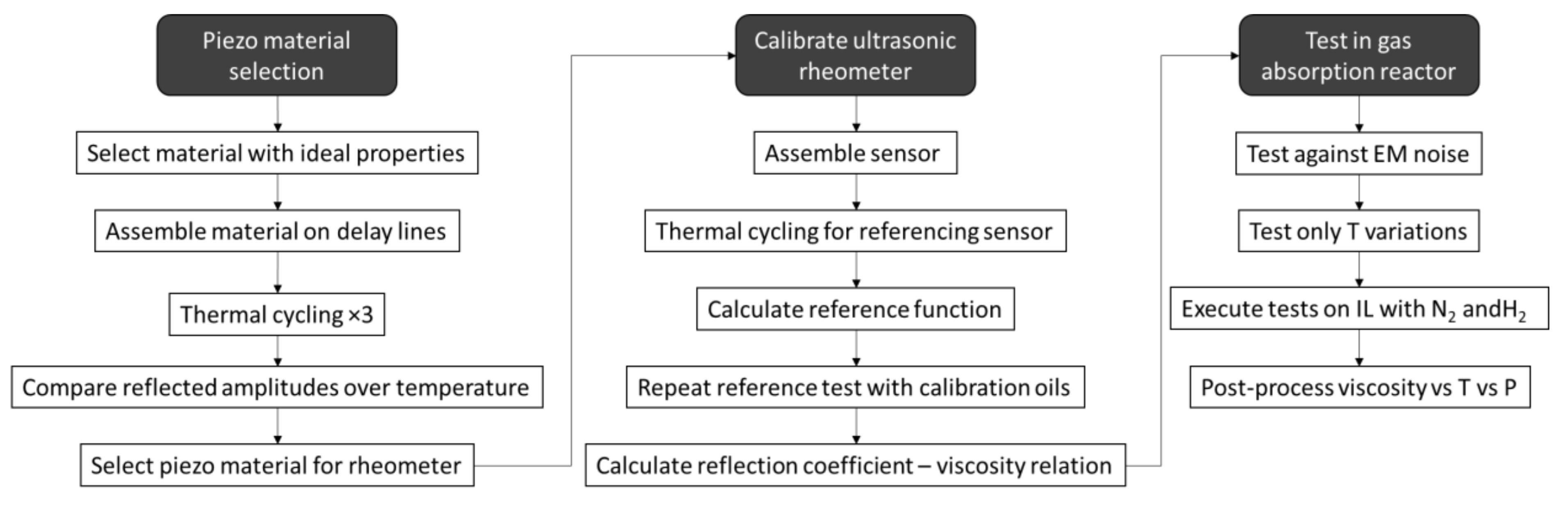
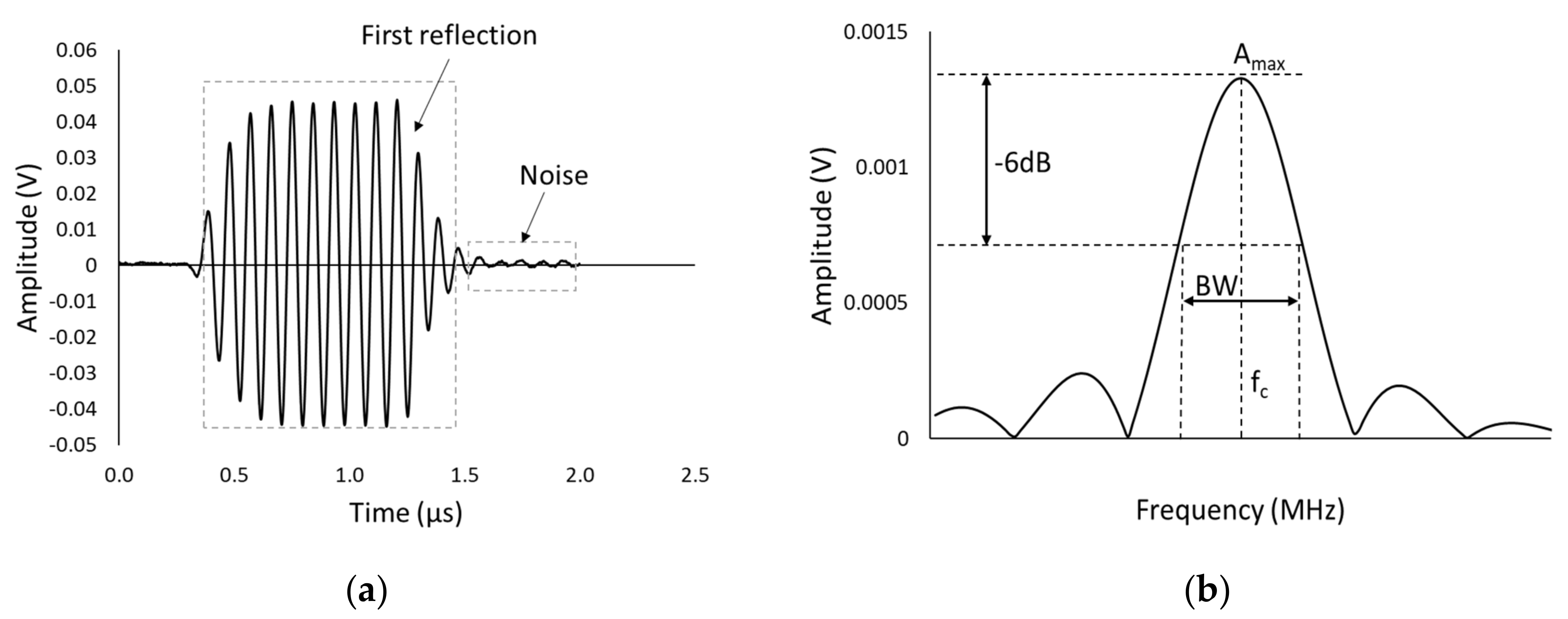
2.4. Testing Methodology and Data Postprocessing
Calibration and Measurement Methodology
- Sample randomization.
- Test randomization.
- Blinding, for stage two only.
- Test repetitions.
- Stage 1: Piezoelectric material and data postprocessing
- Stage 2: Calibration of the rheological sensor
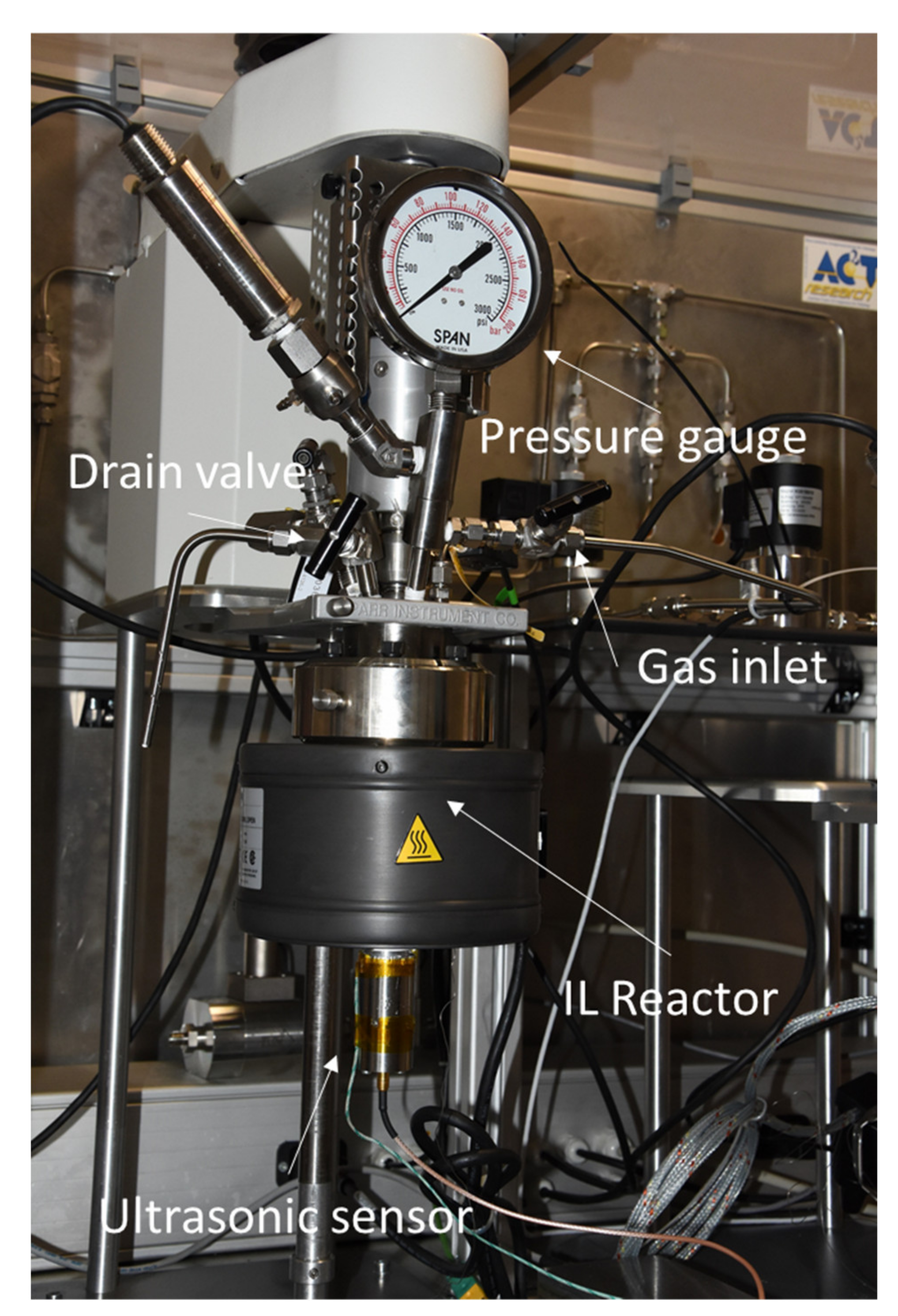
- Stage 3: Gas Absorption Reactor Testing and Postprocessing
3. Results
- Stage 1: Piezoelectric Material Selection
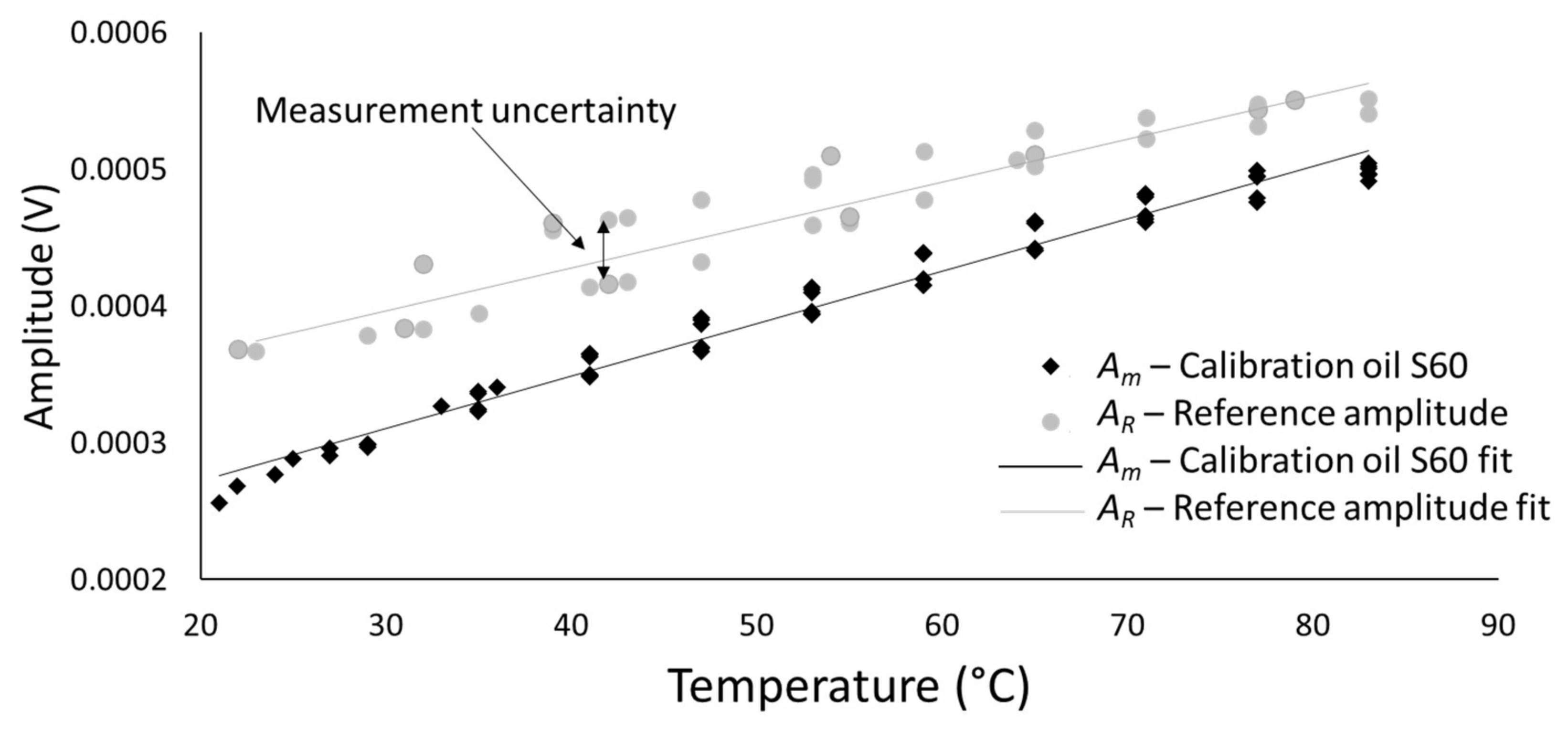
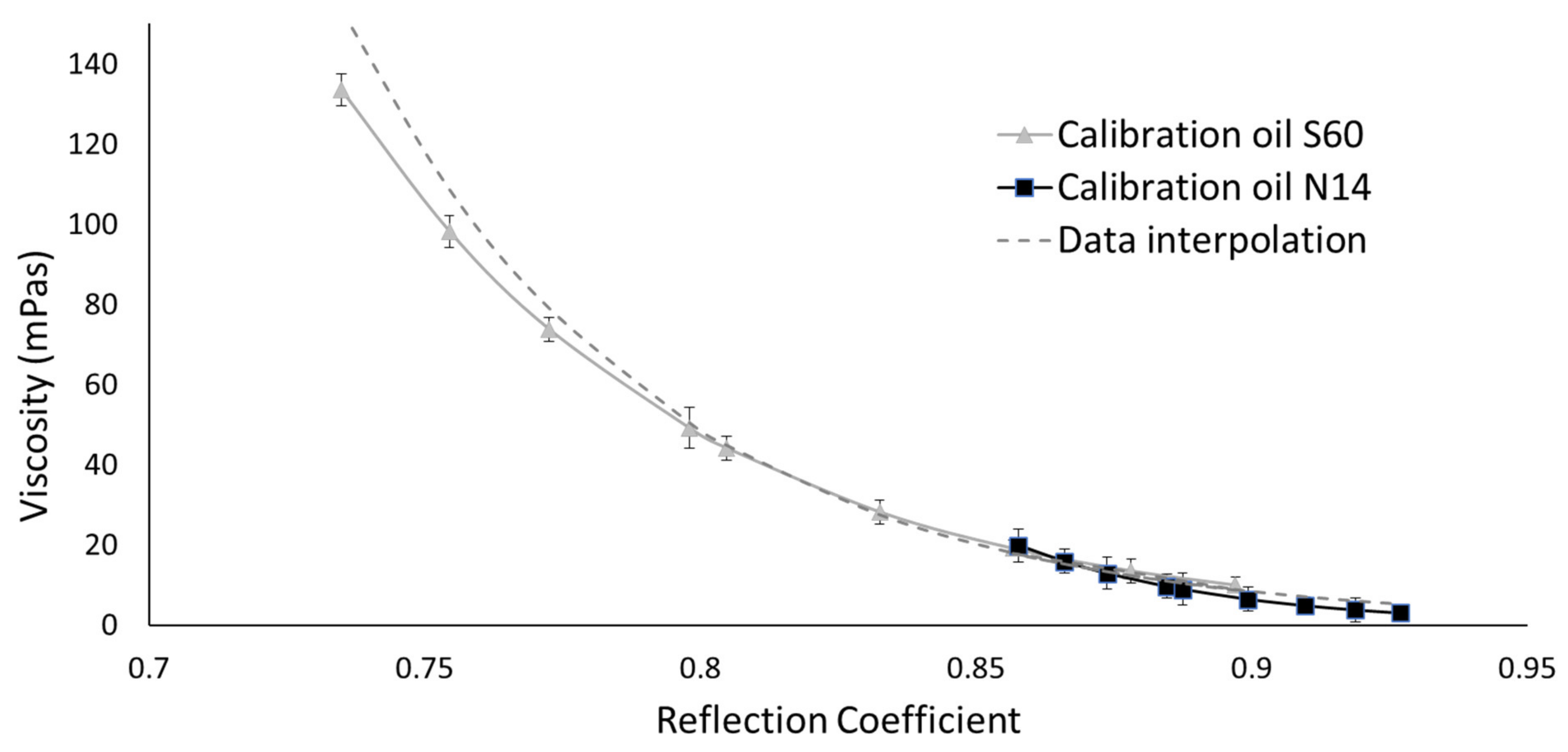
- Stage 2: Referencing Rheometer
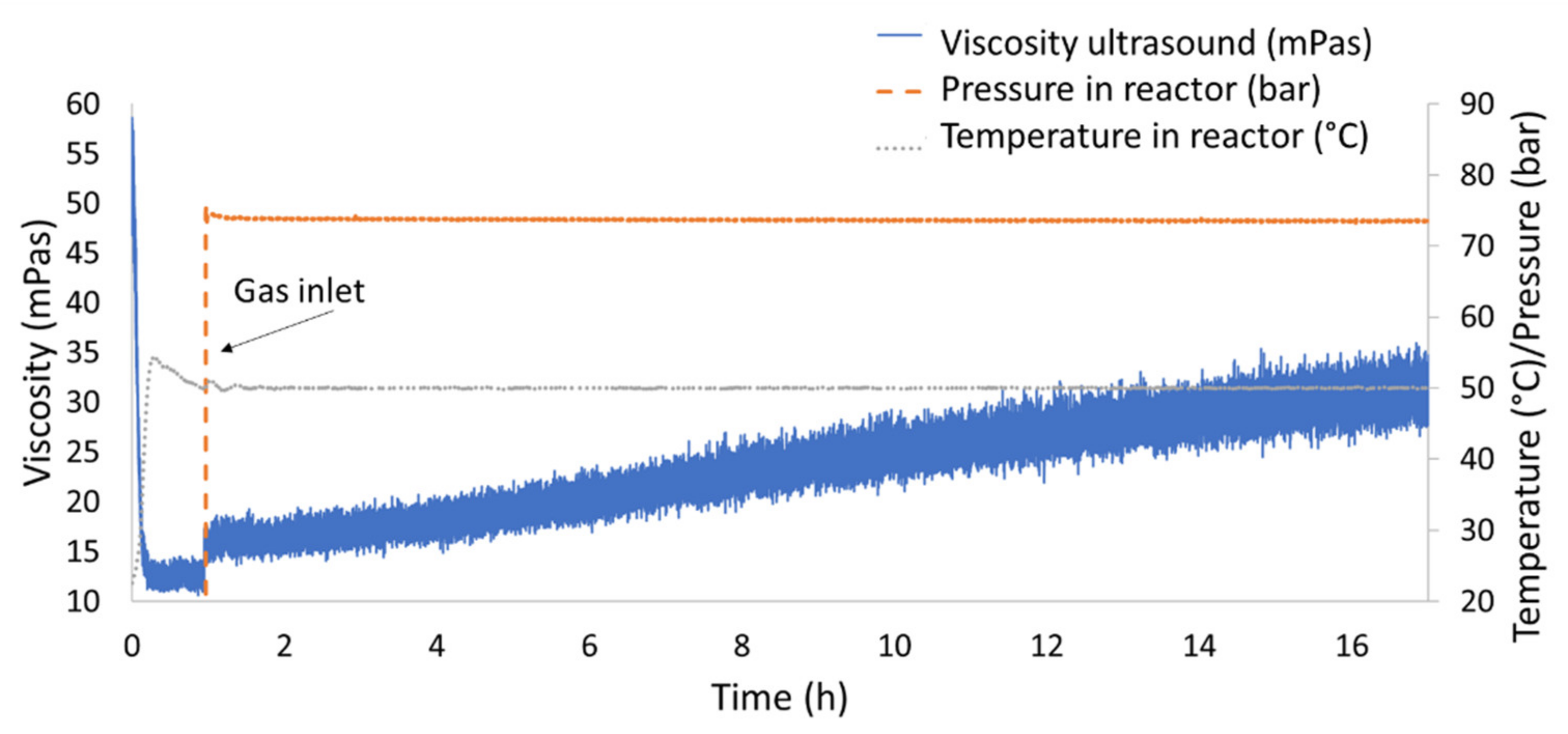
- Stage 3: Test in Gas Absorption Reactor
4. Conclusions
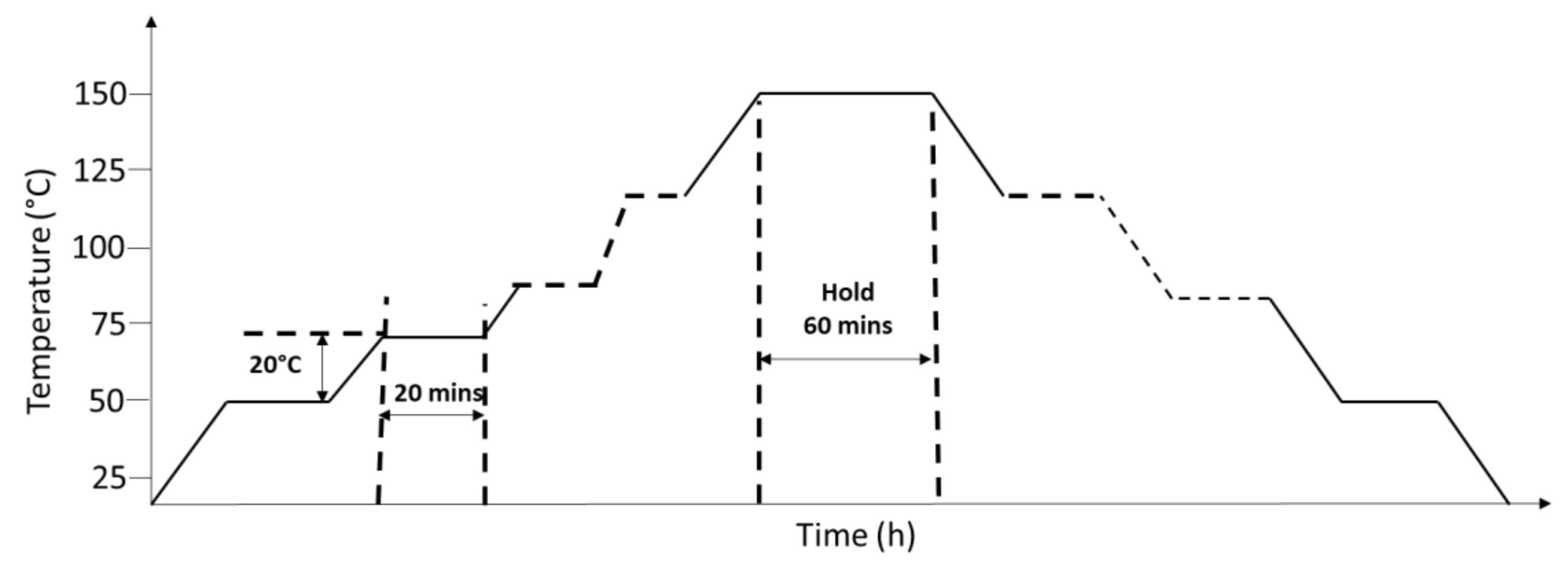
- Thermal cycling within the operating temperature range was required upon constructing reflectance sensor devices to allow the setting of piezoelectric material properties. At least three thermal cycles should be executed to verify the sensor response stability.
- Lead metaniobate in tungsten bronze structure was the ideal piezoelectric material for the construction of ultrasonic reflectance rheometers. This selection was based on overall bandwidth, signal to noise ratio and response linearity in the range of temperature 20 °C to 150 °C. Overall, BiT showed the highest stability to temperature and it was the recommended material for special operations above 400 °C.
- Conventional ultrasonic sensors employing PZT were characterized by reflected amplitudes that change over time with thermal cycling. This makes the referencing of such piezoelectric device impossible for long operation periods.
- The viscosity–reflection coefficient calibration curves were not dependent on temperature. This was proven by the fact that the reflection coefficients for two calibrated oils with identical viscosity at different temperatures overlap in a viscosity–reflection coefficient diagram. This was of high importance to reduce the measurement uncertainty when testing a sample of unknown acoustic and mechanical properties and to simplify referencing procedures for ultrasonic devices.
- The ultrasonic rheometer developed in this work was stable over long-time operations and the corrosive nature of the IL under study did not influence the sensor response. This proves that polymeric front faces were suitable for long term testing of ILs.
- The measurement of the influence of gas absorption to the viscosity of ILs was made possible by implementing the sensor system into a gas absorption reactor. In particular, the reference IL did not show changes in viscosity when absorbing hydrogen, while the absorption of nitrogen at the same conditions led to significant changes in the viscosity measured. This showed the importance to execute gas absorption tests on lubricants as mechanism operating pressures may induce changes in viscosity that were not predicted by conventional rheological testing.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Ye, C.; Gong, Q.; Wang, H.; Wang, P. Tribological performance of room-temperature ionic liquids as lubricant. Tribol. Lett. 2002, 13, 81–85. [Google Scholar] [CrossRef]
- Shannon, M.S.; Tedstone, J.M.; Danielsen, S.P.; Hindman, M.S.; Irvin, A.C.; Bara, J.E. Free volume as the basis of gas solubility and selectivity in imidazolium-based ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 5565–5576. [Google Scholar] [CrossRef]
- Stachowiak, G.; Batchelor, A.W. Engineering Tribology; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Uerdingen, M.; Treber, C.; Balser, M.; Schmitt, G.; Werner, C. Corrosion behaviour of ionic liquids. Green Chem. 2005, 7, 321–325. [Google Scholar] [CrossRef]
- Mason, W.P.; Baker, W.O.; McSkimin, H.J.; Heiss, J.H. Measurement of shear elasticity and viscosity of liquids at ultrasonic frequencies. Phys. Rev. 1949, 75, 936. [Google Scholar] [CrossRef]
- Lamb, J. Paper 18: Physical Properties of Fluid Lubricants: Rheological and Viscoelastic Behaviour. In Proceedings of the Institution of Mechanical Engineers, Conference Proceedings; SAGE Publications: London, UK, 1967; Volume 182. [Google Scholar]
- Bruckenstein, S.; Shay, M. Experimental aspects of use of the quartz crystal microbalance in solution. Electrochim. Acta 1985, 30, 1295–1300. [Google Scholar] [CrossRef]
- Krim, J.; Widom, A. Damping of a crystal oscillator by an adsorbed monolayer and its relation to interfacial viscosity. Phys. Rev. B 1988, 38, 12184. [Google Scholar] [CrossRef]
- Dwyer-Joyce, R.S.; Drinkwater, B.W.; Donohoe, C.J. The measurement of lubricant–film thickness using ultrasound. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2003, 459, 957–976. [Google Scholar] [CrossRef]
- Dwyer-Joyce, R.S.; Reddyhoff, T.; Drinkwater, B.W. Operating limits for acoustic measurement of rolling bearing oil film thickness. Tribol. Trans. 2004, 47, 366–375. [Google Scholar] [CrossRef]
- Wan Ibrahim, M.K.; Gasni, D.; Dwyer-Joyce, R.S. Profiling a ball bearing oil film with ultrasonic reflection. Tribol. Trans. 2012, 55, 409–421. [Google Scholar] [CrossRef]
- Eggers, F.; Funck, T. Ultrasonic measurements with milliliter liquid samples in the 0.5–100 MHz range. Rev. Sci. Instrum. 1973, 44, 969–977. [Google Scholar] [CrossRef]
- Brai, M.; Kaatze, U. Ultrasonic and hypersonic relaxations of monohydric alcohol/water mixtures. J. Phys. Chem. 1992, 96, 8946–8955. [Google Scholar] [CrossRef]
- Shah, V.V.; Balasubramaniam, K. Measuring Newtonian viscosity from the phase of reflected ultrasonic shear wave. Ultrasonics 2000, 38, 921–927. [Google Scholar] [CrossRef]
- Sheen, S.H.; Chien, H.-T.; Raptis, A.C. An in-line ultrasonic viscometer. In Review of Progress in Quantitative Nondestructive Evaluation; Springer: Boston, MA, USA, 1995; pp. 1151–1158. [Google Scholar]
- Franco, E.E.; Adamowski, J.C.; Buiochi, F. Ultrasonic viscosity measurement using the shear-wave reflection coefficient with a novel signal processing technique. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 1133–1139. [Google Scholar] [CrossRef]
- Greenwood, M.S.; Adamson, J.D.; Bond, L.J. Measurement of the viscosity–density product using multiple reflections of ultrasonic shear horizontal waves. Ultrasonics 2006, 44, e1031–e1036. [Google Scholar] [CrossRef]
- Kasolang, S.; Ahmad, M.A.; Joyce, R.S.D. Measurement of circumferential viscosity profile in stationary journal bearing by shear ultrasonic reflection. Tribol. Int. 2011, 44, 1264–1270. [Google Scholar] [CrossRef]
- McSkimin, H.J.; Andreatch, P., Jr. Measurement of dynamic shear impedance of low viscosity liquids at ultrasonic frequencies. J. Acoust. Soc. Am. 1967, 42, 248–252. [Google Scholar] [CrossRef]
- Ting, R.Y. Evaluation of new piezoelectric composite materials for hydrophone applications. Ferroelectrics 1986, 67, 143–157. [Google Scholar] [CrossRef]
- Sohn, H.; Kim, S.B. Development of dual PZT transducers for reference-free crack detection in thin plate structures. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 57, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Wolny, W.W. Application driven industrial development of piezoceramics. J. Eur. Ceram. Soc. 2005, 25, 1971–1976. [Google Scholar] [CrossRef]
- Parr, A.C.S.; O’Leary, R.L.; Hayward, G. Improving the thermal stability of 1-3 piezoelectric composite transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 550–563. [Google Scholar] [CrossRef]
- Pialucha, T.; Cawley, P. The detection of thin embedded layers using normal incidence ultrasound. Ultrasonics 1994, 32, 431–440. [Google Scholar] [CrossRef]
- Kažys, R.; Voleišis, A.; Voleišienė, B. High temperature ultrasonic transducers. Ultragarsas Ultrasound 2008, 63, 7–17. [Google Scholar]
- Huang, J.; Riisager, A.; Berg, R.W.; Fehrmann, R. Tuning ionic liquids for high gas solubility and reversible gas sorption. J. Mol. Catal. A Chem. 2008, 279, 170–176. [Google Scholar] [CrossRef]
- Wu, W.; Han, B.; Gao, H.; Liu, Z.; Jiang, T.; Huang, J. Desulfurization of flue gas: SO2 absorption by an ionic liquid. Angew. Chem. Int. Ed. 2004, 43, 2415–2417. [Google Scholar] [CrossRef]
- Kinsler, L.E.; Frey, A.R.; Coppens, A.B.; Sanders, J.V. Fundamentals of Acoustics, 4th ed.; Wiley-VCH: Princeton, NJ, USA, 1999; p. 560. ISBN 0-471-84789-5. [Google Scholar]
- Brekhovskikh, L. Waves in Layered Media; Elsevier: Amsterdam, The Netherlands, 2012; Volume 16. [Google Scholar]
- Van Kervel, S.J.H.; Thijssen, J.M. A calculation scheme for the optimum design of ultrasonic transducers. Ultrasonics 1983, 21, 134–140. [Google Scholar] [CrossRef]
- Schirru, M.; Li, X.; Cadeddu, M.; Dwyer-Joyce, R.S. Development of a shear ultrasonic spectroscopy technique for the evaluation of viscoelastic fluid properties: Theory and experimental validation. Ultrasonics 2019, 94, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Schirru, M. Development of an Ultrasonic Sensing Technique to Measure Lubricant Viscosity in Engine Journal Bearing In-Situ; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Darbari, G.S.; Singh, R.P.; Verma, G.S. Ultrasonic attenuation in carbon steel and stainless steel at elevated temperatures. J. Appl. Phys. 1968, 39, 2238–2245. [Google Scholar] [CrossRef]
- Harrison, G.; Barlow, A.J. 3. Dynamic viscosity measurement. In Methods in Experimental Physics; Academic Press: Cambridge, MA, USA, 1981; Volume 19, pp. 137–178. [Google Scholar]
- Cox, W.P.; Merz, E.H. Correlation of dynamic and steady flow viscosities. J. Polym. Sci. 1958, 28, 619–622. [Google Scholar] [CrossRef]
- Bair, S.; Yamaguchi, T.; Brouwer, L.; Schwarze, H.; Vergne, P.; Poll, G. Oscillatory and steady shear viscosity: The Cox–Merz rule, superposition, and application to EHL friction. Tribol. Int. 2014, 79, 126–131. [Google Scholar] [CrossRef]
- Marrucci, G. Dynamics of entanglements: A nonlinear model consistent with the Cox-Merz rule. J. Non Newton. Fluid Mech. 1996, 62, 279–289. [Google Scholar] [CrossRef]
- Larson, R.G. Constitutive Equations for Polymer Melts and Solutions: Butterworths Series in Chemical Engineering; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Bair, S.S.; Andersson, O.; Qureshi, F.S.; Schirru, M.M. New EHL modeling data for the reference liquids squalane and squalane plus polyisoprene. Tribol. Trans. 2018, 61, 247–255. [Google Scholar] [CrossRef]
- Voeltzel, N.; Vergne, P.; Fillot, N.; Bouscharain, N.; Joly, L. Rheology of an ionic liquid with variable Carreau exponent: A full picture by molecular simulation with experimental contribution. Tribol. Lett. 2016, 64, 25. [Google Scholar] [CrossRef]
- Daschakraborty, S.; Biswas, R. Dielectric relaxation in ionic liquids: Role of ion-ion and ion-dipole interactions, and effects of heterogeneity. J. Chem. Phys. 2014, 140, 014504. [Google Scholar] [CrossRef] [PubMed]
- Setter, N.; Damjanovic, D.; Eng, L.; Fox, G.; Gevorgian, S.; Hong, S.; Kingon, A.I.; Kohlstedt, H.; Park, N.Y.; Stephenson, G.B.; et al. Ferroelectric thin films: Review of materials, properties, and applications. J. Appl. Phys. 2006, 100, 051606. [Google Scholar] [CrossRef]
- Kasolang, S.; Joyce, R.S.D.; Ahmad, M.A. PZT transducer design and pulsing optimization for film thickness and viscosity measurement. Sens. Actuators A Phys. 2013, 203, 386–393. [Google Scholar] [CrossRef]
- Baptista, F.G.; Filho, J.V. A new impedance measurement system for PZT-based structural health monitoring. IEEE Trans. Instrum. Meas. 2009, 58, 3602–3608. [Google Scholar] [CrossRef]
- Kobayashi, M.; Jen, C.K.; Bussiere, J.F.; Wu, K.T. High-temperature integrated and flexible ultrasonic transducers for nondestructive testing. Ndt E Int. 2009, 42, 157–161. [Google Scholar] [CrossRef]
- Zhu, J.; Ren, L.; Ho, S.C.; Jia, Z.; Song, G. Gas pipeline leakage detection based on PZT sensors. Smart Mater. Struct. 2017, 26, 025022. [Google Scholar] [CrossRef]
- Novotny-Farkas, F.; Böhme, W. The Stabinger viscometer: A unique new instrument for oil service laboratories. In Proceedings of the World Tribology Congress II, Vienna, Austria, 3–7 September 2001. [Google Scholar]
- Liu, F.; Zhong, X.; Xu, J.; Kamali, A.; Shi, Z. Temperature Dependence on Density, Viscosity, and Electrical Conductivity of Ionic Liquid 1-Ethyl-3-Methylimidazolium Fluoride. Appl. Sci. 2018, 8, 356. [Google Scholar] [CrossRef]
- ASTM D7042-14. Standard Test Method for Dynamic Viscosity and Density of Liquids by Stabinger Viscometer (and the Calculation of Kinematic Viscosity); ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Watson, M.; Christoforou, P.; Herrera, P.; Preece, D.; Carrell, J.; Harmon, M.; Krier, P.; Lewis, S.; Maiti, R.; Skipper, W.; et al. An analysis of the quality of experimental design and reliability of results in tribology research. Wear 2019, 426, 1712–1718. [Google Scholar] [CrossRef]
- ASTM. E1065-08 Standard Guide for Evaluating Characteristics of Ultrasonic Search Units; ASTM International: West Conshohocken, PA, USA, 2008. [Google Scholar]
- Schirru, M.; Mills, R.; Dwyer-Joyce, R.; Smith, O.; Sutton, M. Viscosity measurement in a lubricant film using an ultrasonically resonating matching layer. Tribol. Lett. 2015, 60, 42. [Google Scholar] [CrossRef]
- Research Data Repository. Available online: https://triboportal.ac2t.at/ (accessed on 22 June 2020).
- Kariznovi, M.; Nourozieh, H.; Abedi, J. Solubility of carbon dioxide, methane, and ethane in 1-butanol and saturated liquid densities and viscosities. J. Chem. Thermodyn. 2013, 67, 227–233. [Google Scholar] [CrossRef]

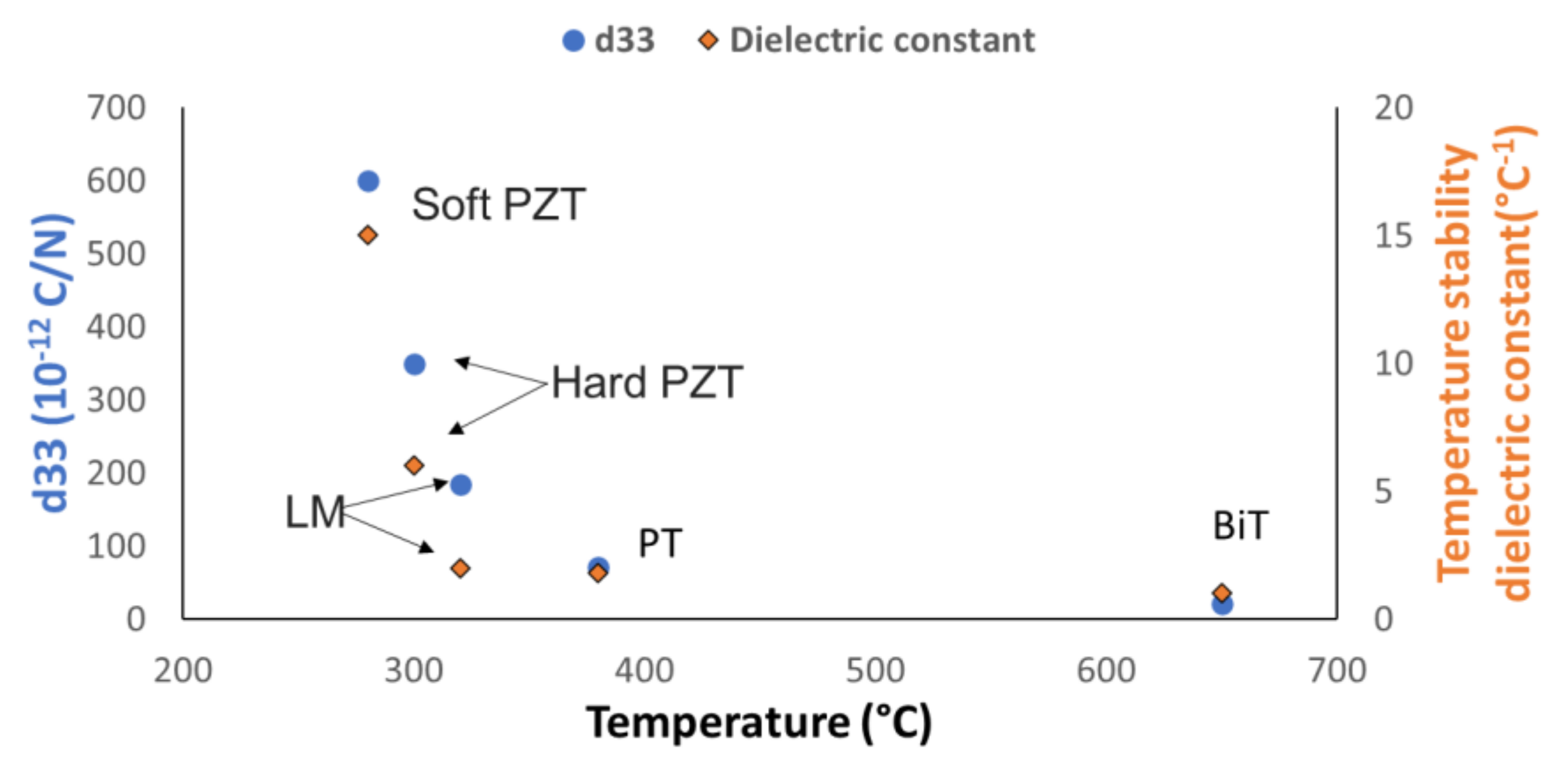








| Material | Structure | Curie T (°C) | d33 (10–12 C/N) | Dielectric Constant, Thermal Stability (°C−1) |
|---|---|---|---|---|
| BiT | Layer | 650 | 21 | 1.0 |
| PT | Perovskite | 380 | 72 | 1.8 |
| LM1 | Tung. Bronze | 480 | 90 | 1.6 |
| LM2 | Tung. Bronze | 320 | 185 | 1.8 |
| LM3 | Tung. Bronze | 280 | 62 | 2.2 |
| Hard PZT | Perovskite | 330 | 245 | 4.0 |
| Ionic Liquid | |
|---|---|
| Property | Value |
| Viscosity @ 40 °C (mPas)—100 Hz | 50.0 |
| Viscosity EMIM base @ 40 °C (mPas)—100 Hz [48] | 20.0 |
| Viscosity @ 100 °C (mPas)—100 Hz | 8.5 |
| VI | 159.4 |
| Density @ 40 °C (kg/m3) | 1242.6 |
| Density @ 100 °C (kg/m3) | 1204.2 |
| Relaxation time EMIM (ns) | 4.0 |
| Carreau viscosity (based on [41,42,48]) @ 50 °C (mPas) | 15.0 to 20.0 |
| Viscosity Standard Oils | ||
|---|---|---|
| Temperature (°C) | Viscosity S60 (mPas) | Viscosity N14 (mPas) |
| 20 | 141.0 | 24.0 |
| 25 | 104.0 | 20.0 |
| 40 | 47.0 | 11.0 |
| 50 | 30.0 | 8.2 |
| 80 | 10.0 | 3.8 |
| 100 | 6.2 | 2.6 |
| Material | R2 | Slope (V/°C) | Bandwidth (%) | SNR (%) |
|---|---|---|---|---|
| BiT | 0.89 ± 0.09 | −1.27 e−6 ± 3.67 e−8 | 21.0 ± 4.0 | 81 ± 15 |
| PT | 0.69 ± 0.24 | 1.37 e−5 ± 3.79 e−6 | 18.0 ± 2.4 | 82 ± 11 |
| LM1 | 0.64 ± 0.28 | 2.00 e−5 ± 1.49 e−5 | 11.5 ± 0.5 | 94 ± 4 |
| LM2 | 0.97 ± 0.02 | −2.50 e−3 ± 3.64 e−4 | 12.5 ± 0.15 | 94 ± 3 |
| LM3 | 0.98 ± 0.01 | 1.63 e−3 ± 2.70 e−4 | 12.5 ± 0.07 | 95 ± 2 |
| Hard PZT | - | - | 12.2 ± 0.15 | 95 ± 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirru, M.; Adler, M. An Ultrasonic Rheometer to Measure Gas Absorption in Ionic Liquids: Design, Calibration and Testing. Sensors 2020, 20, 3544. https://doi.org/10.3390/s20123544
Schirru M, Adler M. An Ultrasonic Rheometer to Measure Gas Absorption in Ionic Liquids: Design, Calibration and Testing. Sensors. 2020; 20(12):3544. https://doi.org/10.3390/s20123544
Chicago/Turabian StyleSchirru, Michele, and Michael Adler. 2020. "An Ultrasonic Rheometer to Measure Gas Absorption in Ionic Liquids: Design, Calibration and Testing" Sensors 20, no. 12: 3544. https://doi.org/10.3390/s20123544
APA StyleSchirru, M., & Adler, M. (2020). An Ultrasonic Rheometer to Measure Gas Absorption in Ionic Liquids: Design, Calibration and Testing. Sensors, 20(12), 3544. https://doi.org/10.3390/s20123544




