Ultra-Low Power Wearable Infant Sleep Position Sensor
Abstract
1. Introduction
2. Materials and Methods
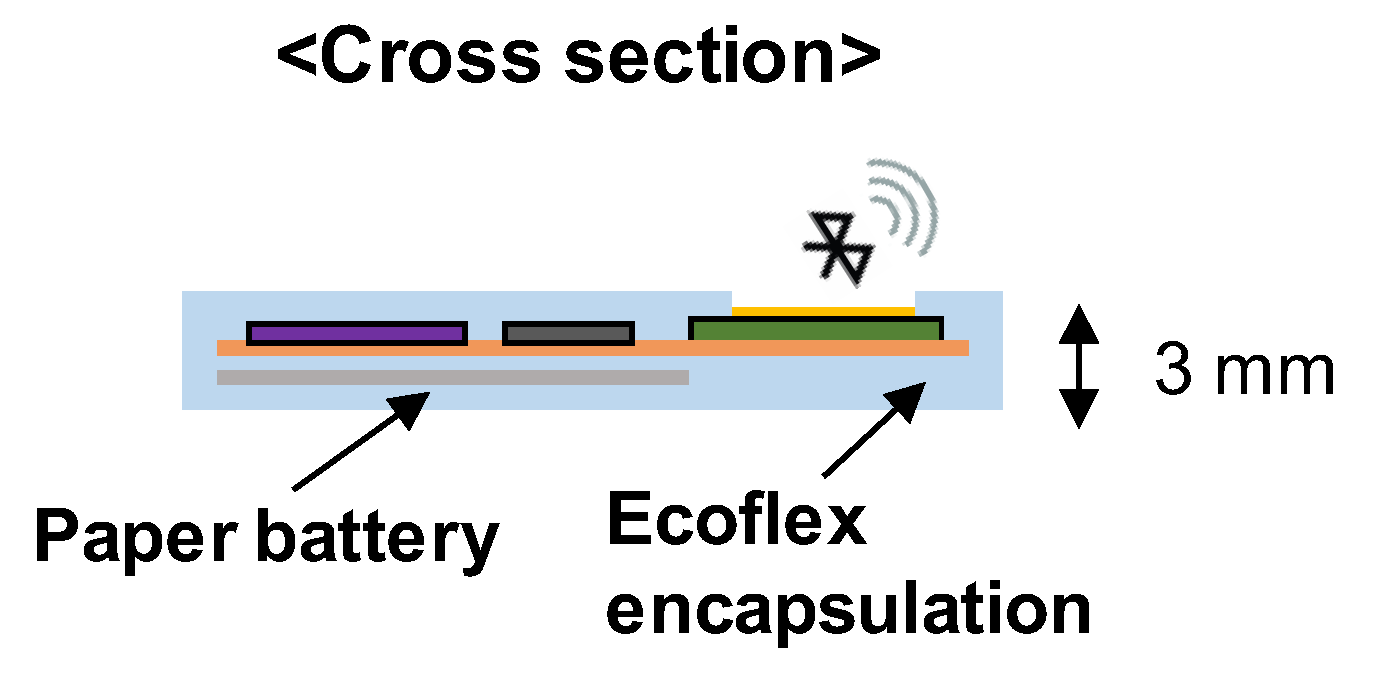
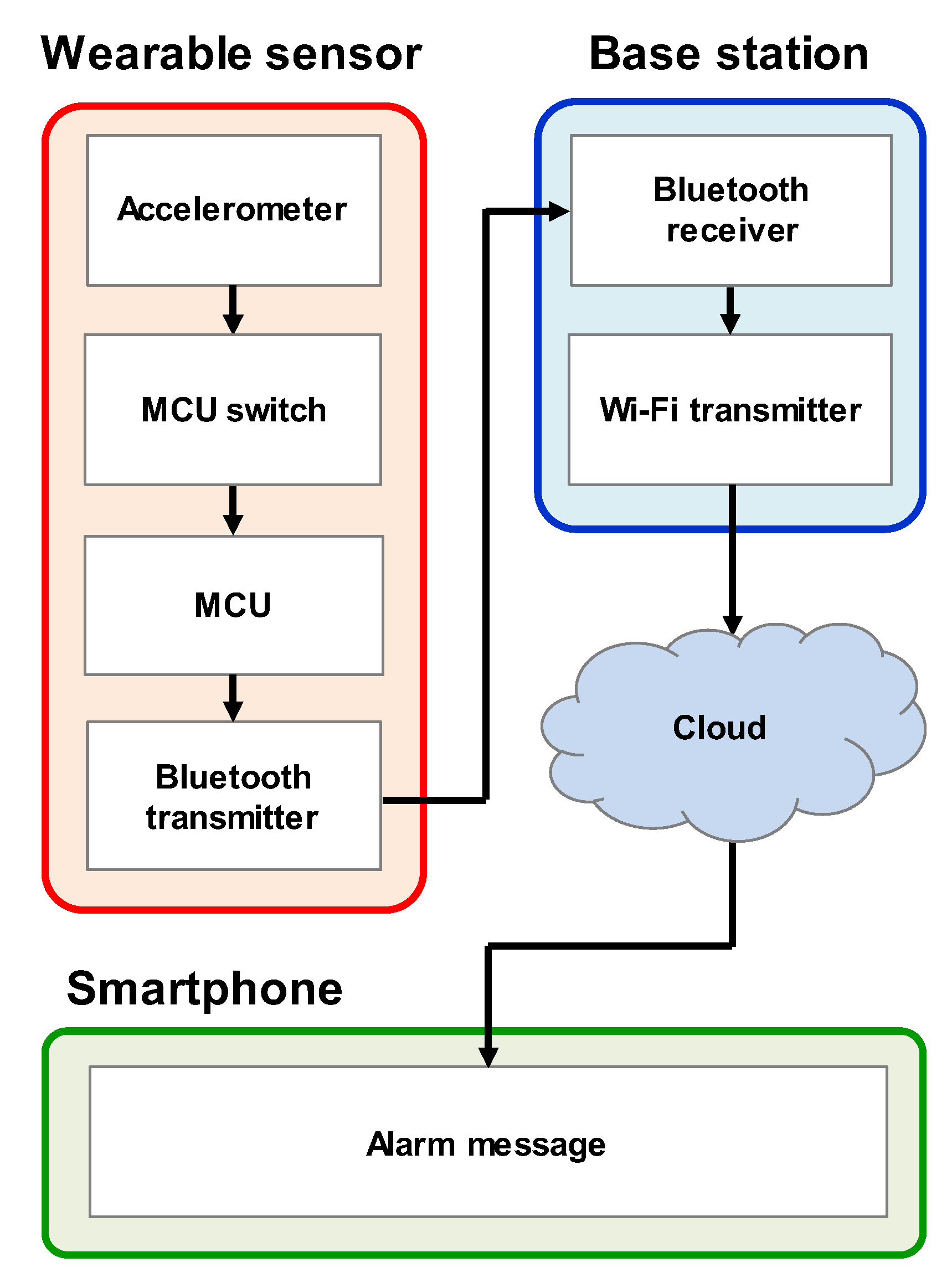
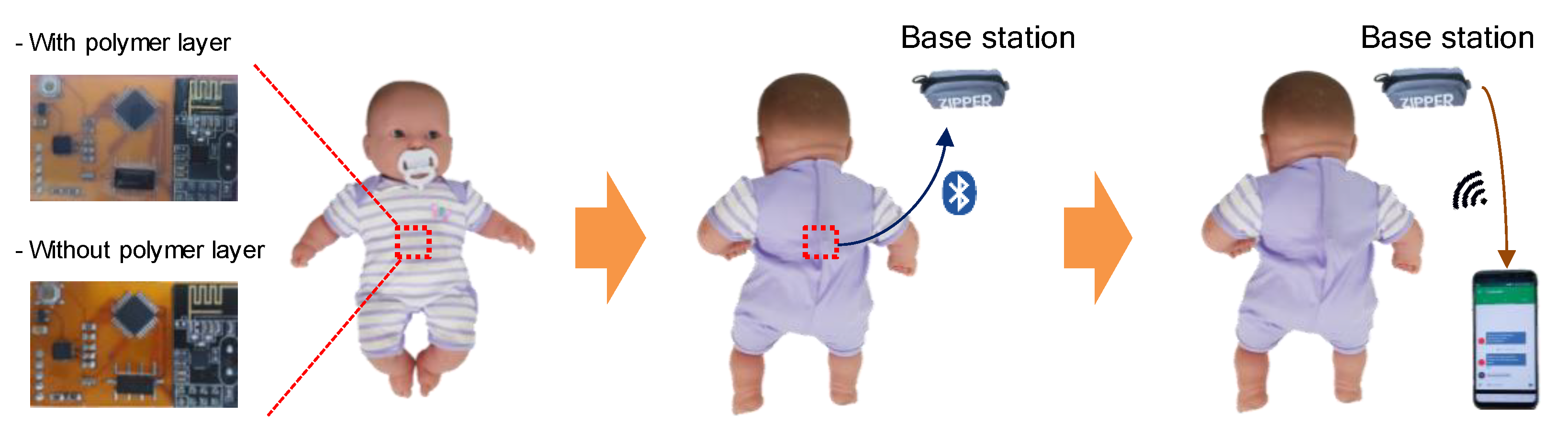
2.1. Fabrication of Wearable Sleep Monitoring Sensor
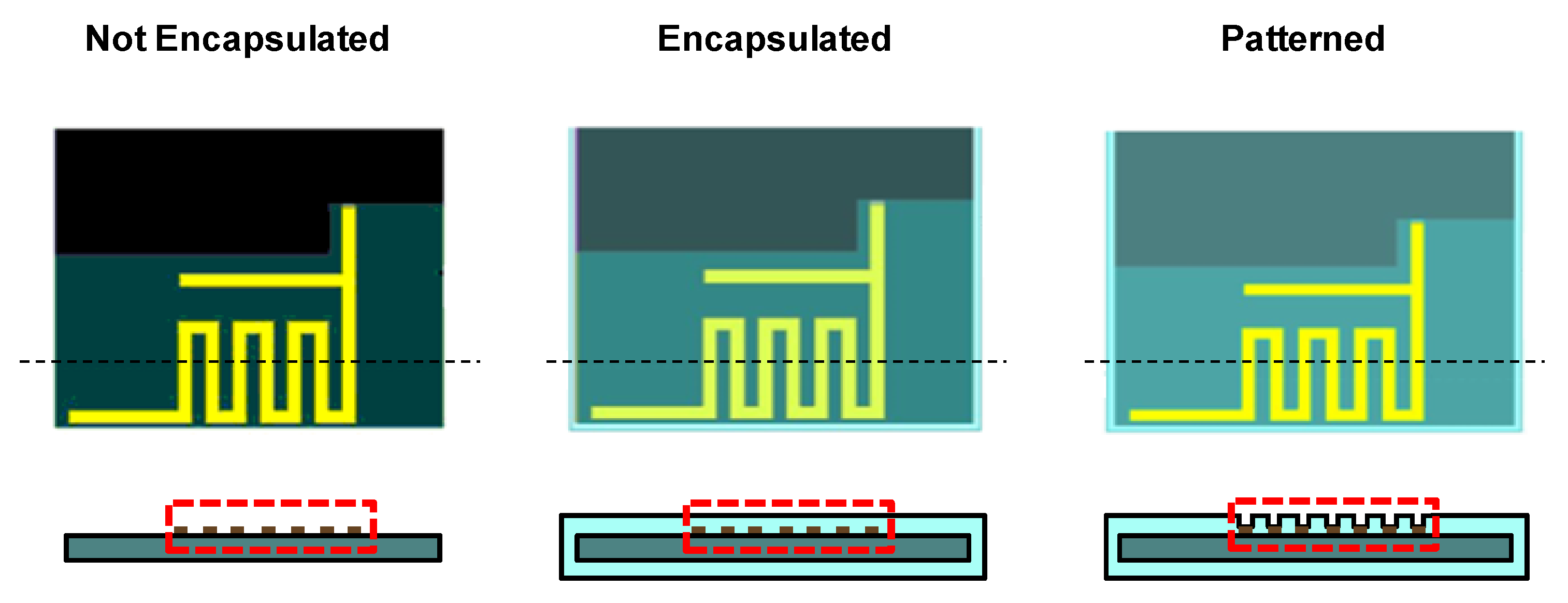
2.2. Measurement of Dielectric Constant and Loss Tangent
2.3. Power Consumption Measurement
3. Results
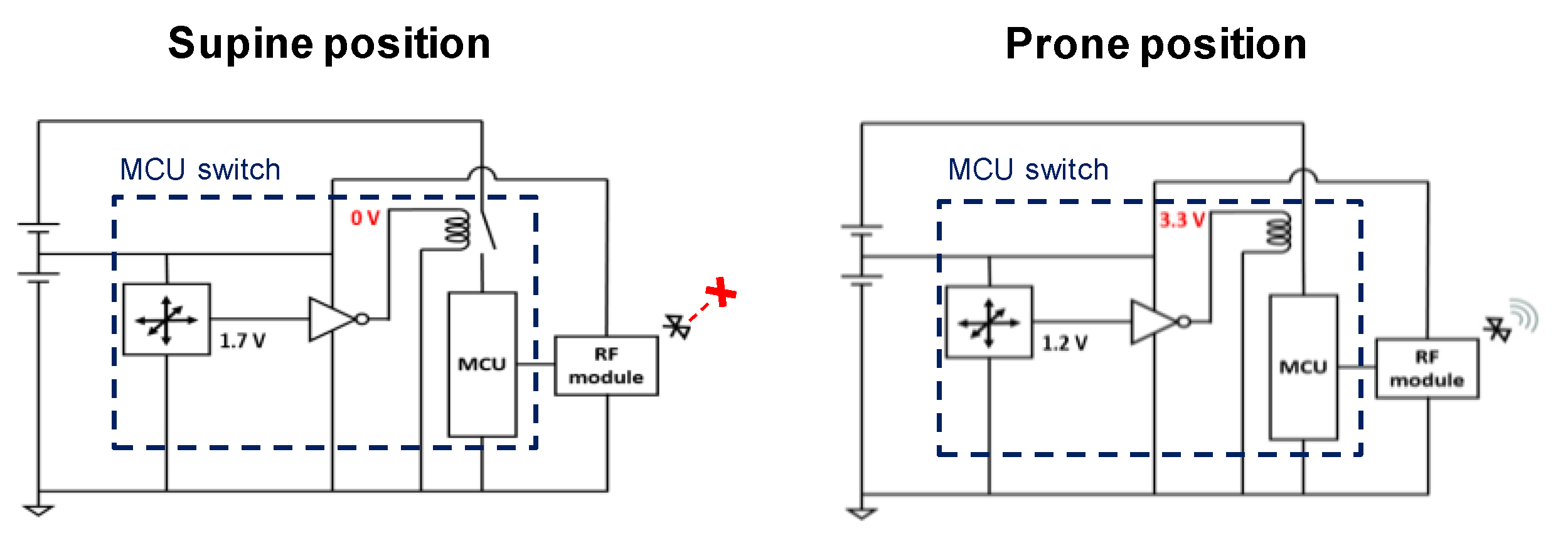
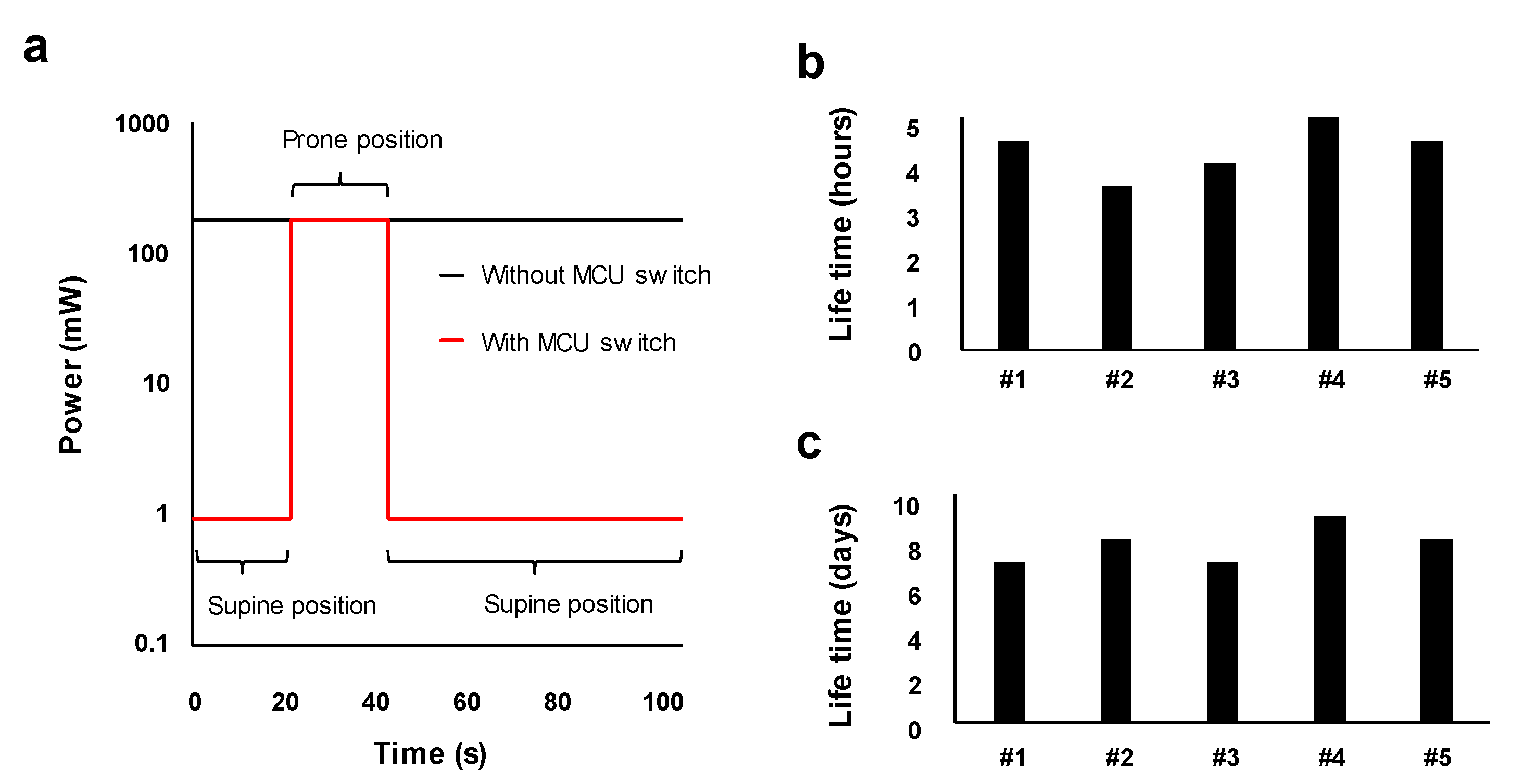
3.1. Low-Power Operation System
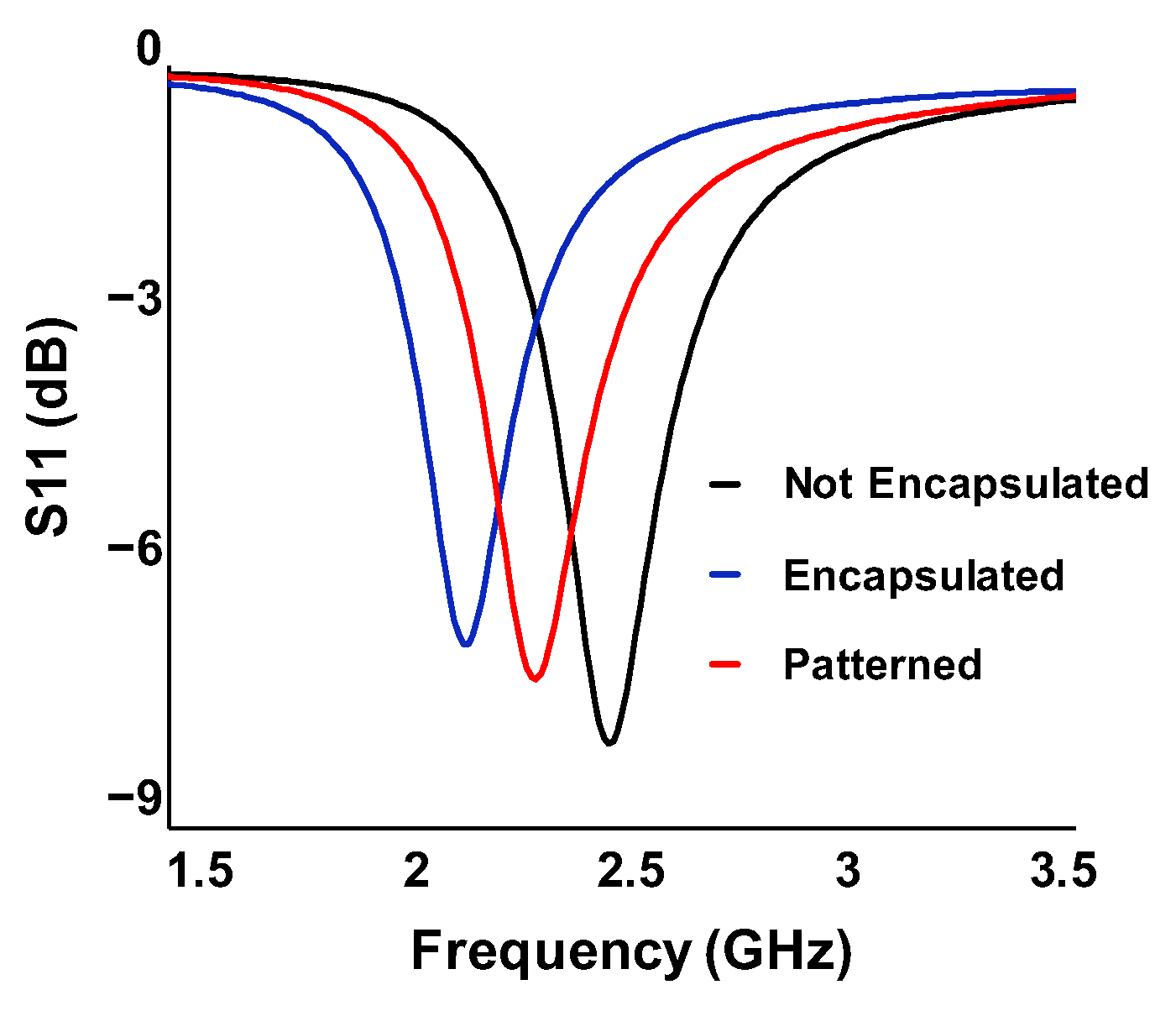
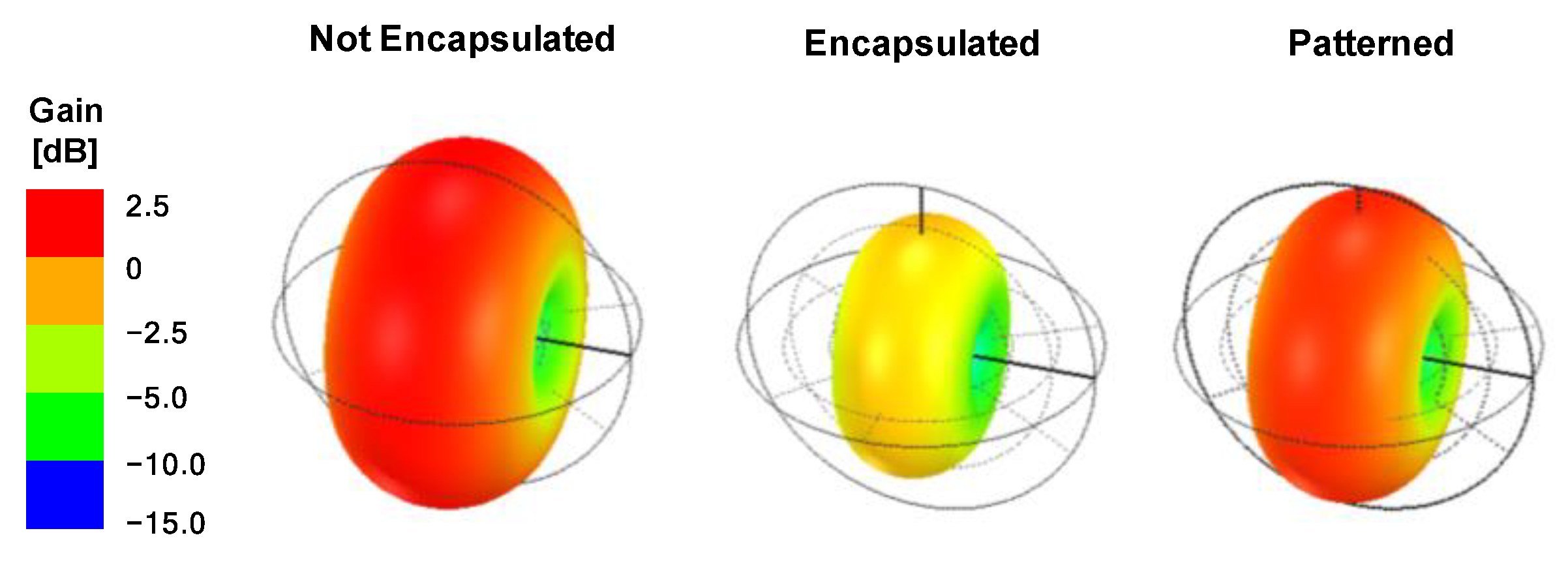
3.2. Antenna Radiation Characteristics
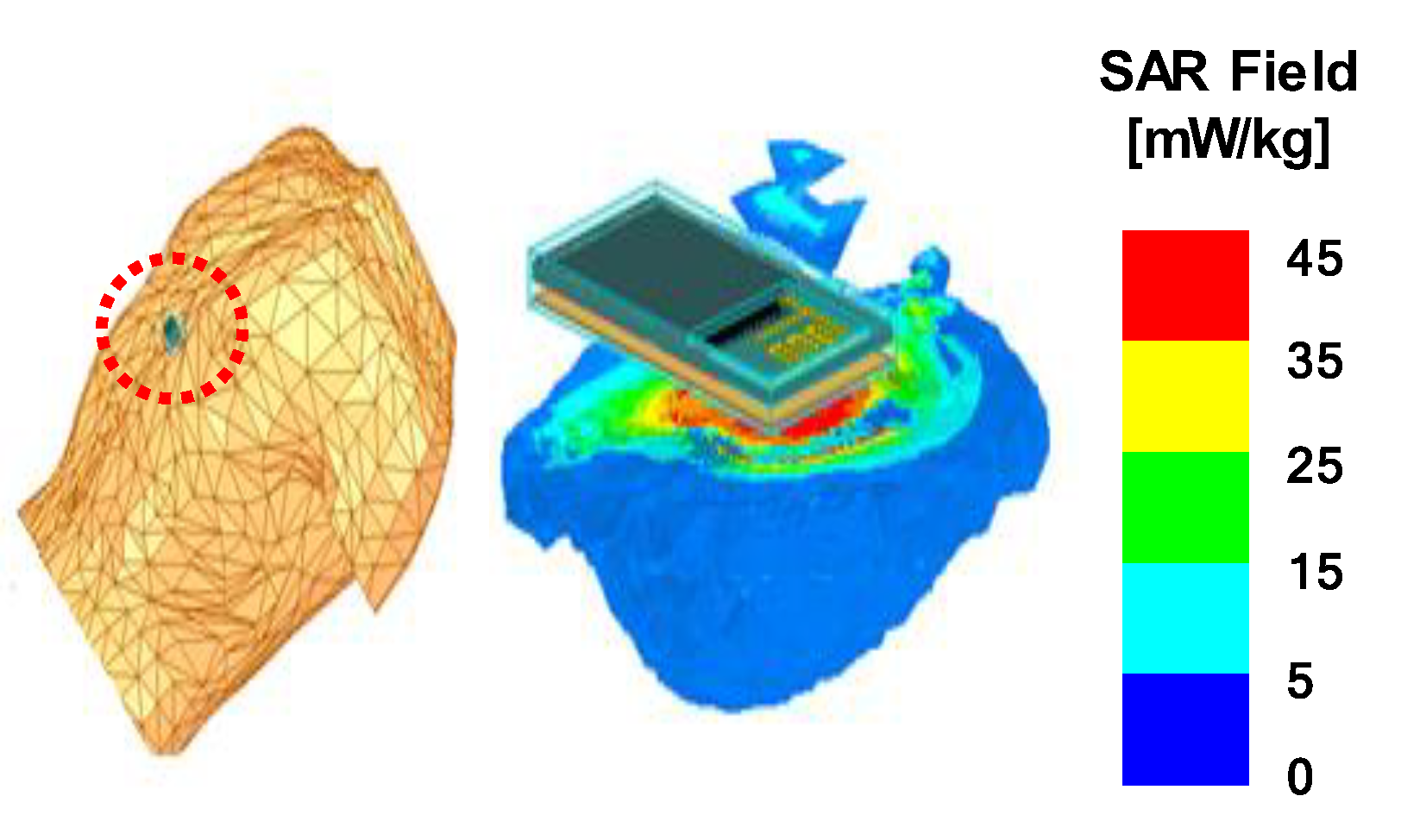
3.3. Specific Absorption Rate (SAR) Simulation
4. Infant Sleep Monitoring System Demonstration
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chung, H.U.; Kim, B.H.; Lee, J.Y.; Lee, J.; Xie, Z.Q.; Ibler, E.M.; Lee, K.; Banks, A.; Jeong, J.Y.; Kim, J.; et al. Binodal, wireless epidermal electronic systems with in-sensor analytics for neonatal intensive care. Science 2019, 363. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J.; Yun, I.; Bae, G.Y.; Kim, D.; Park, S.; Yi, I.M.; Moon, W.; Chung, Y.; Cho, K. An ultrathin conformable vibration-responsive electronic skin for quantitative vocal recognition. Nat. Commun. 2019, 10, 2468. [Google Scholar] [CrossRef] [PubMed]
- Boutry, C.M.; Kaizawa, Y.; Schroeder, B.C.; Chortos, A.; Legrand, A.; Wang, Z.; Chang, J.; Fox, P.; Bao, Z. A stretchable and biodegradable strain and pressure sensor for orthopaedic application. Nat. Electron. 2018, 1, 314–321. [Google Scholar] [CrossRef]
- Jeong, J.W.; Kim, M.K.; Cheng, H.Y.; Yeo, W.H.; Huang, X.; Liu, Y.H.; Zhang, Y.H.; Huang, Y.G.; Rogers, J.A. Capacitive Epidermal Electronics for Electrically Safe, Long-Term Electrophysiological Measurements. Adv. Healthc. Mater. 2014, 3, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lee, B.L.; Chung, W.Y. Smartwatch-Based Wearable EEG System for Driver Drowsiness Detection. IEEE Sens. J. 2015, 15, 7169–7180. [Google Scholar] [CrossRef]
- Jung, M.H.; Namkoong, K.; Lee, Y.; Koh, Y.J.; Eom, K.; Jang, H.; Bae, J.; Park, J. Wrist-wearable bioelectrical impedance analyzer with contact resistance compensation function. In Proceedings of the IEEE Sensors, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]
- Lee, H.; Choi, T.K.; Lee, Y.B.; Cho, H.R.; Ghaffari, R.; Wang, L.; Choi, H.J.; Chung, T.D.; Lu, N.S.; Hyeon, T.; et al. A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 2016, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Spano, E.; Di Pascoli, S.; Iannaccone, G. Low-Power Wearable ECG Monitoring System for Multiple-Patient Remote Monitoring. IEEE Sens. J. 2016, 16, 5452–5462. [Google Scholar] [CrossRef]
- Flores, M.; Glusman, G.; Brogaard, K.; Price, N.D.; Hood, L. P4 medicine: How systems medicine will transform the healthcare sector and society. Pers. Med. 2013, 10, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Samadani, A.; Lee, A.; Kulić, D. A Spinal Motion Measurement Protocol Utilizing Inertial Sensors Without Magnetometers. In Proceedings of the 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018. [Google Scholar]
- Jung, H.T.; Park, J.; Jeong, J.; Ryu, T.; Kim, Y.; Lee, S.I. A wearable monitoring system for at-home stroke rehabilitation exercises: A preliminary study. In Proceedings of the IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Las Vegas, NV, USA, 4–7 March 2018. [Google Scholar]
- Hassan, E.A.; Elbially, M.S.; Morsy, A.A. Monitoring and evaluation of ingestive activities. In Proceedings of the IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Las Vegas, NV, USA, 4–7 March 2018. [Google Scholar]
- Curry, E.J.; Ke, K.; Chorsi, M.T.; Wrobel, K.S.; Miller, A.N.; Patel, A.; Kim, I.; Feng, J.L.; Yue, L.X.; Wu, Q.; et al. Biodegradable Piezoelectric Force Sensor. Proc. Natl. Acad. Sci. USA 2018, 115, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Park, J.; Lee, Y.; Ko, H. Triboelectric Generators and Sensors for Self-Powered Wearable Electronics. ACS Nano 2015, 9, 3421–3427. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Redouté, J.M.; Yuce, M. A Wearable, Low-Power, Real-Time ECG Monitor for Smart T-shirt and IoT Healthcare Applications. In Advances in Body Area Networks I; Springer: Cham, Switzerland, 2019; pp. 165–173. [Google Scholar]
- Bhat, G.; Deb, R.; Chaurasia, V.V.; Shill, H.; Ogras, U.Y. Online human activity recognition using low-power wearable devices, In Proceedings of the International Conference on Computer-Aided Design, San Diego, CA, USA, 5–8 November 2018.
- Yazicioglu, R.F.; Torfs, T.; Merken, P.; Penders, J.; Leonov, V.; Puers, R.; Gyselinckx, B.; Van Hoof, C. Ultra-low-power biopotential interfaces and their applications in wearable and implantable systems. Microelectron. J. 2009, 40, 1313–1321. [Google Scholar] [CrossRef]
- Kim, J.; Gutruf, P.; Chiarelli, A.M.; Heo, S.Y.; Cho, K.; Xie, Z.Q.; Banks, A.; Han, S.; Jang, K.I.; Lee, J.W.; et al. Miniaturized Battery-Free Wireless Systems for Wearable Pulse Oximetry. Adv. Funct. Mater. 2017, 27, 1604373. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Lee, D.; Lee, T.I.; Shin, J.H.; Choi, G.M.; Kim, C.; Lee, S.H.; Lee, J.H.; Kim, Y.H.; Kang, S.M.; et al. Wireless powered wearable micro light-emitting diodes. Nano Energy 2019, 55, 454–462. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Molinnus, D.; Mirza, O.; Guinovart, T.; Windmiller, J.R.; Valdes-Ramirez, G.; Andrade, F.J.; Schoning, M.J.; Wang, J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gutruf, P.; Meacham, K.; Montana, M.C.; Zhao, X.Y.; Chiarelli, A.M.; Vazquez-Guardado, A.; Norris, A.; Lu, L.Y.; Guo, Q.L.; et al. Wireless, battery-free optoelectronic systems as subdermal implants for local tissue oximetry. Sci. Adv. 2019, 5. [Google Scholar] [CrossRef] [PubMed]
- Sudden Unexpected Infant Death and Sudden Infant Death Syndrome. Available online: https://www.cdc.gov/sids/data.htm (accessed on 1 September 2019).
- Zhao, F.; Li, M.; Jiang, Z.Y.; Tsien, J.Z.; Lu, Z.H. Camera-Based, Non-Contact, Vital-Signs Monitoring Technology May Provide a Way for the Early Prevention of SIDS in Infants. Front. Neurol. 2016, 7, 236. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.G.; Fernandes, D.; Branco, S.; Monteiro, J.L.; Cabral, J.; Catarino, A.P.; Rocha, A.M. A Smart Wearable System for Sudden Infant Death Syndrome Monitoring. In Proceedings of the IEEE International Conference on Industrial Technology (ICIT), Taipei, Taiwan, 14–17 March 2016. [Google Scholar]
- Fonseca, A.M.; Horta, E.T.; Sendra, S.; Rodrigues, J.J.P.C.; Moutinho, J.A.F. A Sudden Infant Death Prevention System for Babies. In Proceedings of the IEEE 16th International Conference on E-Health Networking, Applications and Services (Healthcom), Natal, Brazil, 15–18 October 2014. [Google Scholar]
- Lin, W.; Zhang, R.K.; Brittelli, J.; Lehmann, C. Wireless Infant Monitoring Device for the Prevention of Sudden Infant Death Syndrome. In Proceedings of the 11th International Conference & Expo on Emerging Technologies for a Smarter World (CEWIT), Melville, NY, USA, 29–30 October 2014. [Google Scholar]
- Moon, R.Y.; Syn, T.F.S.I.D. SIDS and Other Sleep-Related Infant Deaths: Evidence Base for 2016 Updated Recommendations for a Safe Infant Sleeping Environment. Pediatrics 2016, 138, e20162940. [Google Scholar] [CrossRef] [PubMed]
- Stanford Children’s Health: Infant Sleep. Available online: https://www.stanfordchildrens.org/en/topic/default?id=infant-sleep-90-P02237 (accessed on 16 September 2019).
- Hadjem, A.; Lautru, D.; Dale, C.; Wong, M.F.; Hanna, V.F.; Wiart, J. Study of specific absorption rate (SAR) induced in two child head models and in adult heads using mobile phones. IEEE Trans. Microw. Theory Tech. 2005, 53, 4–11. [Google Scholar] [CrossRef]
- Demir, A.F.; Abbasi, Q.H.; Ankarali, Z.E.; Alomainy, A.; Qaraqe, K.; Serpedin, E.; Arslan, H. Anatomical Region-Specific In Vivo Wireless Communication Channel Characterization. IEEE J. Biomed. Health 2017, 21, 1254–1262. [Google Scholar] [CrossRef]
- NRF24L01 RF Board (A) Overview. Available online: https://www.waveshare.com/nrf24l01-rf-board.htm (accessed on 1 September 2019).
- ICNIRP Guideline. ICNIRP Guidelines for Limiting Exposure to Time-Varying Electric, Magnetic and Electromagnetic Fields. Health Phys. 1998, 74, 494–522. [Google Scholar]
- Specific Absorption Rate (SAR) Summary. Available online: https://rra.go.kr/en/sar/summary.do (accessed on 1 September 2019).
| Power Consumption (mW) | |
|---|---|
| Accelerometer | 0.81 |
| Microcontroller unit (MCU) | 154.14 |
| Radio frequency (RF) module | 39.29 |
| Frequency (MHz) | Ecoflex | |
|---|---|---|
| ε/ε0 | tan δ | |
| 868 1 | 2.814 | 0.018 |
| 900 2 | 2.813 | 0.018 |
| 915 3 | 2.813 | 0.018 |
| 2400 4 | 2.811 | 0.014 |
| 5000 5 | 2.809 | 0.016 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, I.; Jeung, J.; Kim, M.; Kim, Y.-S.; Chung, Y. Ultra-Low Power Wearable Infant Sleep Position Sensor. Sensors 2020, 20, 61. https://doi.org/10.3390/s20010061
Yun I, Jeung J, Kim M, Kim Y-S, Chung Y. Ultra-Low Power Wearable Infant Sleep Position Sensor. Sensors. 2020; 20(1):61. https://doi.org/10.3390/s20010061
Chicago/Turabian StyleYun, Inyeol, Jinpyeo Jeung, Mijung Kim, Young-Seok Kim, and Yoonyoung Chung. 2020. "Ultra-Low Power Wearable Infant Sleep Position Sensor" Sensors 20, no. 1: 61. https://doi.org/10.3390/s20010061
APA StyleYun, I., Jeung, J., Kim, M., Kim, Y.-S., & Chung, Y. (2020). Ultra-Low Power Wearable Infant Sleep Position Sensor. Sensors, 20(1), 61. https://doi.org/10.3390/s20010061






