Exploration of User’s Mental State Changes during Performing Brain–Computer Interface
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
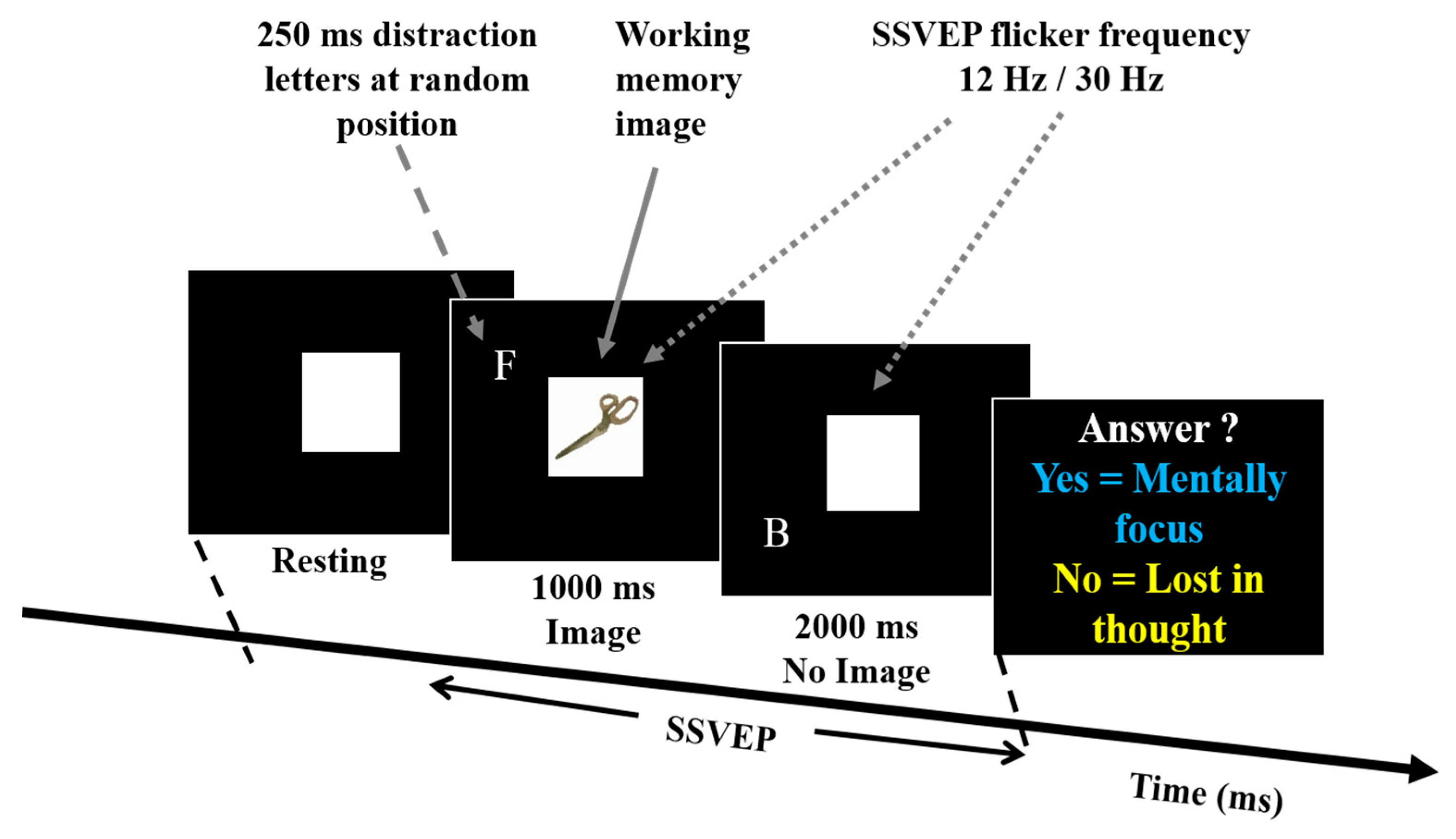
2.2. Experiment Design
2.3. Working Memory Task
2.4. SSVEP Flashing Frequencies at 12 Hz and 30 Hz
2.5. Distraction Task
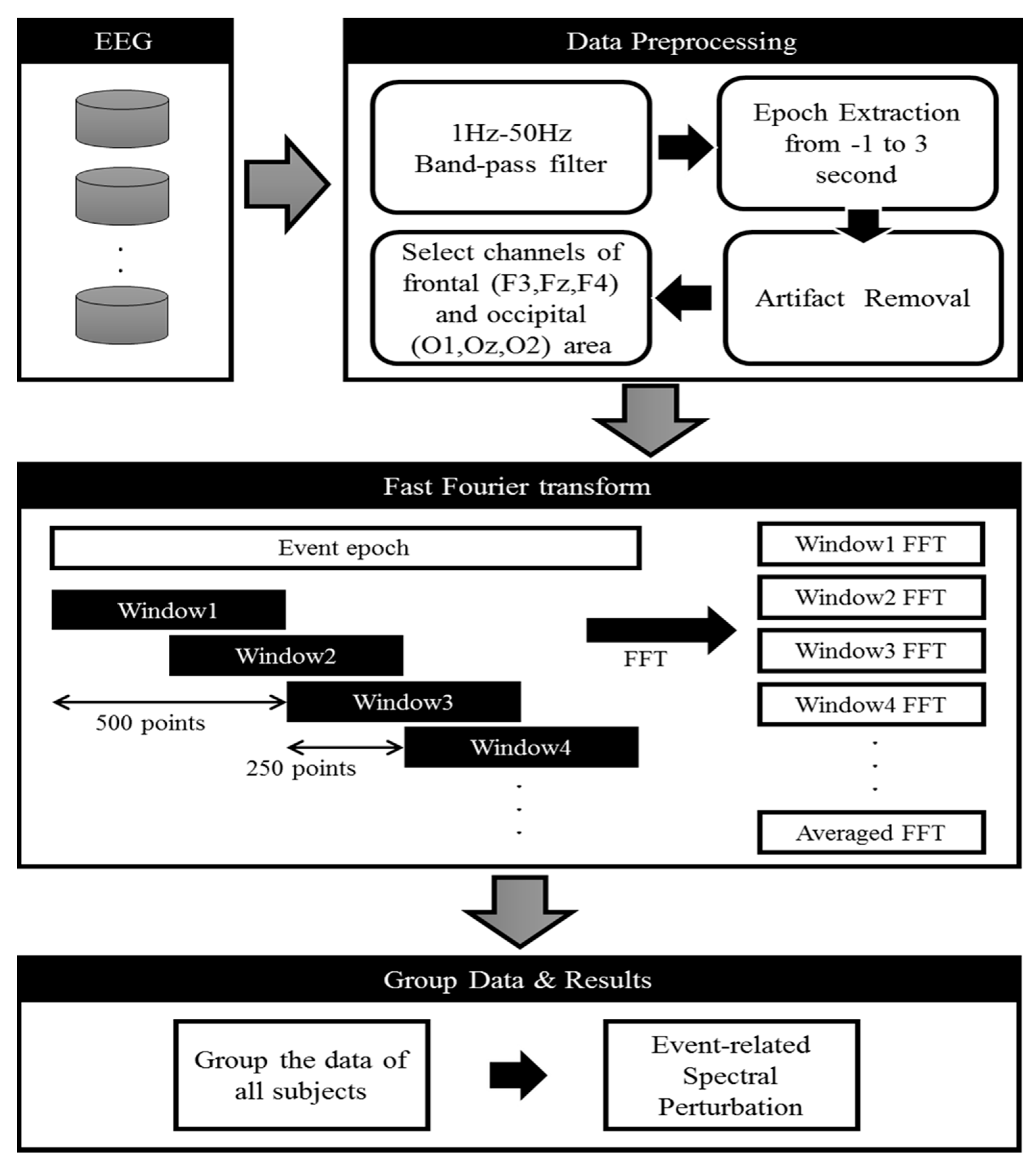
2.6. Acquisition of EEG Signals
2.7. Event-Related Spectral Perturbation (ERSP) Analysis
2.8. Statistical Analysis
2.9. BCI User’s Mental State Changes Monitoring Classification System
3. Results
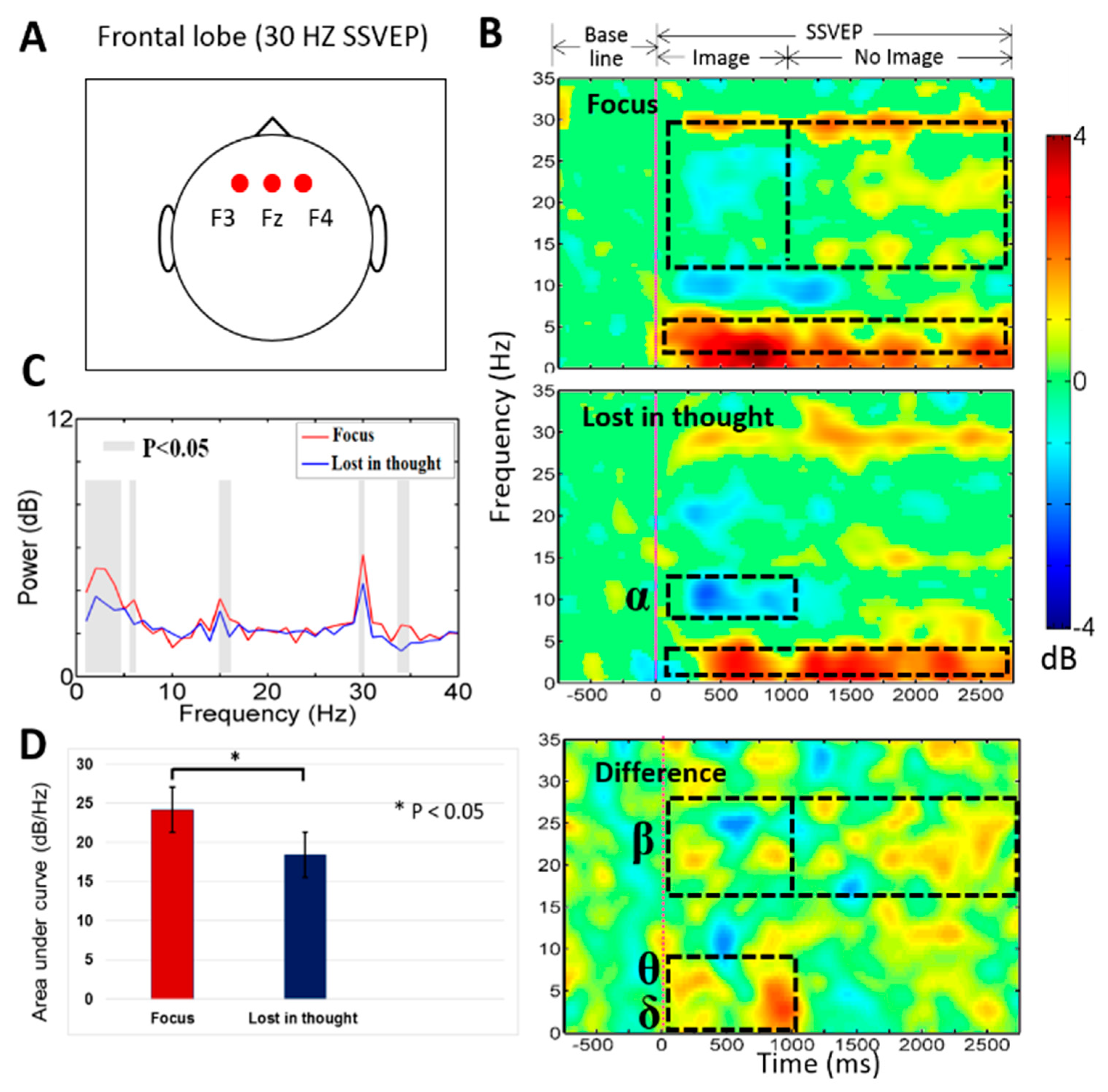
3.1. Neural Activities of Mental Focus and Lost-in-Thought States in the Frontal Lobe
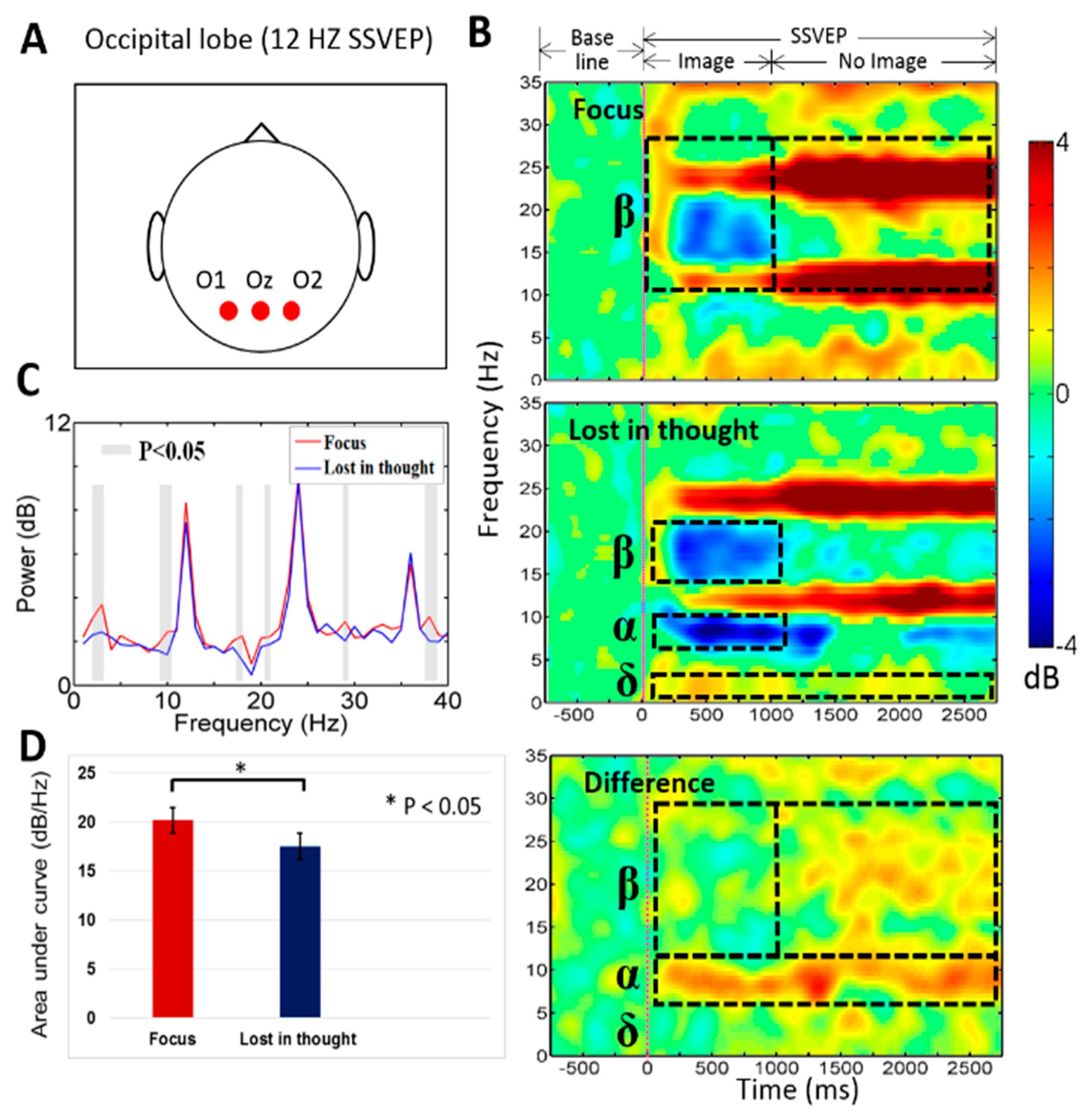
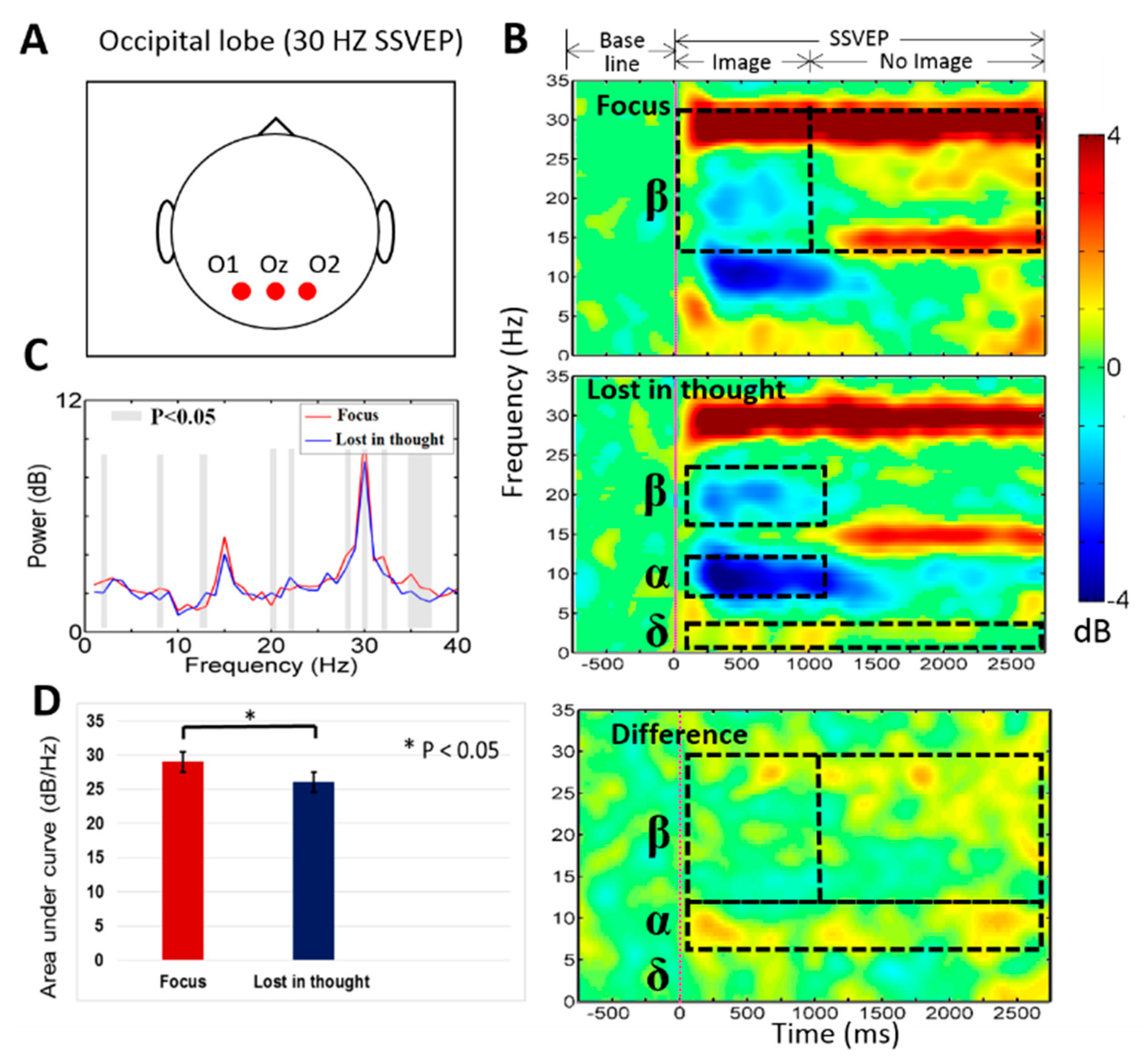
3.2. Neural Activities of Mental Focus and Lost-in-Thought States in the Occipital Lobe
3.3. Power Spectral Density (PSD) during Mental Focus and Lost in Thought States
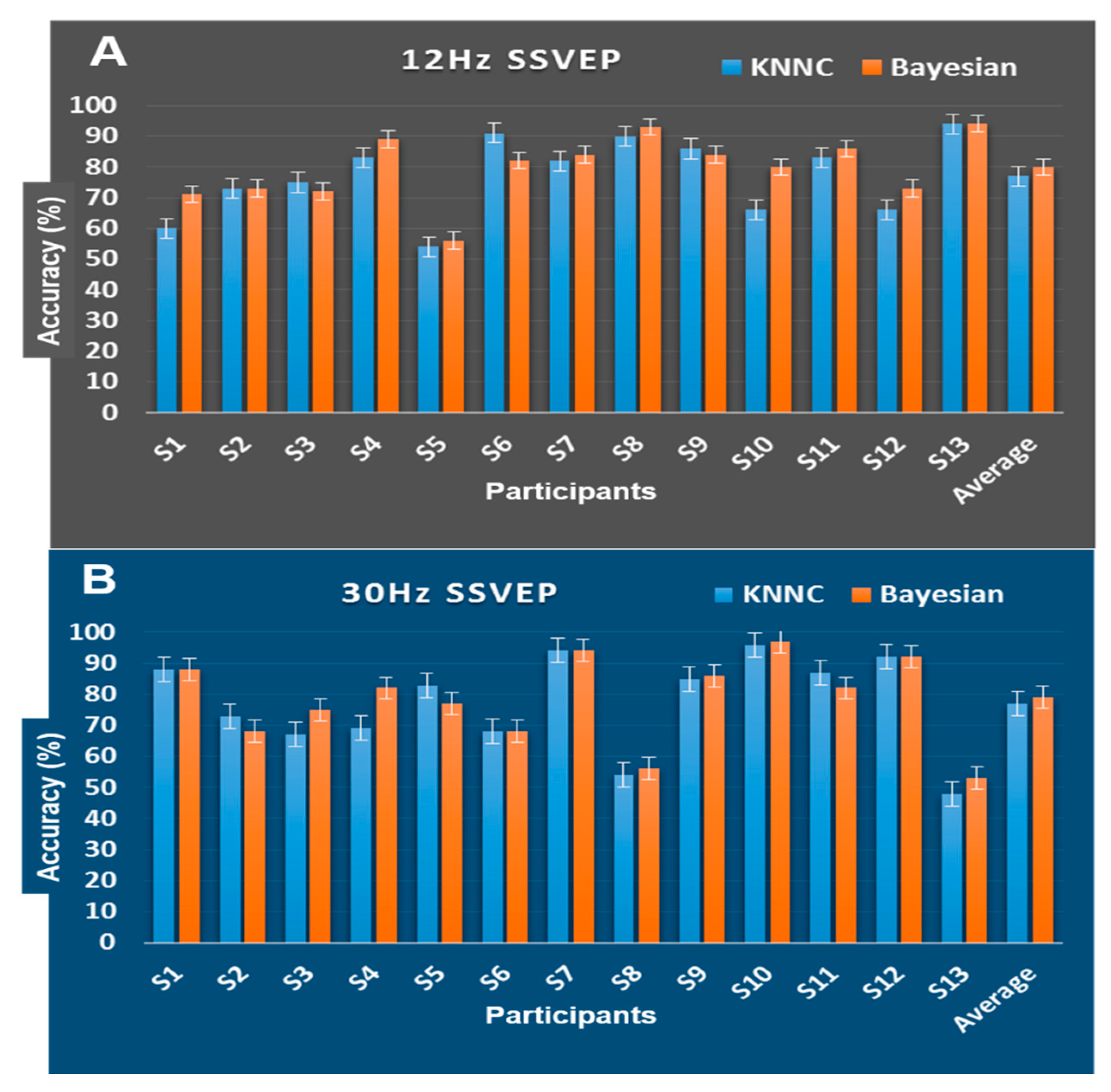
3.4. Mental State Changes Monitoring Classification Results
4. Discussion
4.1. The EEG Power of the Delta (δ), Theta (θ, Beta (β) Bands Increased in the Frontal Lobe during Mental Focus State
4.2. The EEG Power of the Delta (δ), Alpha (α), Beta (β) Bands Increased in the Occipital Lobe at Mental Focus State
4.3. Application and Limitation of This Study
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarah, N.A.; Ayman, A.; Mostafa-Sami, M.M. Brain computer interfacing: Applications and challenges. Egypt. Inf. J. 2015, 16, 213–230. [Google Scholar]
- Tong, J.; Lin, Q.; Xiao, R.; Ding, L. Combining multiple features for error detection and its application in brain-computer interface. Biomed. Eng. Online 2016, 15–17. [Google Scholar] [CrossRef]
- Galloway, N.R. Human brain electrophysiology: Evoked-potentials and evoked magnetic-fields in science and medicine. Br. J. Ophthalmol. 1990, 74, 255. [Google Scholar] [CrossRef]
- Regan, D. Steady-state evoked potentials. J. Opt. Soc. Am. 1977, 67, 1475–1489. [Google Scholar] [CrossRef] [PubMed]
- Richard, B.S.; Mark, A.S.; Andrew, P.; Joseph, C.; Stephen, R.W.; David, G.S. Steady-state visually evoked potential topography associated with a visual vigilance task. Brain Topogr. 1990, 3, 337–347. [Google Scholar]
- Gulbinaite, R.; Johnson, A.; Jong, R.; Morey, C.C.; Rijn, H. Dissociable mechanisms underlying individual differences in visual working memory capacity. Neuroimage 2014, 99, 197–206. [Google Scholar] [CrossRef]
- Smallwood, J.; Schooler, J.W. The restless mind. Psychol. Bull. 2006, 132, 946–958. [Google Scholar] [CrossRef]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering minds: The default network and stimulus-independent thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef]
- Carlstedt, R.A. Handbook of Integrative Clinical Psychology, Psychiatry, and Behavioral Medicine: Perspectives, Practices, and Research; Springer Publishing Company: New York, NY, USA, 2010; p. 887. [Google Scholar]
- Oken, B.S.; Salinsky, M.C.; Elsas, S.M. Vigilance, alertness, or sustained attention: Physiological basis and measurement. Clin. Neurophysiol. 2006, 117, 1885–1901. [Google Scholar] [CrossRef]
- Kane, M.J.; Jarrold, C.; Kane, M.; Miyake, A.; Towse, J. Variation in Working Memory Capacity as Variation in Executive Attention and Control; Oxford University Press: New York, NY, USA, 2007; pp. 21–48. [Google Scholar]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Morgan, S.T.; Hansen, J.C.; Hillyard, S.A. Selective attention to stimulus location modulates the steady-state visual evoked potential. Proc. Natl. Acad. Sci. USA 1996, 93, 4770–4774. [Google Scholar] [CrossRef]
- Muller, M.M.; Pictonb, T.W.; Valdes-Sosac, P.; Rierac, J.; Teder-Sälejärvid, W.A.; Hillyardd, S.A. Effects of spatial selective attention on the steady-state visual evoked potential in the 20–28 Hz range. Brain Res. Cogn. Brain Res. 1998, 6, 249–261. [Google Scholar] [CrossRef]
- Muller, M.M.; Teder-Salejarvi, W.; Hillyard, S.A. The time course of cortical facilitation during cued shifts of spatial attention. Nat. Neurosci. 1998, 1, 631–634. [Google Scholar] [CrossRef]
- Muller, M.M. Sustained division of the attentional spotlight. Nature 2003, 424, 309–312. [Google Scholar] [CrossRef]
- Pei, F.; Pettet, M.W.; Norcia, A.M. Neural correlates of object-based attention. J. Vis. 2002, 2, 588–596. [Google Scholar] [CrossRef]
- Silberstein, R.B.; Ciorciari, J.; Pipingas, A. Steady-state visually evoked potential topography during the Wisconsin card sorting test. Electroencephalogr. Clin. Neurophysiol. 1995, 96, 24–35. [Google Scholar] [CrossRef]
- Hillyard, S.A.; Anllo-Vento, L. Event-related brain potentials in the study of visual selective attention. Proc. Natl. Acad. Sci. USA 1998, 95, 781–787. [Google Scholar] [CrossRef]
- Xie, S.; Liu, C.; Klaus, O.; Zhu, F.; Wang, L.; Xie, X.; Wang, W. Stimulator selection in ssvep-based spatial selective attention study. Comput. Intell. Neurosci. 2016, 9. [Google Scholar] [CrossRef]
- Evain, A.; Argelaguet, F.; Roussel, N.; Casiez, G.; Lecuyer, A. Can I Think of Something Else when Using a BCI? Cognitive demand of an SSVEP-based BCI. In Proceedings of the CHI Conference on Human Factors in Computing Systems (CHI ’17), Denver, CA, USA, 6–11 May 2017; pp. 5120–5125. [Google Scholar]
- Pardo, J.V.; Fox, P.T.; Raichle, M.E. Localization of a human system for sustained attention by positron emission tomography. Nature 1991, 349, 61–64. [Google Scholar] [CrossRef]
- Collette, F.; Van der Linden, M. Brain imaging of the central executive component of working memory. Neurosci. Biobehav. Rev. 2002, 26, 105–125. [Google Scholar] [CrossRef]
- Culham, J.C.; Brandt, S.A.; Cavanagh, P.; Kanwisher, N.G.; Dale, A.M.; Tootell, R.B.H. Cortical fMRI activation produced by attentive tracking of moving targets. J. Neurophysiol. 1998, 80, 2657–2670. [Google Scholar] [CrossRef]
- Bauer, M.; Oostenveld, R.; Peeters, M.; Fries, P. Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J. Neurosci. 2006, 26, 490–501. [Google Scholar] [CrossRef]
- Braboszcz, C.; Delorme, A. Lost in thoughts: Neural markers of low alertness during mind wandering. Neuroimage 2011, 54, 3040–3047. [Google Scholar] [CrossRef]
- Kramberger, I.; Kačič, Z.; Donaj, G. Binocular phase-coded visual stimuli for SSVEP-based BCI. IEEE Access 2019, 7, 48912–48922. [Google Scholar] [CrossRef]
- Schneider, D.; Barth, A.; Wascher, E. On the contribution of motor planning to the retroactive cuing benefit in working memory: Evidence by mu and beta oscillatory activity in the EEG. Neuroimage 2017, 162, 73–85. [Google Scholar] [CrossRef]
- Makeig, S.; Inlow, M. Lapses in alertness: Coherence of fluctuations in performance and EEG spectrum. Electroencephalogr. Clin. Neurophysiol. 1993, 86, 23–35. [Google Scholar] [CrossRef]
- De Gennaro, L.; Ferrara, M.; Bertini, M. The boundary between wakefulness and steep: Quantitative electroencephalographic changes during the sleep onset period. Neuroscience 2001, 107, 1–11. [Google Scholar] [CrossRef]
- Caldwell, J.A.; Prazinko, B.; Caldwell, J.L. Body posture affects electroencephalographic activity and psychomotor vigilance task performance in sleep-deprived subjects. Clin. Neurophysiol. 2003, 114, 23–31. [Google Scholar] [CrossRef]
- Chikara, R.K.; Chang, E.C.; Lu, Y.-C.; Lin, D.-S.; Lin, C.-T.; Ko, L.-W. Monetary Reward and Punishment to Response Inhibition Modulate Activation and Synchronization Within the Inhibitory Brain Network. Front. Hum. Neurosci. 2018, 12, 27. [Google Scholar] [CrossRef]
- Loomis, A.L.; Harvey, E.N.; Hobart, G.A. Cerebral states during sleep, as studied by human brain potential. J. Exp. Psychol. 1937, 21, 127–144. [Google Scholar] [CrossRef]
- Roth, B. The clinical and theoretical importance of EEG rhythms corresponding to states of lowered vigilance. Electroencephalogr. Clin. Neurophysiol. 1961, 13, 395–399. [Google Scholar] [CrossRef]
- Hsu, S.H.; Pion-Tonachini, L.; Palmer, J.; Miyakoshi, M.; Makeig, S.; Jung, T.P. Modeling brain dynamic state changes with adaptive mixture independent component analysis. Neuroimage 2018, 183, 47–61. [Google Scholar] [CrossRef]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Szafir, D.B.; Mutlu, B. Pay attention! Designing adaptive agents that monitor and improve user engagement, in CHI ’12. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, Austin, TX, USA, 5–10 May 2012. [Google Scholar]
- Laufs, H.; Holtd, J.L.; Elfontd, R.; Kramsd, M.; Paulbce, J.S.; Kleinschmidtaf, K.K.A. Where the BOLD signal goes when alpha EEG leaves. Neuroimage 2006, 31, 1408–1418. [Google Scholar] [CrossRef]
- Mantini, D.; Perrucci, M.G.; Del Gratta, C.; Romani, G.L.; Corbetta, M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. USA 2007, 104, 13170–13175. [Google Scholar] [CrossRef]
- Dreyer, A.M.; Herrmann, C.S.; Rieger, J.W. Tradeoff between user experience and BCI classification accuracy with frequency modulated steady-state visual evoked potentials. Front. Hum. Neurosci. 2017, 11, 391. [Google Scholar] [CrossRef]
- Zhang, Q.; Vugt, M.; Borst, J.P.; Anderson, J.R. Mapping working memory retrieval in space and in time: A combined electroencephalography and electrocorticography approach. Neuroimage 2018, 174, 472–484. [Google Scholar] [CrossRef]
- Mathieu, B.B.; Emmanuelle, D.D.; Tina, M.; Martin, L. The bank of standardized stimuli (BOSS), a new set of 480 normative photos of objects to be used as visual stimuli in cognitive research. PLoS ONE 2010, 5, e10773. [Google Scholar]
- Wen, W.; Brann, E.; Costa, S.D.; Haggard, P. Enhanced perceptual processing of self-generated motion: Evidence from steady-state visual evoked potentials. Neuroimage 2018, 175, 438–448. [Google Scholar] [CrossRef]
- Claudio, B.; Robert, J.; Barry, E.B.; Katarzyna, J.; Blinowska, A.C.; Wilhelmus, H.I.M.; Drinkenburg, W.K.; Robert, T.K.; Fernando, L.S.; Paul, N.; et al. International federation of clinical neurophysiology (IFCN)-EEG research workgroup: Recommendations on frequency and topographic analysis of resting state EEG rhythms. Part 1: Applications in clinical research studies. Clin. Neurophysiol. 2020, 131, 285–307. [Google Scholar]
- Nick, K.; Jayant, A.; Sandor, B.; Luis, C.; Simon, F.; Peter, W.K.; Hiroshi, S.; Ronit, P.; Michel, J.A.M.P. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin. Neurophysiol. Pr. 2017, 2, 170–185. [Google Scholar]
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap: Monographs on Statistics & Applied Probability; CRC press: Boca Raton, FL, USA, 1994; ISBN 10: 0412042312. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Lotte, F.; Congedo, M.; Lecuyer, A.; Lamarche, F.; Arnaldi, B. A review of classification algorithms for EEG-based brain-computer interfaces. J. Neural Eng. 2007, 4, 2. [Google Scholar] [CrossRef]
- Rezaei, S.; Tavakolian, K.; Nasrabadi, A.M.; Setarehdan, S.K. Different classification techniques considering brain computer interface applications. J. Neural Eng. 2006, 3, 139. [Google Scholar] [CrossRef]
- Culham, J.C.; Cavanagh, P.; Kanwisher, N.G. Attention response functions: Characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron 2001, 32, 737–745. [Google Scholar] [CrossRef]
- Wittevrongel, B.; Khachatryan, E.; Hnazaee, M.F.; Carrette, E.; Taeye, L.D.; Meurs, A.; Boon, P.; Roost, D.V. Representation of steady-state visual evoked potentials elicited by luminance flicker in human occipital cortex: An electrocorticography study. Neuroimage 2018, 175, 315–326. [Google Scholar] [CrossRef]
- Onton, J.; Delorme, A.S.; Makeig, S. Frontal midline EEG dynamics during working memory. Neuroimage 2005, 27, 341–356. [Google Scholar] [CrossRef]
- Jensen, O.; Tesche, C.D. Frontal theta activity in humans increases with memory load in a working memory task. Eur. J. Neurosci. 2002, 15, 1395–1399. [Google Scholar] [CrossRef]
- Harmony, T.; Fernándeza, T.; Silvaa, J.; Bernala, J.; Díaz-Comasb, L.; Reyesa, L.; Marosia, E.; Rodrígueza, M.; Rodrígueza, M. EEG delta activity: An indicator of attention to internal processing during performance of mental tasks. Int. J. Psychophysiol. 1996, 24, 161–171. [Google Scholar] [CrossRef]
- Labecki, M.; Nowicka, M.M.; Suffczynski, P. Temporal modulation of steady-state visual evoked potentials. Int. J. Neural. Syst. 2019, 29, 1850050. [Google Scholar] [CrossRef]
- Chikara, R.K.; Ko, L.W. Neural Activities Classification of Human Inhibitory Control Using Hierarchical Model. Sensors 2019, 19, 3791. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, B.; Wang, Y.; Xu, S.; Ga, X. Control of a 7-DOF robotic arm system with an SSVEP-based BCI. Int. J. Neural. Syst. 2018, 28, 1850018. [Google Scholar] [CrossRef]
- Zhang, S.; Han, X.; Chen, X.; Wang, Y.; Gao, S.; Gao, X. A study on dynamic model of steady-state visual evoked potentials. J. Neural Eng. 2018, 15, 46010. [Google Scholar] [CrossRef]
- Astrand, E. A continuous time-resolved measure decoded from EEG oscillatory activity predicts working memory task performance. J. Neural Eng. 2018, 15, 36021. [Google Scholar] [CrossRef]
- Dreze, X.; Hussherr, F.X. Internet advertising: Is anybody watching? J. Interact. Market. 2003, 17, 2–72. [Google Scholar] [CrossRef]
- Malheiros, M.; Jennett, C.; Patelm, S.; Brostoff, S.; Angela, M. Too close for comfort: A study of the effectiveness and acceptability of rich-media personalized advertising. In Proceedings of the CHI ’12, SIGCHI Conference on Human Factors in Computing Systems, Austin, TX, USA, 5 May 2012. [Google Scholar]
- Zhang, T.; Liu, T.F.; Li, F.; Li, M.; Liu, D.; Zhang, R.; He, H.; Li, P.; Gong, J.; Luo, C. Structural and functional correlates of motor imagery BCI performance: Insights from the patterns of fronto-parietal attention network. Neuroimage 2016, 134, 475–485. [Google Scholar] [CrossRef]
- Ko, L.-W.; Shih, Y.-C.; Chikara, R.K.; Chuang, Y.-T.; Chang, E.C. Neural Mechanisms of Inhibitory Response in a Battlefield Scenario: A Simultaneous fMRI-EEG Study. Front. Hum. Neurosci. 2016, 10, 185. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, L.-W.; Chikara, R.K.; Lee, Y.-C.; Lin, W.-C. Exploration of User’s Mental State Changes during Performing Brain–Computer Interface. Sensors 2020, 20, 3169. https://doi.org/10.3390/s20113169
Ko L-W, Chikara RK, Lee Y-C, Lin W-C. Exploration of User’s Mental State Changes during Performing Brain–Computer Interface. Sensors. 2020; 20(11):3169. https://doi.org/10.3390/s20113169
Chicago/Turabian StyleKo, Li-Wei, Rupesh Kumar Chikara, Yi-Chieh Lee, and Wen-Chieh Lin. 2020. "Exploration of User’s Mental State Changes during Performing Brain–Computer Interface" Sensors 20, no. 11: 3169. https://doi.org/10.3390/s20113169
APA StyleKo, L.-W., Chikara, R. K., Lee, Y.-C., & Lin, W.-C. (2020). Exploration of User’s Mental State Changes during Performing Brain–Computer Interface. Sensors, 20(11), 3169. https://doi.org/10.3390/s20113169



