Abstract
The unique properties of MoS2 nanosheets make them a promising candidate for high-performance room temperature gas detection. Herein, few-layer MoS2 nanosheets (FLMN) prepared via mechanical exfoliation are coated on a substrate with interdigital electrodes for room-temperature NO2 detection. Interestingly, compared with other NO2 gas sensors based on MoS2, FLMN gas sensors exhibit high responsivity for room-temperature NO2 detection, and NO2 is easily desorbed from the sensor surface with an ultrafast recovery behavior, with recovery times around 2 s. The high responsivity is related to the fact that the adsorbed NO2 can affect the electron states within the entire material, which is attributed to the very small thickness of the MoS2 nanosheets. First-principles calculations were carried out based on the density functional theory (DFT) to verify that the ultrafast recovery behavior arises from the weak van der Waals binding between NO2 and the MoS2 surface. Our work suggests that FLMN prepared via mechanical exfoliation have a great potential for fabricating high-performance NO2 gas sensors.
1. Introduction
In recent years, transition metal dichalcogenides (TMDs) have attracted much interest due to their unique layered structure, electronic and energy storage properties, which can be exploited in numerous devices such as sensors, field-effect transistors and supercapacitors [1,2,3,4]. MoS2, as the frontrunner in the TMDs family, has also been extensively investigated as a potential gas-sensing material because of its tunable band gap, large surface-to-volume ratio, and various active sites [5,6,7]. Gas-sensing properties are closely related to the size of the gas-sensing materials. According to reports, all electrons inside the gas sensing channel can be affected by the adsorbed gas when the grain size is smaller than two times the Debye length (so-called grain-size control). The space-charge layer then penetrates into the whole sensing channels, and the response is drastically promoted [8,9]. Structurally, MoS2 is a layered material in which S-Mo-S atoms are closely packed in a hexagonal arrangement, and each neighboring layer is connected by van der Waals forces [10]. Due to the strong intra-layer interactions and the relatively weak interactions between these layers, the synthesis of single or few-layer MoS2 nanosheets (FLMN) becomes possible by mechanical exfoliation from bulk MoS2 [11,12]. With this method which is the easiest and fastest way to produce the pristine, highly crystalline and atomic thickness layered materials [13], single or few-layer MoS2 nanosheets can be obtained without introducing too many defects. Compared with single-layer MoS2, few-layer MoS2 exhibits much higher electronic mobility due to lower Schottky barriers, which makes it more attractive for gas sensing [14,15,16]. Moreover, it is reported that the physical adsorption of gas molecules on MoS2 surface can overcome the shortcoming of difficult desorption from two-dimensional materials [17], which is beneficial to improve the recovery characteristics of gas sensors. Hence, the FLMN prepared via mechanical exfoliation show great potential in fabricating high-performance NO2 gas sensors.
In this work, the FLMN prepared via mechanical exfoliation is transferred to an Al2O3 ceramic substrate with Ag-Pd interdigital electrodes. Exfoliated MoS2 nanosheets are interconnected among interdigital electrodes to form sensitive channels. The planar gas sensors based on FLMN show high responsivity to NO2 and ultrafast recovery behavior without heating unit. We hope that this work can provide a useful guideline for the application of two-dimensional (2D) MoS2 in high-performance gas sensors.
2. Experimental Details
A typical mechanical exfoliation process is shown in Figure 1. The bulk MoS2 crystal and scotch tape were purchased from XFNANO Materials Tech Co. (Nanjing, China). First, a piece of scotch tape was adhered onto a bulk MoS2 crystal for about 5 s and then the scotch tape was removed carefully with as small angles as possible. Secondly, the scotch tape with the MoS2 flakes was folded and separated repeatedly many times to get thin MoS2 flakes, and a metallic luster can be clearly seen on the scotch tape surface. Then, the scotch tape with MoS2 thin flakes adhered tightly onto a clean Al2O3 ceramic substrate with Ag-Pd interdigital electrodes, and this adhesion state was maintained for 6 hours before the scotch tape was removed. Subsequently, the acetone was dripped onto the substrate to remove adhesive residue from the scotch tape. Finally, an FLMN gas sensor without the heating unit was obtained and used for further characterization.
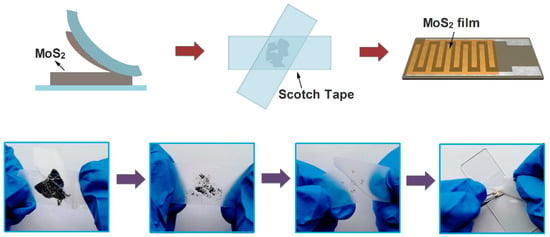
Figure 1.
Schematic diagram of the preparation process for the mechanically exfoliated MoS2 nanosheets.
The morphologies of exfoliated MoS2 nanosheets were observed by scanning electron microscope (SEM, Hitachi SU5000, Tokyo, Japan), transmission electron microscope (TEM, JEM-2100, JEOL, Tokyo, Japan) and atomic force microscope (AFM, Cypher S, Asylum Research, Oxford Instruments, Abingdon, UK). Molecular structure of exfoliated MoS2 nanosheets was evaluated by Raman spectra (Renishaw inVia, Renishaw, Gloucestershire, UK). The gas sensor surface was observed through a biological microscope (Eclipse-E200, Nikon, Tokyo, Japan). All the electrical measurements were carried out on a CGS-8 intelligent gas sensing analysis system (Beijing Elite Tech Co., Ltd, Beijing, China) at room temperature of 25 °C. The laboratory humidity is 55% relative humidity (RH), and the volume of our test chamber is 1000 mL. The NO2 gas sources of different concentrations (5 ppm, 10 ppm, 20 ppm, 50 ppm, 100 ppm, and 200 ppm, 21%vol O2 with 79%vol N2 as balanced gas) were bought from Dalian Special Gases co. LTD (Dalian, China), which had been calibrated by Fourier transform infrared spectrometer (spectrum 100, PerkinElmer, Waltham, MA, USA). The response of the gas sensors is defined as the ratio of the resistance of the sensors in tested gases (Rg) to that in the air (R0). For oxidizing tested gases, that is response = Rg/R0, while for the reducing tested gases, response = R0/Rg [18,19,20]. The time taken by a sensor to reach 90% of the total resistance change was defined as the response/recovery times [21].
3. Results and Discussion
In order to further verify the few-layer microstructure of MoS2 nanosheets, SEM, TEM, Raman spectra and AFM images of MoS2 nanosheets are shown in Figure 2. From Figure 2a, a sheet with a smooth surface is observed on the substrate, and the sheet surface shows the same pattern as the substrate, which looks transparent. This phenomenon, that the fuzzy pattern of the substrate reveals on the sheet surface, can be mainly attributed to the fact that the thickness of the sheet is thin enough to allow the electron beam to penetrate through it at the acceleration voltage of 30 kV, resulting in the reception of signals from the substrates. A high-resolution TEM (HRTEM) image is shown in Figure 2b, and a lattice fringe spacing of 0.27 nm corresponding to the crystal planes (100) of MoS2. The ordered lattice arrangement of MoS2 indicates that MoS2 has good crystallinity [22]. In Figure 2c, Raman spectra of bulk MoS2 and exfoliated MoS2 nanosheets are shown as measured using a 532 nm laser at room temperature of 25 °C. Two characteristic vibration modes can be observed in the spectrum of bulk MoS2, the in-plane E12g mode at 381.8 cm−1 results from opposite vibration of two S atoms with respect to the Mo atom while the A1g mode at 407.3 cm−1 is associated with the out-of-plane vibration of only S atoms in opposite directions [23,24]. Generally, the frequency difference (Δ) of the two dominant modes can be used to estimate the number of MoS2 layers [25].
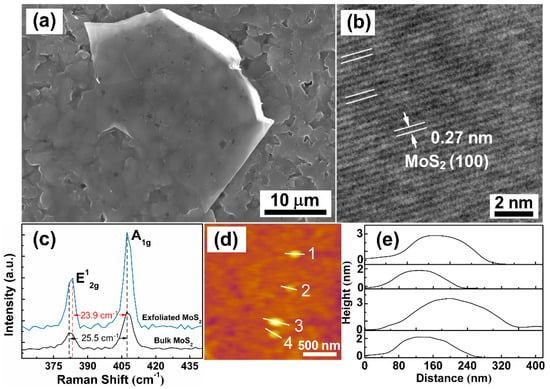
Figure 2.
(a) SEM and (b) HRTEM images of MoS2 nanosheets. (c) Raman spectra of MoS2 nanosheets and bulk MoS2. (d) AFM image of MoS2 nanosheets. (e) Height profiles of the AFM image.
Compared to the bulk MoS2, the E12g peak of MoS2 nanosheets shifts from 381.8 cm−1 to 383.4 cm−1, whereas the A1g mode experiences almost zero shift. The value of Δ is 23.9 cm−1 for the exfoliated MoS2 nanosheets which consisted of about four-monolayer MoS2, agrees well with the reported results in the literature [23]. Figure 2d,e shows the AFM image and the corresponding quantitative AFM height profiles. The thicknesses of the randomly distributed MoS2 nanosheets are about 1.5 to 3.2 nm. With the thickness of a MoS2 monolayer of about 0.65 nm [26,27], this suggests that the as-prepared FLMN are composed of 2–5 monolayers MoS2.
The optical images of the gas sensor without and with FLMN are displayed in Figure 3a–c. The blank Al2O3 ceramic substrate with Ag-Pd interdigital electrodes is clearly seen in Figure 3a, and the bare gap is quite clean without any materials. From Figure 3b,c, the FLMN with metallic luster are randomly dispersed on the Al2O3 ceramic substrate with interdigital electrodes, and the interlaced MoS2 nanosheets are bridged between the adjacent electrodes, which form the sensing channels on the gap. In order to support the issue that the sensing channels are composed of bridged MoS2 nanosheets, gas sensors with and without FLMN were exposed to 5 and 200 ppm NO2 at room temperature and their response and recovery behaviors are shown in Figure 3d. It can be seen that the resistance of the gas sensor coated with FLMN increases upon injection of NO2 and that it returns to its original value after removing the NO2. The higher the concentration, the larger the resistance change. The resistance responses of the uncoated gas sensor to 5 and 200 ppm NO2 are shown in the inset of Figure 3d. No matter whether the uncoated gas sensor is exposed to 5 ppm NO2, 200 ppm NO2 or air, there is no change in the resistance, and its resistance is much higher than the resistance of the gas sensor coated with FLMN. These results prove that bridging MoS2 nanosheets between adjacent electrodes form a conduction channel that reduces the resistance of the coated gas sensor below its resistance before coating. The observed NO2 response patterns can, therefore, be ascribed to the MoS2 nanosheets.
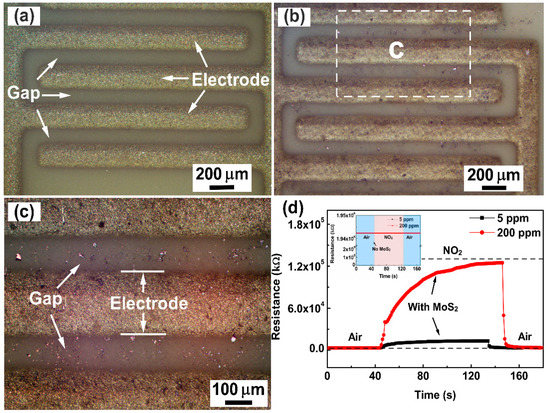
Figure 3.
Optical images of the gas sensor (a) without FLMN and (b) with FLMN, and (c) optical images of the encircled region in FLMN gas sensor of Figure 3b with higher magnification. (d) Response/recovery characteristic curves of the gas sensor with and without FLMN to 5 ppm and 200 ppm NO2.
The FLMN gas sensor was repeatedly exposed to gas pulses with concentrations ranging from 5 to 200 ppm NO2, separated by periods of fresh air in between. The resulting transient response-recovery curve of the FLMN gas sensor is shown in Figure 4a. The resistance of the gas sensor gradually increases with the NO2 concentrations as it is exposed to NO2, implying that the N-type response behavior of MoS2 nanosheets is found in the detection of NO2. According to this figure, the response of the gas sensor continuously increases as the NO2 concentration is ramped up from 5 to 200 ppm at room temperature, and the responses are about 4.4, 6.1, 9.3, 15.8, 29.1 and 41.7 corresponding to 5, 10, 20, 50, 100 and 200 ppm NO2, respectively. After removing the NO2 from the gas sensor, the resistance of the gas sensor can return completely each time with almost no drift. The recovery behavior is very fast and recovery time constants are as short as 2–4 s. As the MoS2 gas sensors reported in the previous works often fail to recover [28,29,30], the degree of recovery is an important indicator of the quality of the gas sensor. Herein, the recovery characteristic of the MoS2 gas sensor is investigated by calculating the recovery rate, defined as follows [31,32].
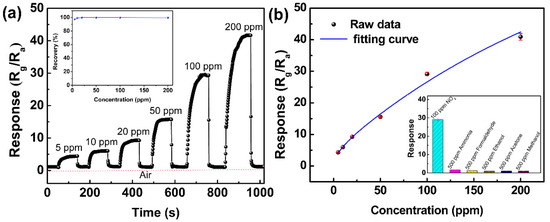
Figure 4.
(a) Transient response characteristics at an NO2 concentration range of 5 to 200 ppm, and the inset shows the recovery rate of the FLMN gas sensor at different NO2 concentrations. (b) Index fitting curve of the response versus NO2 concentration and the inset shows the cross sensitivity of the FLMN gas sensor with regard to various target gases.
Here, R0 and Rg are the resistances of the gas sensor before and after exposure to the target gas, and Rr is the stable resistances after putting the gas sensor back to air. As shown in the inset of Figure 4a, the FLMN gas sensor shows an outstanding recovery rate greater than 97%, which implies good recovery behavior for the FLMN gas sensor to detect NO2 gas.
In order to illustrate the contrast between our gas sensor and the state of the art, the performance of our gas sensor is compared to other reported MoS2 gas sensor in Table 1. The gas sensor based on FLMN presents a very high response of 4.4 and a very fast recovery time of ~2 s at 5 ppm NO2 gas, which does not require any heating unit to realize the detection of NO2 at room temperature.

Table 1.
Compared gas-sensing performances of few-layer MoS2 nanosheets with previous works based on different MoS2 nanostructures toward NO2.
As far as we know, our gas sensor features the fastest recovery time and the highest response for the detection of NO2 at room temperature. In order to illustrate the data reliability, error bars have been calculated by the standard deviation formula [40], and the response versus NO2 concentration index fitting curve with error bars is shown in Figure 4b. It exhibits small deviations for the FLMN gas sensor, indicating the data are reliable in the whole concentration range from 5 to 200 ppm. Based on the least squares method [41], the fitting equation of the response Y and NO2 concentration X can be represented as Y = 81.68 − 77.26 × e(−X/295.29) − 2.44 × e(−X/10.48), and the regression coefficient R2 is 0.965 at the concentration range from 5 to 200 ppm. The response curve shows optimal linear dependence in range of 5 to 100 ppm and then sign of slight saturation behavior at the NO2 concentration larger than 100 ppm. From the inset of Figure 4b, the FLMN gas sensor shows a high response to NO2 at room temperature while only minimal responses toward other gases such as ammonia, formaldehyde, ethanol, acetone and methanol, which is of an excellent cross sensitivity toward NO2.
In order to evaluate the repeatability and reversibility, the FLMN gas sensor is continuously placed into and removed from NO2 of the same concentration, and Figure 5a shows the response and recovery curves for three cycles when the gas sensors alternately change between air and 100 ppm NO2. Generally, the target gas is difficult to desorb completely from gas sensor surface without any stimulation of external field such as thermal field [36], optical field [42], etc., resulting in the long recovery time of gas sensor and the large drifting baseline at room temperature [31,32]. The above phenomenon is mainly caused by the chemical adsorption formed on the surface of sensing materials which makes it difficult for gas molecules to desorption [43]. In our work, for each cycle, the response of the gas sensor is 29 (the resistance changes from about 3 MΩ to 88 MΩ in the case of NO2 adsorption) and the response/recovery times are 42/2 s as shown in Figure 5a. It is worth mentioning that the recovery curve can return quickly to the baseline for each time with almost no drift, namely, the gaseous NO2 can be completely desorbed from the gas sensor without any extra stimulus like optical or thermal sources. The results illustrate that our FLMN gas sensors are superior in terms of repeatability and reversibility compared to other gas sensors working at room temperature. Interestingly, and contrary to many reported works [20,32,44,45,46], the recovery time of our sensors is far shorter than response time. The reason for this abnormal behavior is attributed to the fact that physical adsorption of gases is dominant on the mechanically exfoliated FLMN surface which is reported to have few defects [47]. While chemical adsorption mainly takes place on the other reported gas sensors due to the formation of defects on the surface of MoS2 synthesized by the wet chemical method [48,49]. In order to verify the physical adsorption behavior of NO2 on the MoS2 surface mentioned above, the parameters related to the adsorption configuration, such as the adsorption energy, the distance between NO2 and MoS2, the bond lengths of gas molecules, etc., are calculated based on density-functional theory (DFT). All DFT calculations were performed as implemented in the Vienna ab-initio simulation package (VASP) [50,51], and the exchange-correlation potential is treated with the Perdew-Burke-Eznerh of generalized-gradient approximation (PBE-GGA) [52,53]. The projector augmented wave (PAW) method is used to describe the electron–ion interaction [54]. For the structural relaxations and energy calculations, we employ the D2 method of Grimme (DFT-D2), which includes van der Waals (vdW) interactions [52,55]. All calculations are performed with a 3 × 3 × 1 supercell of MoS2 containing 27 atoms, and the cut off energy for plane-wave expansion is 400 eV. The Brillouin zone is sampled with a grid of 9 × 9 × 1 conducted by the Monkhorst-Pack special k-point scheme [56]. For geometry optimization, all the internal coordinates are fully relaxed until the Hellmann-Feynman forces are less than 0.005 eV/Å. The three adsorption configurations of NO2 molecules on MoS2 surface, including the NO2 adsorbed on the hollow, Mo-top and S-top sites of MoS2, are shown in Figure 5b–d, and Table 2 gives the adsorption parameters for three adsorption configurations including total energy (Etot), adsorption energy (Ead), the distance of adsorbed NO2 to the MoS2 monolayer (dzN-S) and the bond length of gas molecules (lN-O). The adsorption energy of gas molecules on MoS2 surface is calculated as Ead = Etot − EMoS2 − EGas, where Etot is the total energy of MoS2 with a molecule absorbed, EMoS2 and EGas are the energies of the pristine MoS2 single layer and isolated gas molecule. From Table 2, the total adsorption energies of the three adsorption configurations are almost the same, indicating that three adsorption configurations are all possible for the adsorption of NO2 on MoS2 surface.
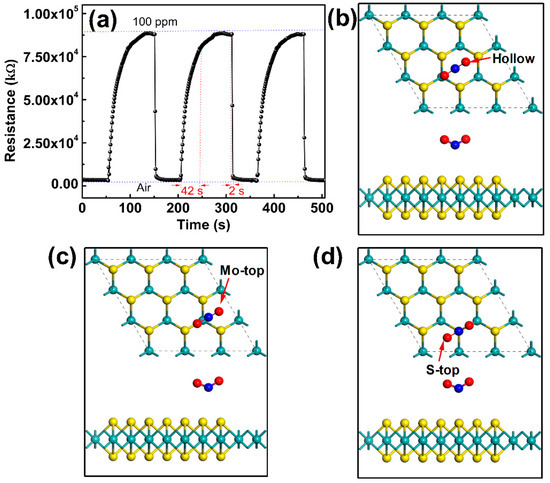
Figure 5.
(a) Repeatability and reversibility of the FLMN gas sensor at 100 ppm NO2 concentration. (b−d) Three adsorption configurations of NO2 molecules on MoS2 surface.

Table 2.
Calculated adsorption parameters of NO2 molecule in its three adsorption configurations.
The Ead of NO2 in the three adsorption configurations is very small, which the maximum Ead (NO2 adsorbed on hollow) is only 0.05 eV, and the distances of adsorbed NO2 to the MoS2 surface are large in all three adsorption configurations (dzN-S are all greater than 3 Å). The small Ead and the large dzN-S indicate that no chemical bond is formed between NO2 and MoS2 on MoS2 surface. Moreover, no matter at which adsorption site NO2 is adsorbed, the lN-O of NO2 gas molecules is approximately 1.218 Å, which lN-O is almost unchanged compared with lN-O (l~1.213 Å) of the free NO2 gas molecule. This further illustrates that the NO2 gas is not chemically adsorbed on the MoS2 surface, because the bond length will be greatly affected by the gas chemically adsorbed on the surface of the material [57,58]. Therefore, from the calculation results of Ead, dzN-S and lN-O, it is concluded that the NO2 gas is adsorbed on the MoS2 surface by a weak van der Waals interaction, i.e. the physical adsorption is the main factor for the adsorption of NO2 on MoS2 surface.
The response of gas sensing materials against target gas is mainly dependent on the electronic interaction between gases and materials, which occurs mainly on the surface of materials, i.e., it is a surface-controlled process [59]. As only a certain thickness of material surface can interact with the gas, only a certain depth of electron depletion layer can be formed with an order of 2–100 nm when the gas adsorbs on the surface of materials, which is usually called the Debye length (L) [8,9,60]. When the crystallite size (D) is much larger than 2L, grain-boundary contacts display higher resistance and govern the electric gas sensitivity of the chain (grain-boundary control) [59]. When D decreases to come closer to 2L, the necks become the most resistant, controlling the gas sensitivity (neck control) [61]. Finally, when D is smaller than 2L, each constituent grain is fully depleted of conduction electrons as a whole. In this situation, the resistance of grains dominates the whole resistance of the chain and the gas sensitivity, in this case, is controlled by grains themselves (grain control) [9]. The reported results mentioned above illustrate that the gas sensing properties are closely related to the size of gas sensing materials.
Figure 6 shows the schematic diagram of the gas sensing mechanism and equivalent circuit, and the exfoliated FLMNs are bridged randomly between the adjacent electrodes, which form the sensing channels on the gap. In our work, the gas sensor based on FLMN exhibits a higher response than other reported MoS2 gas sensors which have been shown in Table 1, and their high responsivity can be attributed to the following two reasons. First, the thickness of MoS2 is about 1.5–3.2 nm in Figure 6b, which belongs to the type of grain control, so the electrons of all over the sensing channels (including the bridging contact and the FLMN itself) can be affected by the adsorbed NO2, and the space-charge layer then penetrates into the whole sensing channels, which leads to a sharp decrease in conductivity and great improvement of the response [8,9]. As shown in the equivalent circuit diagram, all the electronic transport paths through the FLMN are controlled by NO2 gas, so the total resistance of FLMN gas sensor can change greatly when cycling it between air and NO2. As we have shown in the schematic of Figure 6b, the main reason why FLMN have a large response is that the NO2 gas controls all the conduction channels of carriers in the material, and the thickness of MoS2 is an important factor determining its gas sensing properties. Secondly, the gas molecules are generally confined to adsorb on the active sites of materials in the case of chemical adsorption, which limits the number of gas molecules adsorbed on the surface of the material [62,63]. By means of physical adsorption and a small amount of chemical adsorption, NO2 gas molecules can easily diffuse to the whole surface of the material, leading to the increase of the adsorption quantity of gas molecules on the materials surface [63]. When gas molecules are physically adsorbed the material surface, the electrostatic attraction between the material and gas causes the transformation of electrons from the surface of the material to form a dipole moment [47,64,65]. As the gas concentration increases, the continuous transfer of electrons will increase the resistance of materials, and the increase of dipole moment further promotes physical adsorption of gas molecules. Therefore, the resistance of material increases with the increase in gas concentration. In addition, there may be a few chemisorptions of NO2 gas molecules on the mechanically exfoliated FLMN due to the existence of few defects on the surface of MoS2 [66]. Here, chemisorption of NO2 gas molecules on FLMN does not affect the fast desorption behavior, and the main reason is that Brunauer–Emmett–Teller (BET) water layers formed on FLMN surface may dissolve the chemisorbed NO2 which can promote the rapid desorption of chemisorbed NO2 [67].
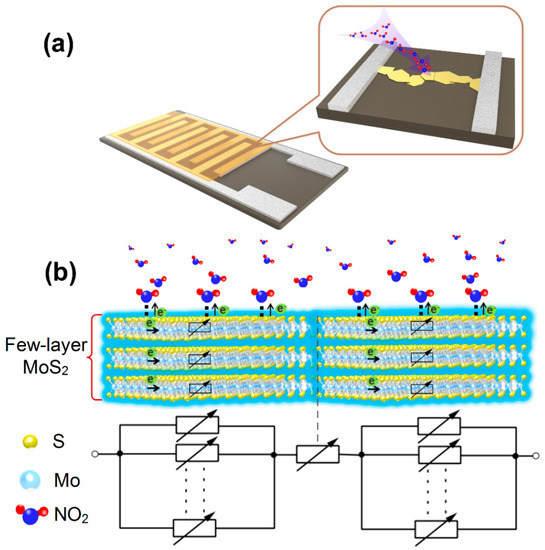
Figure 6.
(a) Schematic diagram of the FLMN gas sensor structure. (b) Schematic diagram of NO2 gas sensing mechanism and equivalent circuit for the FLMN.
4. Conclusions
In summary, an FLMN gas sensor via a facile way (mechanical exfoliation) was demonstrated to have excellent performance, which enables overcoming the limitations of 2D TMDs gas sensors such as low response and poor recovery. Through the comparison with the state of the art, the performances, including high response against NO2 at room temperature and the quick and complete recovery behaviors (the recovery time of 2 s, the recovery rate greater than 97%), are confirmed in this work. Based on density-functional theory (DFT), the calculation shows that the excellent performances at room temperature are mainly attributed to the physical adsorption of NO2 on FLMN surface and size effect from extremely thin thickness of FLMN. Thus, an FLMN gas sensor via mechanical exfoliation can resolve the low NO2-sensing performance issues in terms of response and recovery, and potentially open up a new avenue for gas sensing applications.
Author Contributions
W.L. designed the experiments, analyzed the data and wrote the paper. Y.Z. revised the paper. X.L., J.C. and X.G. performed the theoretical analysis. X.X. gave advice on the experiments. J.P. and X.Z. provided characterization instruments.
Funding
This work was supported by the National Natural Science Foundation of China (51502255, 11474245 and 11772285) and Hunan Provincial Natural Science Foundation of China (2018JJ2404).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Choi, S.-Y.; Kim, D.-H.; Lee, K.H.; Lee, B.H.; Ko, H.C.; Hahm, M.G. Metal decoration effects on the gas-sensing properties of 2D hybrid-structures on flexible substrates. Sensors 2015, 10, 24903–24913. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, J.; Kang, Y.; Ai, Y.; Li, C.M. Two dimensional atomically thin MoS2 nanosheets and their sensing applications. Nanoscale 2015, 7, 19358–19376. [Google Scholar] [CrossRef] [PubMed]
- Bhimanapati, G.R.; Lin, Z.; Meunier, V.; Jung, Y.; Das, S.; Cha, J.; Xiao, D.; Son, Y.; Strano, M.S.; Cooper, V.R.; et al. Recent advances in two-dimensional materials beyond graphene. ACS Nano 2015, 9, 11509–11539. [Google Scholar] [CrossRef]
- Shang, M.; Du, C.; Huang, H.; Mao, J.; Liu, P.; Song, W. Direct electrochemical growth of amorphous molybdenum sulfide nanosheets on Ni foam for high-performance supercapacitors. J. Colloid Interface Sci. 2018, 532, 24–31. [Google Scholar] [CrossRef]
- Su, S.; Lv, W.; Zhang, T.; Tan, Q.; Zhang, W.; Xiong, J. A MoS2 Nanoflakes-Based LC Wireless Passive Humidity Sensor. Sensors 2018, 18, 4466. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, Y.H.; Lee, S.Y.; Sohn, W.; Lee, J.E.; Kim, D.H.; Shim, Y.-S.; Kwon, K.C.; Choi, K.S.; Yoo, H.J.; et al. Highly selective and sensitive chemoresistive humidity sensors based on rGO/MoS2 van der Waals composites. J. Mater. Chem. A 2018, 6, 5016–5024. [Google Scholar] [CrossRef]
- Cho, S.-Y.; Kim, S.J.; Lee, Y.; Kim, J.-S.; Jung, W.-B.; Yoo, H.-W.; Kim, J.; Jung, H.-T. Highly enhanced gas adsorption properties in vertically aligned MoS2 layers. ACS Nano 2015, 9, 9314–9321. [Google Scholar] [CrossRef]
- Yamazoe, N. New approaches for improving semiconductor gas sensors. Sens. Actuators B Chem. 1991, 5, 7–19. [Google Scholar] [CrossRef]
- Seal, S.; Shukla, S. Nanocrystalline SnO gas sensors in view of surface reactions and modifications. JOM 2002, 54, 35–38. [Google Scholar] [CrossRef]
- Pujari, R.B.; Lokhande, A.C.; Shelke, A.R.; Kim, J.H.; Lokhande, C.D. Chemically deposited nano grain composed MoS2 thin films for supercapacitor application. J. Colloid Interface Sci. 2017, 496, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-dimensional atomic crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef]
- Li, M.-Y.; Chen, C.-H.; Shi, Y.; Li, L.-J. Heterostructures based on two-dimensional layered materials and their potential applications. Mater. Today 2016, 19, 322–335. [Google Scholar] [CrossRef]
- Perera, M.M.; Lin, M.-W.; Chuang, H.-J.; Chamlagain, B.P.; Wang, C.; Tan, X.; Cheng, M.M.-C.; Tománek, D.; Zhou, Z. Improved carrier mobility in few-layer MoS2 field-effect transistors with ionic-liquid gating. ACS Nano 2013, 7, 4449–4458. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Ye, P.D. Channel Length Scaling of MoS2 MOSFETs. ACS Nano 2012, 6, 8563–8569. [Google Scholar] [CrossRef]
- Das, S.; Chen, H.-Y.; Penumatcha, A.V.; Appenzeller, J. High-performance Multilayer MoS2 Transistors with Scandium Contacts. Nano Lett. 2012, 13, 100–105. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Yu, H.; Wei, Z.; Liao, M.; Chen, P.; Wang, S.; Shi, D.; Sun, Q.; Zhang, G. Highly sensitive MoS2 humidity sensors array for noncontact sensation. Adv. Mater. 2017, 29, 1702076. [Google Scholar] [CrossRef]
- Xu, H.; Ju, D.; Li, W.; Gong, H.; Zhang, J.; Wang, J.; Cao, B. Low-working-temperature, fast-response-speed NO2 sensor with nanoporous-SnO2/polyaniline double-layered film. Sens. Actuators B Chem. 2016, 224, 654–660. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Q.; Wang, Z.; Zhang, R.; Gao, Y.; Sun, P.; Lu, G. Improvement of NO2 gas sensing performance based on discoid tin oxide modified by reduced graphene oxide. Sens. Actuators B Chem. 2016, 227, 419–426. [Google Scholar] [CrossRef]
- Cho, J.-H.; Yu, J.-B.; Kim, J.-S.; Sohn, S.-O.; Lee, D.-D.; Huh, J.-S. Sensing behaviors of polypyrrole sensor under humidity condition. Sens. Actuators B Chem. 2005, 108, 389–392. [Google Scholar] [CrossRef]
- Feng, J.; Sun, X.; Wu, C.; Peng, L.; Lin, C.; Hu, S.; Yang, J.; Xie, Y. Metallic few-layered VS2 ultrathin nanosheets: High two-dimensional conductivity for in-plane supercapacitors. J. Am. Chem. Soc. 2011, 133, 17832–17838. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From bulk to monolayer MoS2: Evolution of raman scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Lin, H.; Wang, J.; Luo, Q.; Peng, H.; Luo, C.; Qi, R.; Huang, R.; Travas-Sejdic, J.; Duan, C.-G. Rapid and highly efficient chemical exfoliation of layered MoS2 and WS2. J. Alloy. Compd. 2017, 699, 222–229. [Google Scholar] [CrossRef]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous lattice vibrations of single-and few-layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-layer MoS2 transistors. Nat. Nanotechnol. 2011, 6, 147. [Google Scholar] [CrossRef]
- Lee, H.S.; Min, S.-W.; Park, M.K.; Lee, Y.T.; Jeon, P.J.; Kim, J.H.; Ryu, S.; Im, S. MoS2 nanosheets for top-gate nonvolatile memory transistor channel. Small 2012, 8, 3111–3115. [Google Scholar] [CrossRef]
- Shim, Y.-S.; Kwon, K.C.; Suh, J.M.; Choi, K.S.; Song, Y.G.; Sohn, W.; Choi, S.; Hong, K.; Jeon, J.-M.; Hong, S.-P.; et al. Synthesis of Numerous Edge Sites in MoS2 via SiO2 Nanorods Platform for Highly Sensitive Gas Sensor. ACS Appl. Mater. Interfaces 2018, 10, 31594–31602. [Google Scholar] [CrossRef]
- Kanaujiya, N.; Anupam; Golimar, K.; Pandey, P.C.; Jyoti; Varma, G.D. Investigating NO2 gas sensing behavior of flower-like MoS2 and rGO based nano-composite. AIP Conf. Proc. 2018, 1953, 030142. [Google Scholar]
- Kumar, R.; Kulriya, P.K.; Mishra, M.; Singh, F.; Gupta, G.; Kumar, M. Highly selective and reversible NO2 gas sensor using vertically aligned MoS2 flake networks. Nanotechnology 2018, 29, 464001. [Google Scholar] [CrossRef]
- Chatterjee, A.P.; Mitra, P.; Mukhopadhyay, A.K. Chemically deposited zinc oxide thin film gas sensor. J. Mater. Sci. 1999, 34, 4225–4231. [Google Scholar] [CrossRef]
- Ko, K.Y.; Song, J.-G.; Kim, Y.; Choi, T.; Shin, S.; Lee, C.W.; Lee, K.; Koo, J.; Lee, H.; Kim, J.; et al. Improvement of gas-sensing performance of large-area tungsten disulfide nanosheets by surface functionalization. ACS Nano 2016, 10, 9287–9296. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Goel, N.; Kumar, M. UV-Activated MoS2 Based Fast and Reversible NO2 Sensor at Room Temperature. ACS Sens. 2017, 2, 1744–1752. [Google Scholar] [CrossRef]
- Xu, T.; Pei, Y.; Liu, Y.; Wu, D.; Shi, Z.; Xu, J.; Tian, Y.; Li, X. High-response NO2 resistive gas sensor based on bilayer MoS2 grown by a new two-step chemical vapor deposition method. J. Alloy. Compd. 2017, 725, 253–259. [Google Scholar] [CrossRef]
- Han, Y.; Huang, D.; Ma, Y.; He, G.; Hu, J.; Zhang, J.; Hu, N.; Su, Y.; Zhou, Z.; Zhang, Y.; et al. Design of Heteronanostructures on MoS2 Nanosheets to Boost NO2 Room Temperature Sensing. ACS Appl. Mater. Interfaces 2018, 10, 22640–22649. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Goel, N.; Kumar, M. High-performance NO2 sensor using MoS2 nanowires network. Appl. Phys. Lett. 2018, 112, 053502. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, R.; Venkatesan, S.; Zakhidov, A.; Yang, G.; Bao, J.; Kumar, M.; Kumar, M. Photoactivated Mixed In-Plane and Edge-Enriched p-Type MoS2 Flake-Based NO2 Sensor Working at Room Temperature. ACS Sens. 2018, 3, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, Z.; Li, Y.; Chen, S.; Li, S.; Li, Y.; Wang, H.; Wang, Z. Hierarchical hollow MoS2 microspheres as materials for conductometric NO2 gas sensors. Sens. Actuators B Chem. 2019, 282, 259–267. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, J.-G.; Ryu, G.H.; Ko, K.Y.; Woo, W.J.; Kim, Y.; Kim, D.; Lim, J.H.; Lee, S.; Lee, Z.; et al. Low-temperature synthesis of 2D MoS2 on a plastic substrate for a flexible gas sensor. Nanoscale 2018, 10, 9338–9345. [Google Scholar] [CrossRef]
- Zhang, H.G.; Han, X.J.; Yao, B.F.; Li, G.X. Study on the effect of engine operation parameters on cyclic combustion variations and correlation coefficient between the pressure-related parameters of a CNG engine. Appl. Energy 2013, 104, 992–1002. [Google Scholar] [CrossRef]
- Li, J.; Hou, C.; Huo, D.; Yang, M.; Fa, H.B.; Yang, P. Development of a colorimetric sensor array for the discrimination of aldehydes. Sens. Actuators B Chem. 2014, 196, 10–17. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, C.; Guo, Y. UV assisted ultrasensitive trace NO2 gas sensing based on few-layer MoS2 nanosheet-ZnO nanowire heterojunctions at room temperature. J. Mater. Chem. A 2018, 6, 10286–10296. [Google Scholar] [CrossRef]
- Lee, G.; Yang, G.; Cho, A.; Han, J.W.; Kim, J. Defect-engineered graphene chemical sensors with ultrahigh sensitivity. Phys. Chem. Chem. Phys. 2016, 18, 14198–14204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Liu, S.; Zhang, T. Preparation of Ag nanoparticles-SnO2 nanoparticles-reduced graphene oxide hybrids and their application for detection of NO2 at room temperature. Sens. Actuators B Chem. 2016, 222, 893–903. [Google Scholar] [CrossRef]
- Gu, D.; Li, X.; Zhao, Y.; Wang, J. Enhanced NO2 sensing of SnO2/SnS2 heterojunction based sensor. Sens. Actuators B Chem. 2017, 244, 67–76. [Google Scholar] [CrossRef]
- Randeniya, L.K.; Shi, H.; Barnard, A.S.; Fang, J.; Martin, P.J.; Ostrikov, K. Harnessing the Influence of Reactive Edges and Defects of Graphene Substrates for Achieving Complete Cycle of Room-Temperature Molecular Sensing. Small 2013, 9, 3993–3999. [Google Scholar] [CrossRef] [PubMed]
- Ricciardella, F.; Vollebregt, S.; Polichetti, T.; Miscuglio, M.; Alfano, B.; Miglietta, M.L.; Massera, E.; Francia, G.D.; Sarro, P.M. Effects of graphene defects on gas sensing properties towards NO2 detection. Nanoscale 2017, 9, 6085–6093. [Google Scholar] [CrossRef] [PubMed]
- Huo, N.; Yang, S.; Wei, Z.; Li, S.S.; Xia, J.B.; Li, J. Photoresponsive and gas sensing field-effect transistors based on multilayer WS2 nanoflakes. Sci. Rep. 2014, 4, 5209. [Google Scholar] [CrossRef]
- Kang, J.; Ikram, M.; Zhao, Y.; Zhang, J.; Rehman, A.U.; Gong, L.; Shi, K. Three-dimensional flower-like Mg(OH)2@MoS2 nanocomposite: Fabrication, characterization and high-performance sensing properties for NOx at room temperature. New J. Chem. 2017, 41, 12071–12078. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Bucko, T.; Hafner, J.; Lebegue, S.; Angyan, J.G. Improved description of the structure of molecular and layered crystals: Ab initio DFT calculations with van der Waals corrections. J. Phys. Chem. A 2010, 114, 11814–11824. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Zhou, M.; Lu, Y.-H.; Cai, Y.-Q.; Zhang, C.; Feng, Y.-P. Adsorption of gas molecules on transition metal embedded graphene: A search for high-performance graphene-based catalysts and gas sensors. Nanotechnology 2011, 22, 385502. [Google Scholar] [CrossRef]
- Gao, G.; Park, S.H.; Kang, H.S. A first principles study of NO2 chemisorption on silicon carbide nanotubes. Chem. Phys. 2009, 355, 50–54. [Google Scholar] [CrossRef]
- Liu, B.; Chen, L.; Liu, G.; Abbas, A.N.; Fathi, M.; Zhou, C. High-performance chemical sensing using Schottky-contacted chemical vapor deposition grown monolayer MoS2 transistors. ACS Nano 2014, 8, 5304–5314. [Google Scholar] [CrossRef]
- Tang, W.; Wang, J. Enhanced gas sensing mechanisms of metal oxide heterojunction gas sensors. Acta Phys.-Chim. Sin. 2016, 32, 1087–1104. [Google Scholar]
- Sharma, S.; Madou, M. A new approach to gas sensing with nanotechnology. Phil. Trans. R. Soc. A 2012, 370, 2448–2473. [Google Scholar] [CrossRef]
- Xu, C.; Tamaki, J.; Miura, N.; Yamazoe, N. Grain size effects on gas sensitivity of porous SnO2-based elements. Sens. Actuators B Chem. 1991, 3, 147–155. [Google Scholar] [CrossRef]
- Cho, Y.; Sohn, A.; Kim, S.; Kim, D.-W.; Cho, B.; Hahm, M.G.; Kim, D.-H. Influences of gas adsorption and Au nanoparticles on the electrical properties of CVD-grown MoS2 thin films. ACS Appl. Mater. Interfaces 2016, 8, 21612–21617. [Google Scholar] [CrossRef]
- Qi, L.; Wang, Y.; Shen, L.; Wu, Y. Chemisorption-induced n-doping of MoS2 by oxygen. Appl. Phys. Lett. 2016, 108, 063103. [Google Scholar] [CrossRef]
- Yang, J.H.; Ji, J.L.; Li, L.; Wei, S.H. Hydrogen Chemisorption and Physisorption on the Two-Dimensional TiC Sheet Surface. Acta Phys.-Chim. Sin. 2014, 30, 1821–1826. [Google Scholar]
- Zhao, S.; Xue, J.; Kang, W. Gas adsorption on MoS2 monolayer from first-principles calculations. Chem. Phys. Lett. 2014, 595, 35–42. [Google Scholar] [CrossRef]
- Fang, H.; Chuang, S.; Chang, T.C.; Takei, K.; Takahashi, T.; Javey, A. High-performance single layered WSe2 p-FETs with chemically doped contacts. Nano Lett. 2012, 12, 3788–3792. [Google Scholar] [CrossRef]
- Maier, K.; Helwig, A.; Müller, G.; Hille, P.; Eickhoff, M. Effect of water vapor and surface morphology on the low temperature response of metal oxide semiconductor gas sensors. Materials 2015, 8, 6570–6588. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).