Vertically Coupled Plasmonic Racetrack Ring Resonator for Biosensor Applications
Abstract
:1. Introduction
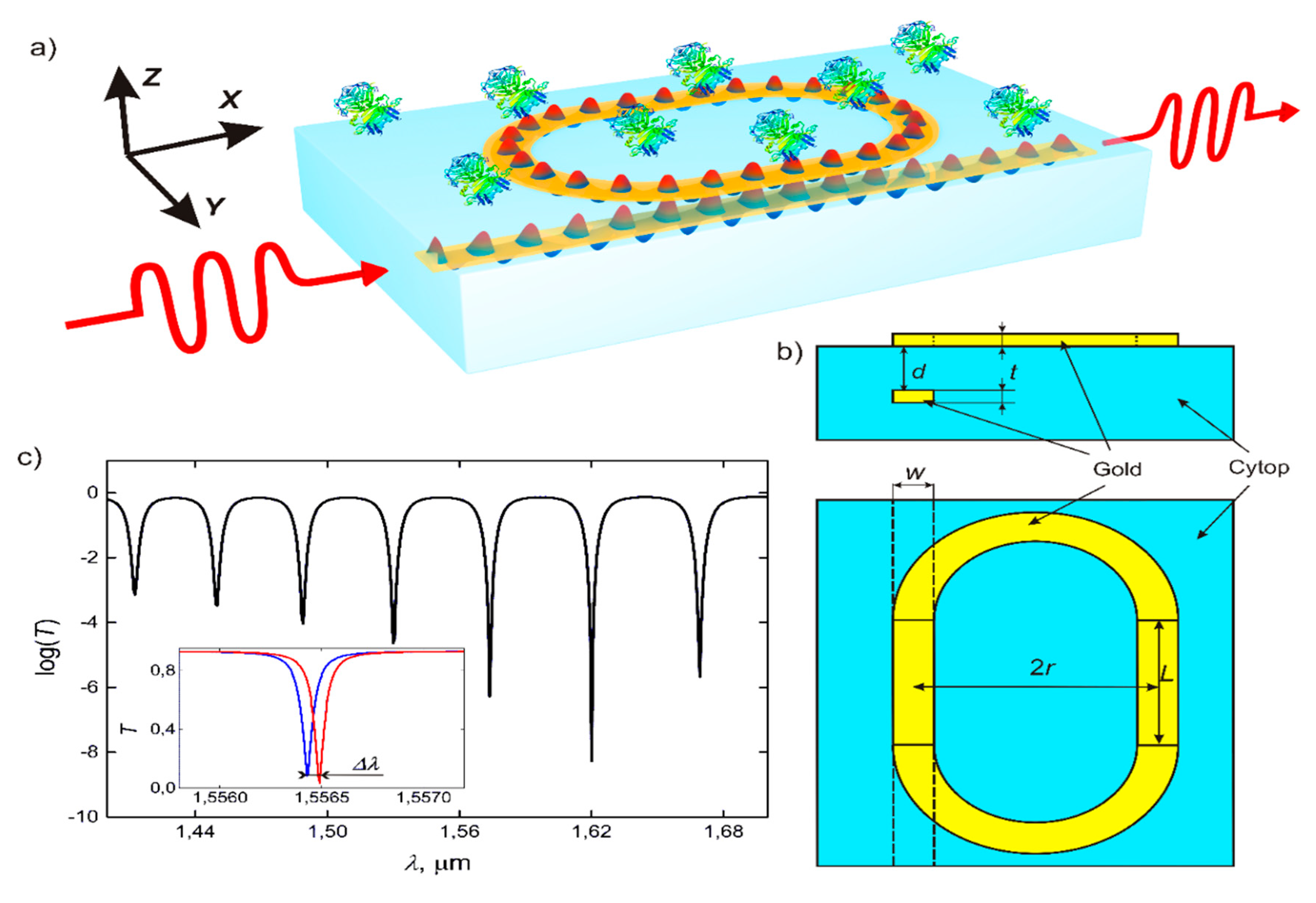
2. Device Configuration and Operation Principles
3. Simulation of a Resonator and Waveguide Coupling
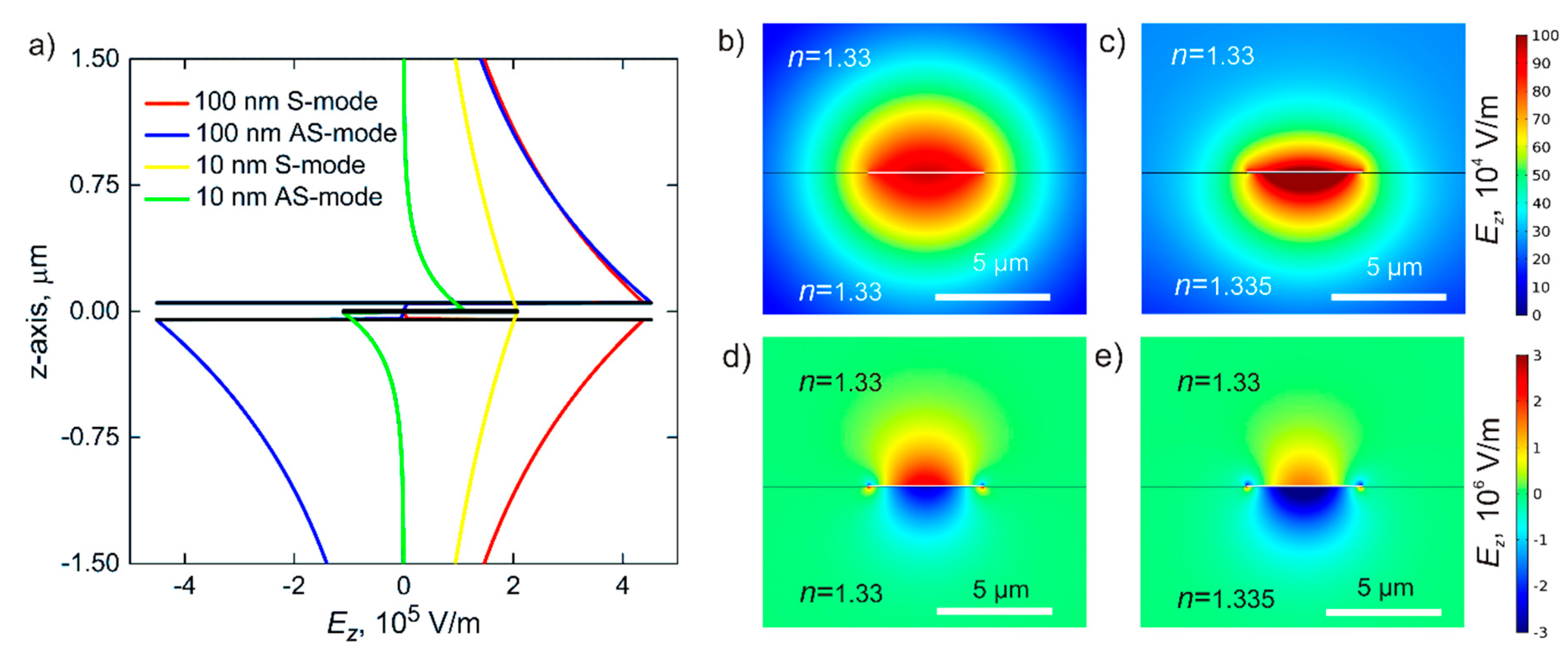
3.1. Waveguide
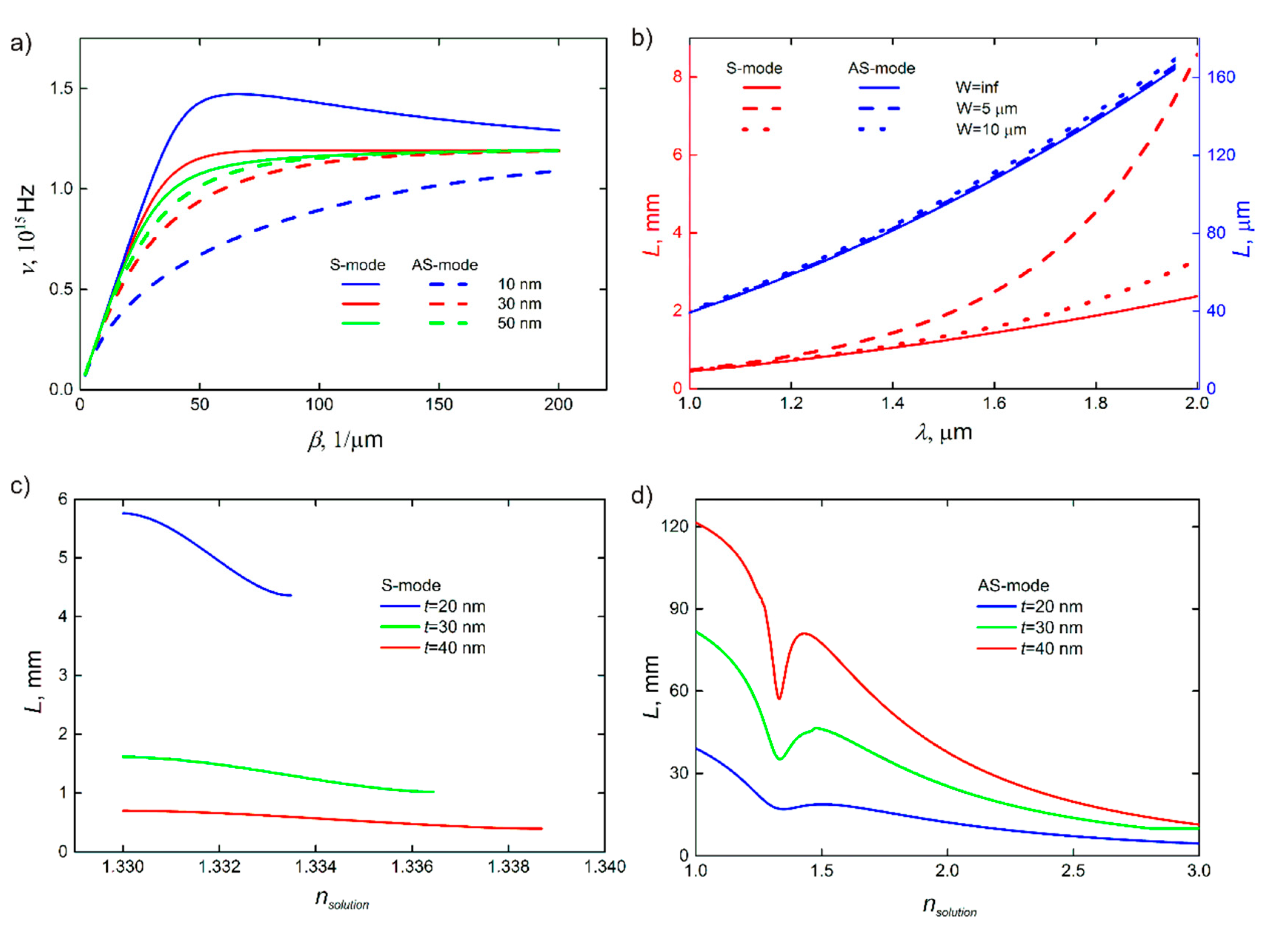
3.2. Coupling of Modes
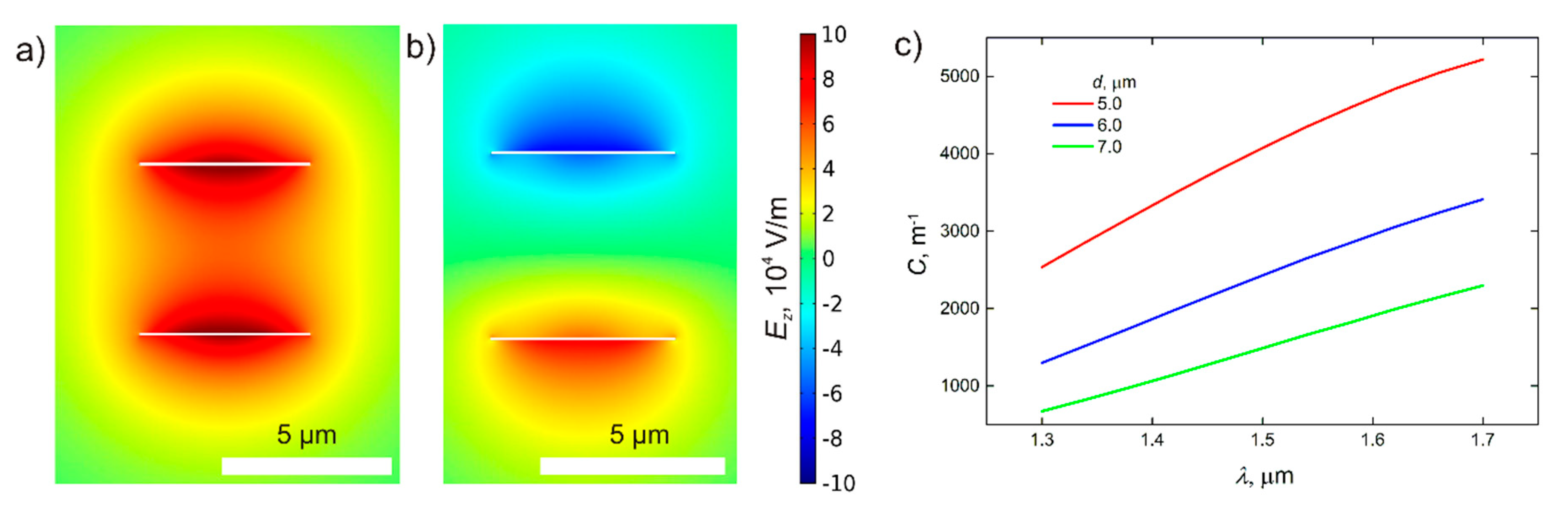
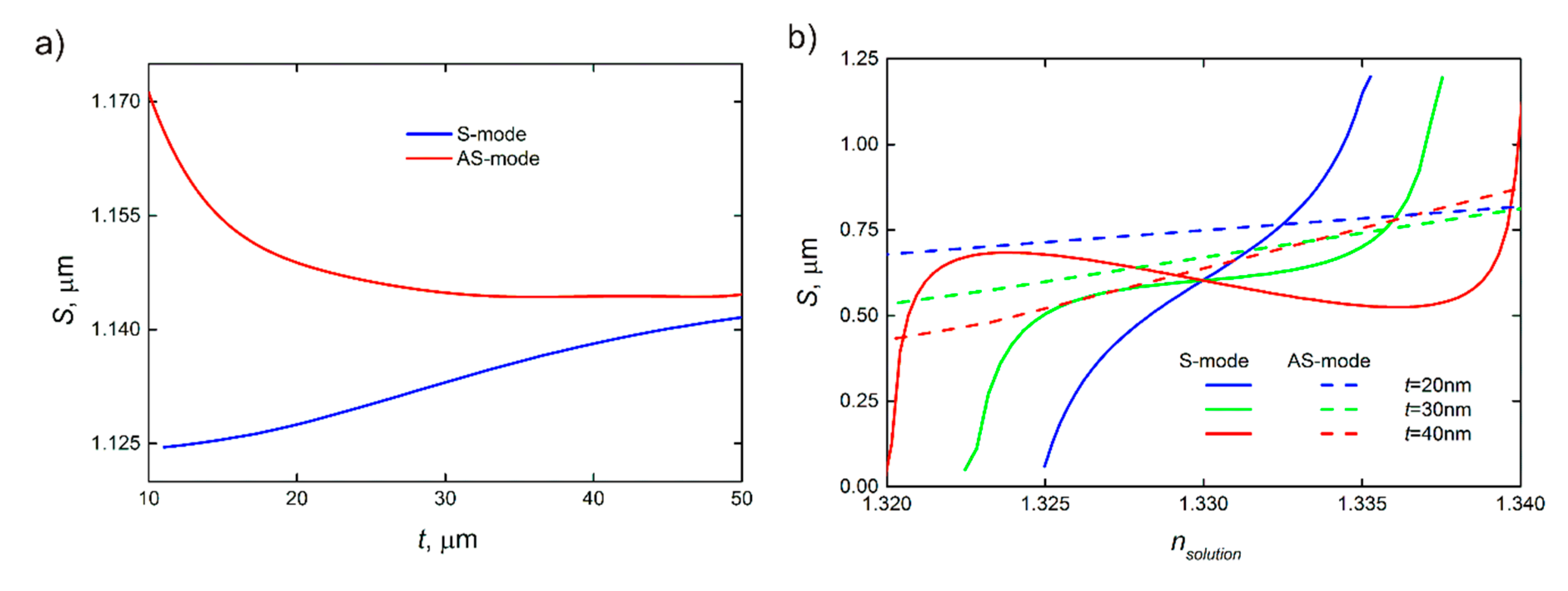
4. Sensitivity
5. Possible Modification and Upgrades
- The scheme with two waveguides coupled to the ring resonator [48] provides more efficient biosensing when using resonators characterized by low optical losses such as photonic ones. In this case, light transmission through the device is characterized by narrower resonant peaks with zero transmission at resonance.
- In theory, the AS-mode provides higher sensitivity than the S-mode as shown in Figure 5. However, the AS-mode propagation length is significantly less than that for the S-mode and even less than the resonator length, which makes optical biosensing highly inefficient. The situation can be different for optical biosensors exploiting plasmonic schemes based on ultrathin metal films and plasmonic two-dimensional (2D) materials [49,50,51]. As shown in Figure 5, the sensitivity to RI increases with the decreasing thickness of the plasmonic waveguide.
- In the case of SRSPP, the intensity transmitted through the device is strongly suppressed due to absorption in the waveguide; thus, the plasmonic waveguide can be partially (except the coupling area) replaced with a photonic waveguide. To realize this, materials and geometry parameters of the dielectric waveguide should be chosen to match the propagation constant for them with the propagation constant for the plasmonic waveguide [52,53,54].
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roh, S.; Chung, T.; Lee, B. Overview of the characteristics of micro- and nano-structured surface plasmon resonance sensors. Sensors 2011, 11, 1565–1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zanchetta, G.; Lanfranco, R.; Giavazzi, F.; Bellini, T.; Buscaglia, M. Emerging applications of label-free optical biosensors. Nanophotonics 2017, 6, 19. [Google Scholar] [CrossRef]
- Yang, G.; White, I.M.; Fan, X. An opto-fluidic ring resonator biosensor for the detection of organophosphorus pesticides. Sens. Actuators B Chem. 2008, 133, 105–112. [Google Scholar] [CrossRef]
- Xu, D.X.; Densmore, A.; Delâge, A.; Waldron, P.; McKinnon, R.; Janz, S.; Lapointe, J.; Lopinski, G.; Mischki, T.; Post, E.; et al. Folded cavity SOI microring sensors for high sensitivity and real time measurement of biomolecular binding. Opt. Express 2008, 16, 15137–15148. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Gleeson, M.A.; Spaugh, B.; Tybor, F.; Gunn, W.G.; Hochberg, M.; Baehr-Jones, T.; Bailey, R.C.; Cary Gunn, L. Label-free biosensor arrays based on silicon ring resonators and high-speed optical scanning instrumentation. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 654–661. [Google Scholar] [CrossRef]
- Boyd, R.W.; Heebner, J.E. Sensitive disk resonator photonic biosensor. Appl. Opt. 2001, 40, 5742–5747. [Google Scholar] [CrossRef]
- Claes, T.; Molera, J.G.; De Vos, K.; Schacht, E.; Baets, R.; Bienstman, P. Label-free biosensing with a slot-waveguide-based ring resonator in silicon on insulator. IEEE Photonics J. 2009, 1, 197–204. [Google Scholar] [CrossRef]
- Barrios, C.A.; Bañuls, M.J.; González-Pedro, V.; Gylfason, K.B.; Sánchez, B.; Griol, A.; Maquieira, A.; Sohlström, H.; Holgado, M.; Casquel, R. Label-free optical biosensing with slot-waveguides. Opt. Lett. 2008, 33, 708–710. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Li, M.; He, J.-J. Highly-sensitive silicon-on-insulator sensor based on two cascaded micro-ring resonators with vernier effect. Opt. Commun. 2011, 284, 156–159. [Google Scholar] [CrossRef]
- Claes, T.; Bogaerts, W.; Bienstman, P. Experimental characterization of a silicon photonic biosensor consisting of two cascaded ring resonators based on the Vernier-effect and introduction of a curve fitting method for an improved detection limit. Opt. Express 2010, 18, 22747–22761. [Google Scholar] [CrossRef] [Green Version]
- Nemova, G.; Kabashin, A.V.; Kashyap, R. Surface plasmon-polariton Mach-Zehnder refractive index sensor. J. Opt. Soc. Am. B 2008, 25, 1673. [Google Scholar] [CrossRef]
- Kozma, P.; Kehl, F.; Ehrentreich-Förster, E.; Stamm, C.; Bier, F.F. Integrated planar optical waveguide interferometer biosensors: A comparative review. Biosens. Bioelectron. 2014, 58, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Mullett, W.; Lai, E.P.; Yeung, J.M. Immunoassay of fumonisins by a surface plasmon resonance biosensor. Anal. Biochem. 1998, 258, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Kondoh, J. Tuning and sensitivity enhancement of surface plasmon resonance sensor. Sens. Actuators B Chem. 2007, 122, 381–388. [Google Scholar] [CrossRef]
- Stebunov, Y.V.; Yakubovsky, D.I.; Fedyanin, D.Y.; Arsenin, A.V.; Volkov, V.S. Superior sensitivity of copper-based plasmonic biosensors. Langmuir 2018, 34, 4681–4687. [Google Scholar] [CrossRef] [Green Version]
- Stebunov, Y.V.; Arsenin, A.V.; Volkov, V.S. Functionalization of chemically derived graphene for surface plasmon resonance (SPR) biosensors. In Chemically Derived Graphene: Functionalization, Properties and Applications; The Royal Society of Chemistry: London, UK, 2018; pp. 328–353. [Google Scholar]
- Stebunov, Y.V.; Aftenieva, O.A.; Arsenin, A.V.; Volkov, V.S. Highly sensitive and selective sensor chips with graphene-oxide linking layer. ACS Appl. Mater. Interfaces 2015, 7, 21727–21734. [Google Scholar] [CrossRef]
- Volkov, V.S.; Stebunov, Y.V.; Yakubovsky, D.I.; Fedyanin, D.Y.; Arsenin, A.V. Novel graphene-oxide-coated SPR interfaces for biosensing applications. AIP Conf. Proc. 2017, 1874, 020008. [Google Scholar]
- Homola, J.; Yee, S.S.; Gauglitz, G. Surface plasmon resonance sensors: Review. Sens. Actuators B Chem. 1999, 54, 3–15. [Google Scholar] [CrossRef]
- Debackere, P.; Taillaert, D.; De Vos, K.; Scheerlinck, S.; Bienstman, P.; Baets, R. Si based waveguide and surface plasmon sensors. Proc. Silicon Photonics II 2007, 6477, 647719. [Google Scholar]
- Dostálek, J.; Čtyroký, J.; Homola, J.; Brynda, E.; Skalský, M.; Nekvindová, P.; Špirková, J.; Škvor, J.; Schröfel, J. Surface plasmon resonance biosensor based on integrated optical waveguide. Sens. Actuators B Chem. 2001, 76, 8–12. [Google Scholar] [CrossRef]
- Lee, J.; Song, J.; Sung, G.Y.; Shin, J.H. Plasmonic waveguide ring resonators with 4 nm air gap and λ0(2)/15,000 mode-area fabricated using photolithography. Nano Lett. 2014, 14, 5533–5538. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.A.; Khonina, S.N.; Kazanskiy, N.L. A serially cascaded micro-ring resonator for simultaneous detection of multiple analytes. Laser Phys. 2019, 29, 046208. [Google Scholar] [CrossRef]
- Su, Y.; Chang, P.; Lin, C.; Helmy, A.S. Record Purcell factors in ultracompact hybrid plasmonic ring resonators. Sci. Adv. 2019, 5, eaav1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Headley, W.R.; Reed, G.T.; Howe, S.; Liu, A.; Paniccia, M. Polarization-independent optical racetrack resonators using rib waveguides on silicon-on-insulator. Appl. Phys. Lett. 2004, 85, 5523–5525. [Google Scholar] [CrossRef] [Green Version]
- Ramirez, J.M.; Liu, Q.; Vakarin, V.; Le Roux, X.; Frigerio, J.; Ballabio, A.; Alonso-Ramos, C.; Simola, E.T.; Vivien, L.; Isella, G.; et al. Broadband integrated racetrack ring resonators for long-wave infrared photonics. Opt. Lett. 2019, 44, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.A.; Kazanskiy, N.L.; Khonina, S.N. Hybrid plasmonic waveguide race-track µ-ring resonator: Analysis of dielectric and hybrid mode for refractive index sensing applications. Laser Phys. 2020, 30, 016202. [Google Scholar] [CrossRef]
- Ginzburg, P. Cavity quantum electrodynamics in application to plasmonics and metamaterials. Rev. Phys. 2016, 1, 120–139. [Google Scholar] [CrossRef] [Green Version]
- Feigenbaum, E.; Orenstein, M. Ultrasmall volume plasmons, yet with complete retardation effects. Phys. Rev. Lett. 2008, 101, 163902. [Google Scholar] [CrossRef] [Green Version]
- Maier, S.A. Plasmonics: Fundamentals and Applications, 1st ed.; Springer: New York, NY, USA, 2007; p. 224. [Google Scholar]
- Bozhevolnyi, S.I.; Volkov, V.S.; Devaux, E.; Laluet, J.-Y.; Ebbesen, T.W. Channel plasmon subwavelength waveguide components including interferometers and ring resonators. Nature 2006, 440, 508–511. [Google Scholar] [CrossRef]
- Righini, G.C.; Chiappini, A. Glass optical waveguides: a review of fabrication techniques. Opt. Eng. 2014, 53, 071819. [Google Scholar] [CrossRef]
- West, P.R.; Ishii, S.; Naik, G.V.; Emani, N.K.; Shalaev, V.M.; Boltasseva, A. Searching for better plasmonic materials. Laser Photonics Rev. 2010, 4, 795–808. [Google Scholar] [CrossRef] [Green Version]
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative plasmonic materials: Beyond gold and silver. Adv. Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef] [PubMed]
- McPeak, K.M.; Jayanti, S.V.; Kress, S.J.P.; Meyer, S.; Iotti, S.; Rossinelli, A.; Norris, D.J. Plasmonic films can easily be better: rules and recipes. ACS Photonics 2015, 2, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Babar, S.; Weaver, J.H. Optical constants of Cu, Ag, and Au revisited. Appl. Opt. 2015, 54, 477–481. [Google Scholar] [CrossRef]
- Yakubovsky, D.I.; Arsenin, A.V.; Stebunov, Y.V.; Fedyanin, D.Y.; Volkov, V.S. Optical constants and structural properties of thin gold films. Opt. Express 2017, 25, 25574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakubovsky, D.I.; Fedyanin, D.Y.; Arsenin, A.V.; Volkov, V.S. Optical constant of thin gold films: Structural morphology determined optical response. AIP Conf. Proc. 2017, 1874, 040057. [Google Scholar]
- Enami, Y.; Yuan, B.; Tanaka, M.; Luo, J.; Jen, A.K.-Y. Electro-optic polymer/TiO2 multilayer slot waveguide modulators. Appl. Phys. Lett. 2012, 101, 123509. [Google Scholar] [CrossRef] [Green Version]
- Daviau, R.; Lisicka-Skrzek, E.; Niall Tait, R.; Berini, P. Broadside excitation of surface plasmon waveguides on Cytop. Appl. Phys. Lett. 2009, 94, 091114. [Google Scholar] [CrossRef]
- Berini, P. Long-range surface plasmon polaritons. Adv. Opt. Photonics 2009, 1, 484–588. [Google Scholar] [CrossRef]
- Degiron, A.; Cho, S.-Y.; Harrison, C.; Jokerst, N.M.; Dellagiacoma, C.; Martin, O.J.F.; Smith, D.R. Experimental comparison between conventional and hybrid long-range surface plasmon waveguide bends. Phys. Rev. A 2008, 77, 021804. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Du, Y.; Xiao, Y.; Li, H.; Zhai, Y.; Zhang, J.; Chen, Z. High performance micro-fiber coupler-based polarizer and band-rejection filter. Opt. Express 2012, 20, 17258. [Google Scholar] [CrossRef]
- White, I.M.; Fan, X. On the performance quantification of resonant refractive index sensors. Opt. Express 2008, 16, 1020–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlborg, C.F.; Gylfason, K.B.; Kaźmierczak, A.; Dortu, F.; Bañuls Polo, M.J.; Maquieira Catala, A.; Kresbach, G.M.; Sohlström, H.; Moh, T.; Vivien, L.; et al. A packaged optical slot-waveguide ring resonator sensor array for multiplex label-free assays in labs-on-chips. Lab Chip 2010, 10, 281–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Sun, X.; Li, X.; Chen, J. Miniature microring resonator sensor based on a hybrid plasmonic waveguide. Sensors 2011, 11, 6856–6867. [Google Scholar] [CrossRef] [Green Version]
- Nenninger, G.G.; Piliarik, M.; Homola, J. Data analysis for optical sensors based on spectroscopy of surface plasmons. Meas. Sci. Technol. 2002, 13, 2038–2046. [Google Scholar] [CrossRef]
- Yalcin, A.; Popat, K.C.; Aldridge, J.C.; Desai, T.A.; Hryniewicz, J.; Chbouki, N.; Little, B.E.; King, O.; Van, V.; Chu, S.; et al. Optical sensing of biomolecules using microring resonators. IEEE J. Sel. Top. Quantum Electron. 2006, 12, 148–155. [Google Scholar] [CrossRef]
- Yakubovsky, D.I.; Stebunov, Y.V.; Kirtaev, R.V.; Ermolaev, G.A.; Mironov, M.S.; Novikov, S.M.; Arsenin, A.V.; Volkov, V.S. Ultrathin and ultrasmooth gold films on monolayer MoS2. Adv. Mater. Interfaces 2019, 6, 1900196. [Google Scholar]
- Volkov, V.S.; Yakubovsky, D.I.; Stebunov, Y.V.; Kirtaev, R.V.; Voronin, K.V.; Arsenin, A.V. Hybrid graphene-nanometallic structures. J. Phys. Conf. Ser. 2018, 1092, 012161. [Google Scholar] [CrossRef]
- Malureanu, R.; Lavrinenko, A. Ultra-thin films for plasmonics: A technology overview. Nanotechnol. Rev. 2015, 4, 1–17. [Google Scholar] [CrossRef]
- Briggs, R.M.; Grandidier, J.; Burgos, S.P.; Feigenbaum, E.; Atwater, H.A. Efficient coupling between dielectric-loaded plasmonic and silicon photonic waveguides. Nano Lett. 2010, 10, 4851–4857. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zenin, V.A.; Leosson, K.; Shi, X.; Nielsen, M.G.; Bozhevolnyi, S.I. Efficient interfacing photonic and long-range dielectric-loaded plasmonic waveguides. Opt. Express 2015, 23, 9100–9108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosciniak, J.; Volkov, V.S.; Bozhevolnyi, S.I.; Markey, L.; Massenot, S.; Dereux, A. Fiber-coupled dielectric-loaded plasmonic waveguides. Opt. Express 2010, 18, 5314–5319. [Google Scholar] [CrossRef] [PubMed]
| Source | Sensitivity, nm/RIU | DL |
|---|---|---|
| Our scheme | 1200 | 8 × 10−7 |
| Interferometer [11] | 250 | 4 × 10−5 |
| Photonic resonator biosensor [45] | 200–250 | 5 × 10−6 |
| Plasmonic resonator biosensor [46] | 580 | 1.5 × 10−4 |
| Kretschman configuration [47] | 7500 | 10−8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronin, K.V.; Stebunov, Y.V.; Voronov, A.A.; Arsenin, A.V.; Volkov, V.S. Vertically Coupled Plasmonic Racetrack Ring Resonator for Biosensor Applications. Sensors 2020, 20, 203. https://doi.org/10.3390/s20010203
Voronin KV, Stebunov YV, Voronov AA, Arsenin AV, Volkov VS. Vertically Coupled Plasmonic Racetrack Ring Resonator for Biosensor Applications. Sensors. 2020; 20(1):203. https://doi.org/10.3390/s20010203
Chicago/Turabian StyleVoronin, Kirill V., Yury V. Stebunov, Artem A. Voronov, Aleksey V. Arsenin, and Valentyn S. Volkov. 2020. "Vertically Coupled Plasmonic Racetrack Ring Resonator for Biosensor Applications" Sensors 20, no. 1: 203. https://doi.org/10.3390/s20010203






