Estimation of Heart Rate Recovery after Stair Climbing Using a Wrist-Worn Device
Abstract
:1. Introduction
2. Materials and Methods
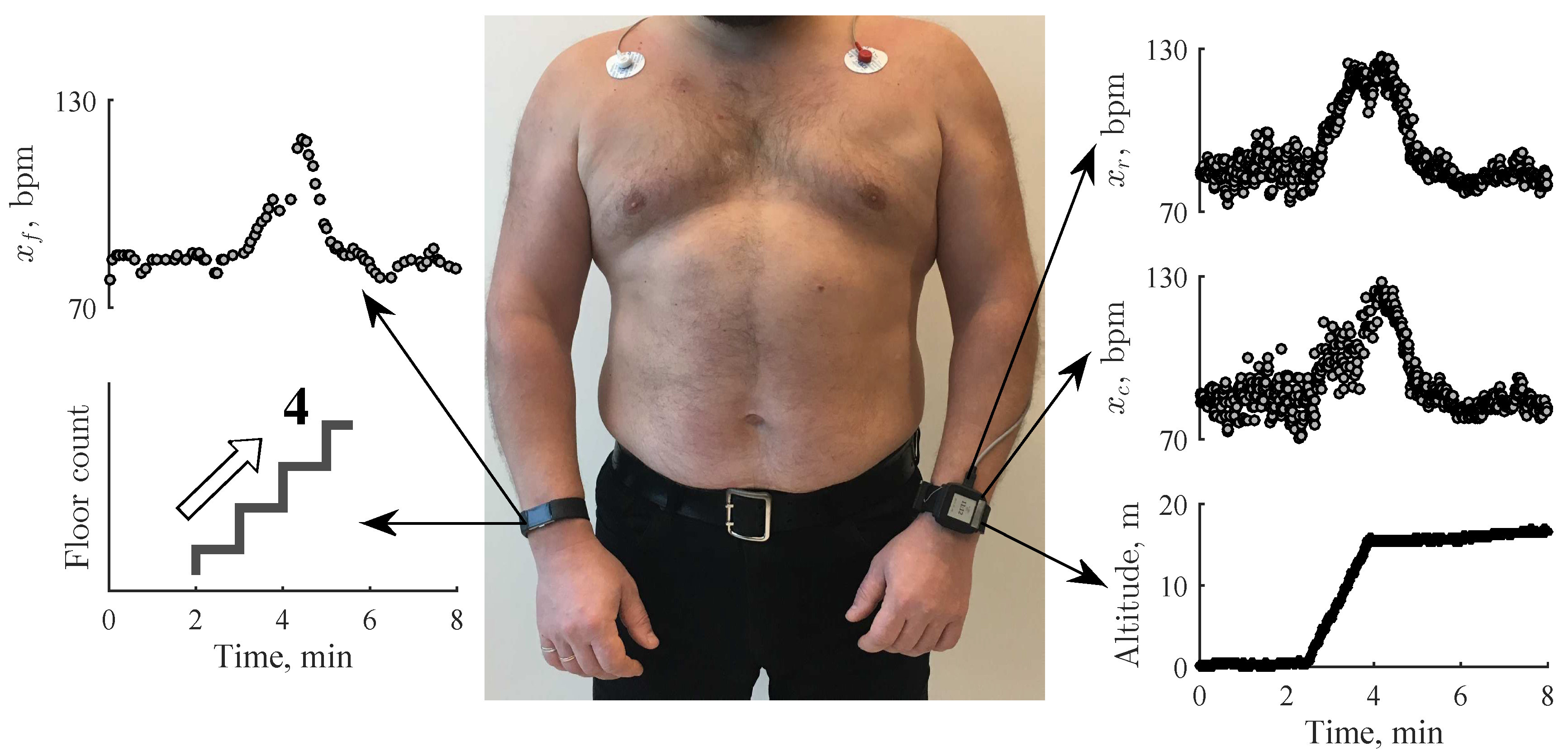
2.1. Data Acquisition
2.2. Study Population
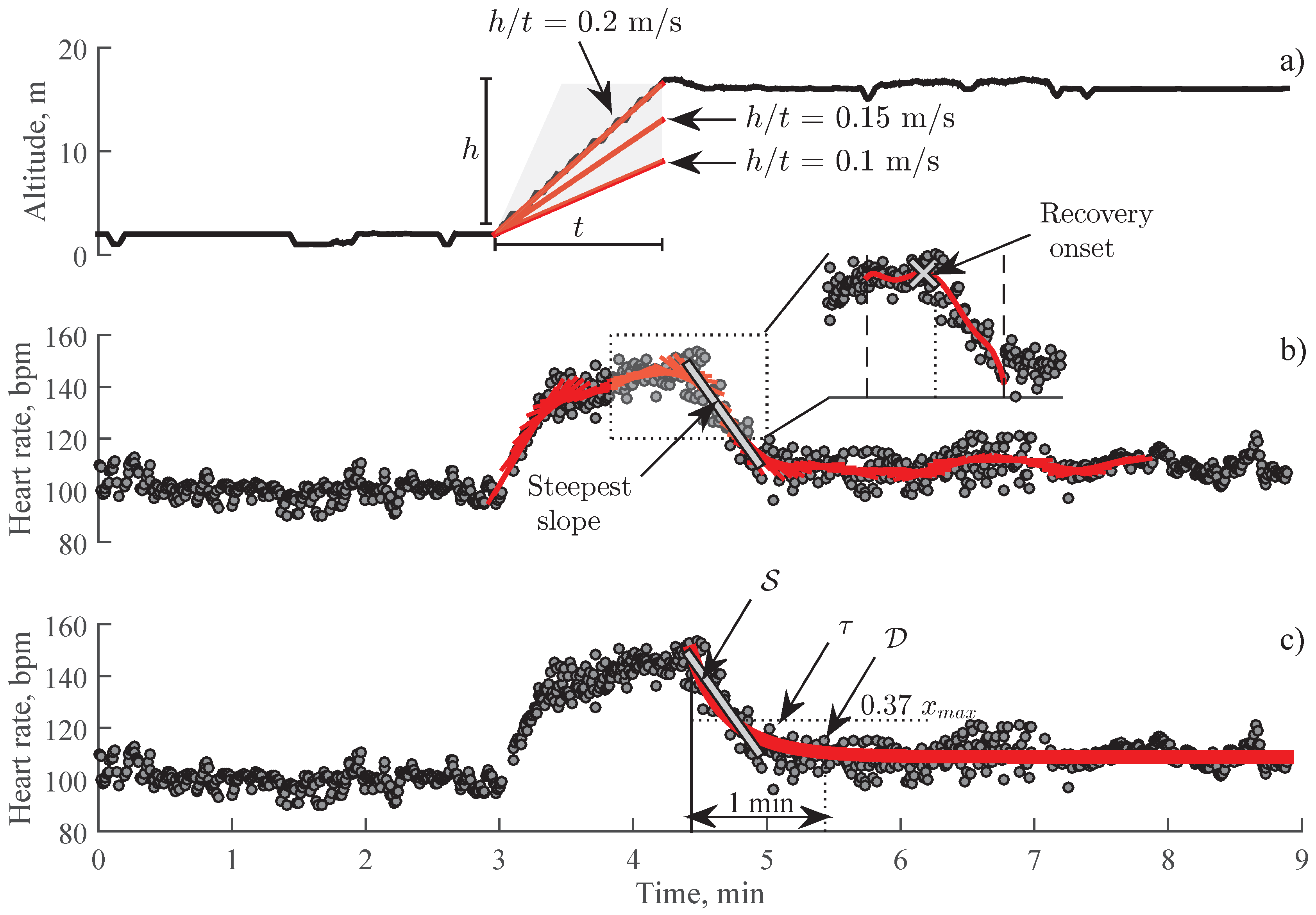
2.3. Detection of Stair Climbing Activity
2.4. Detection of Recovery Onset
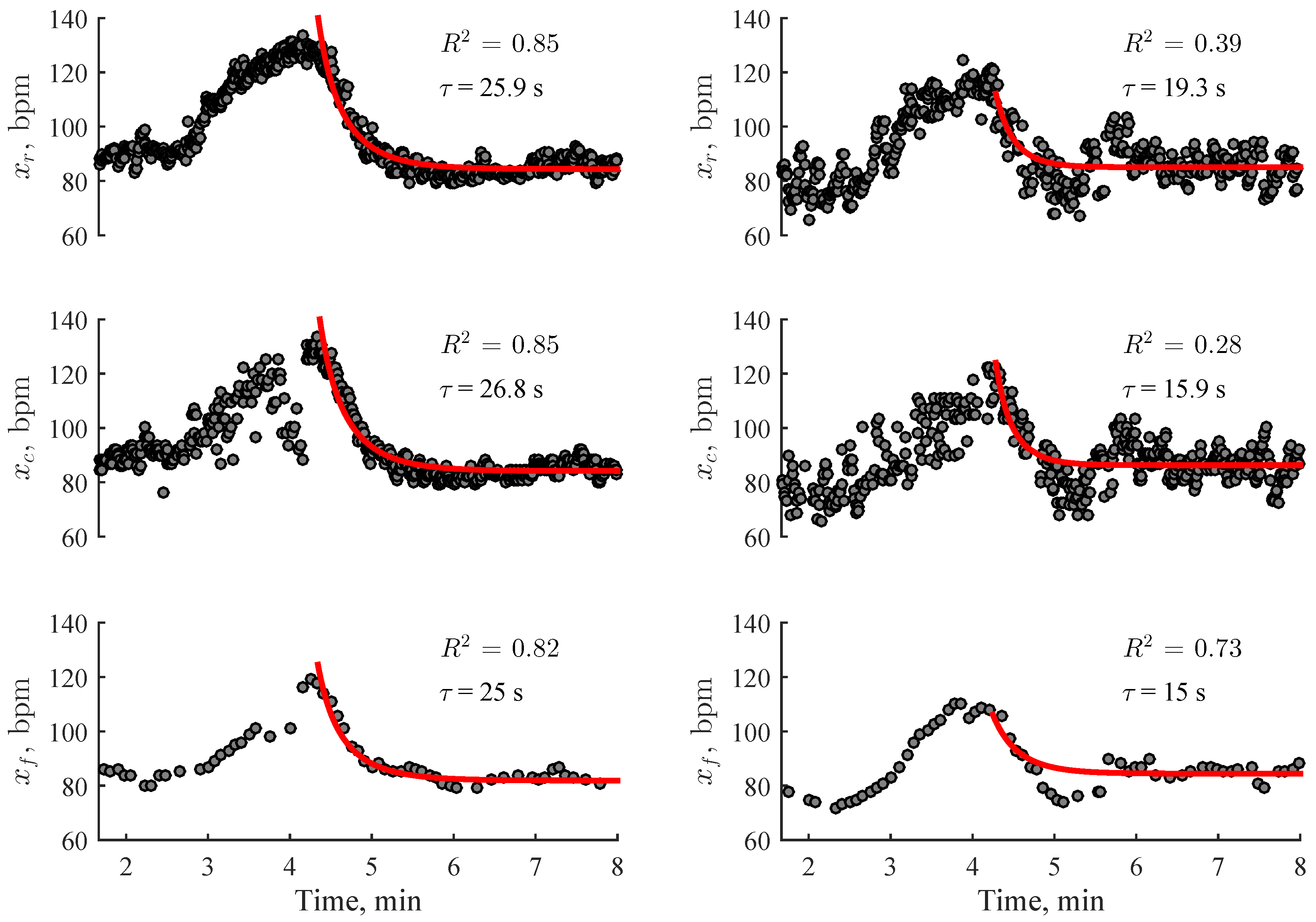
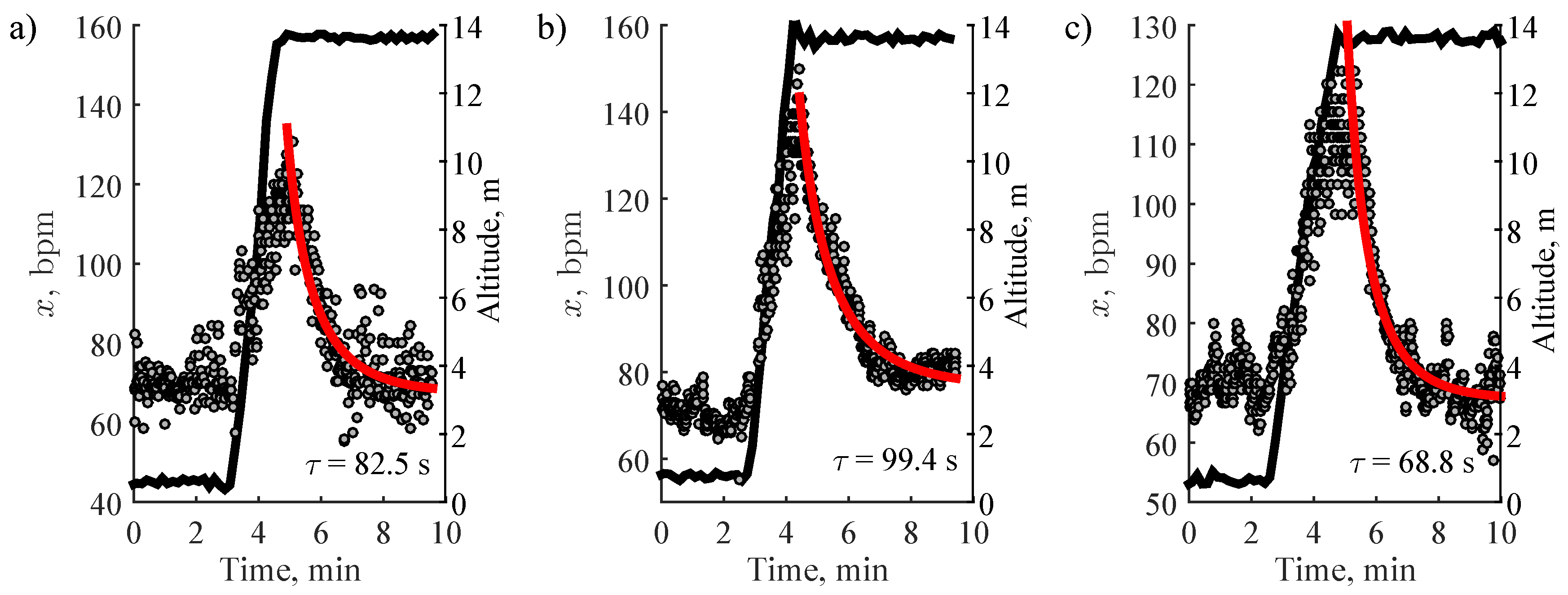
2.5. Estimation of Heart Rate Recovery
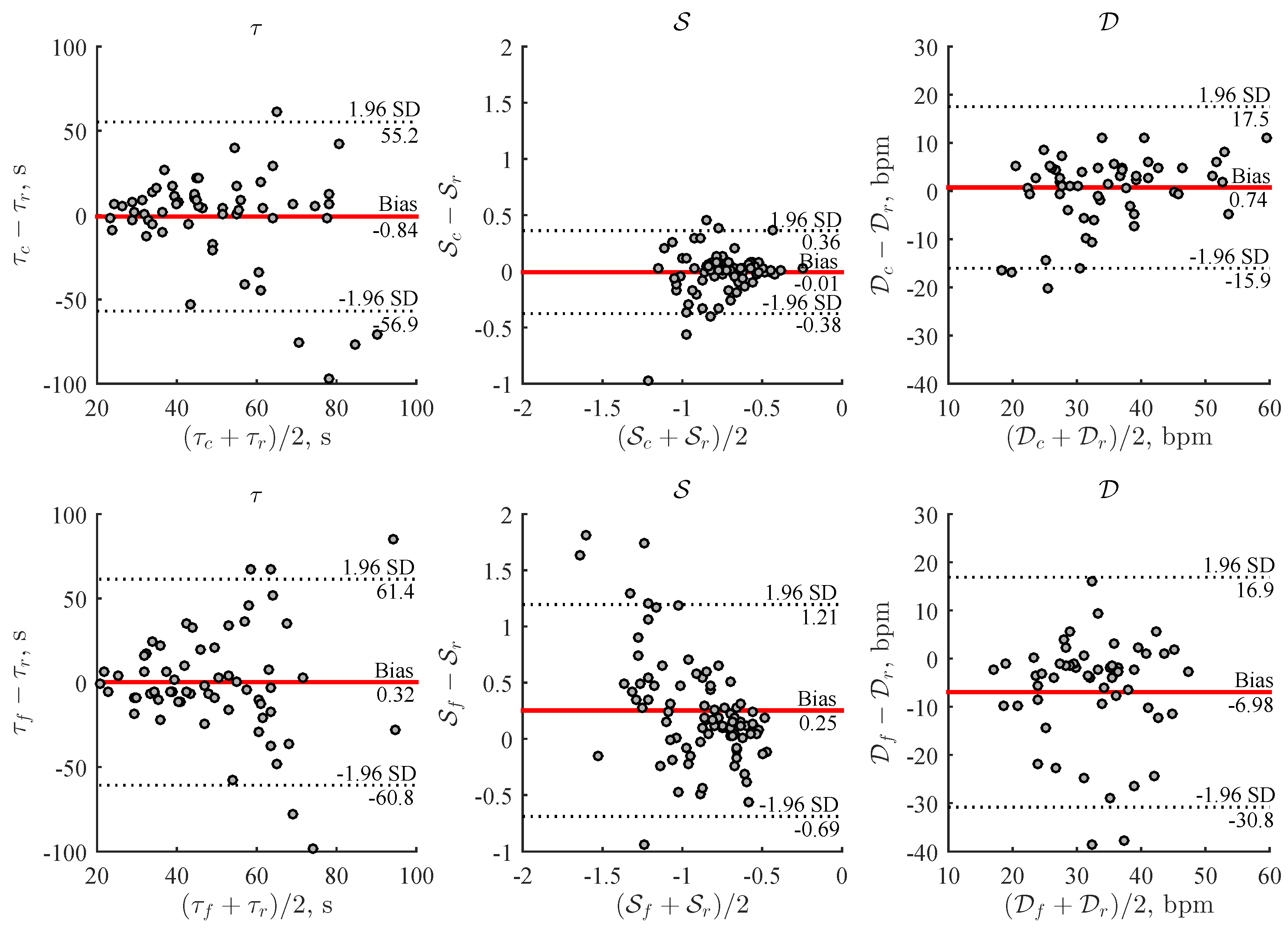
2.6. Performance Evaluation
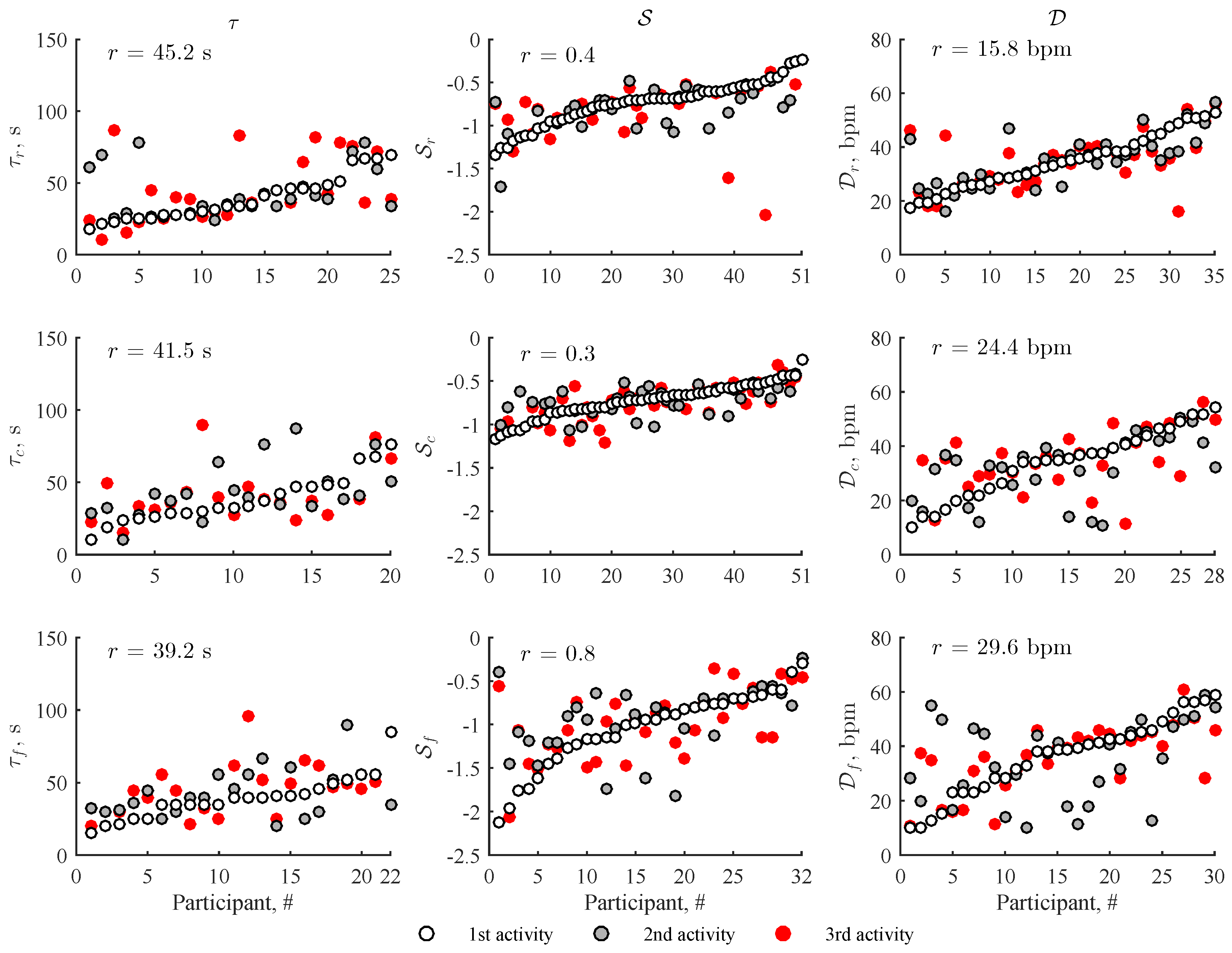
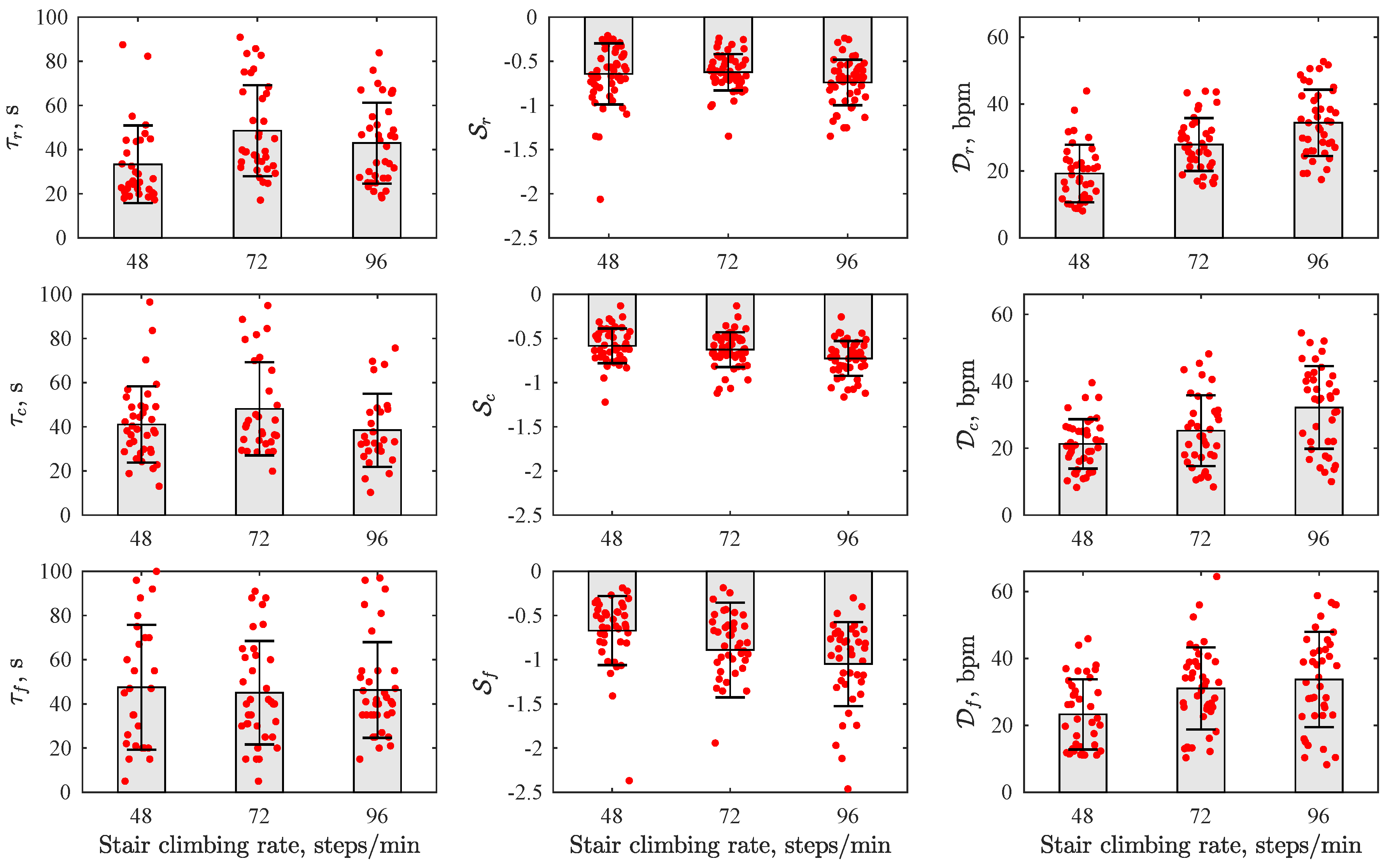
3. Results
4. Discussion
5. Conclusions
Sample Availability
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HRR | Heart rate recovery |
| PPG | Photoplethysmogram |
| ECG | Electrocardiogram |
References
- Vivekananthan, D.P.; Blackstone, E.H.; Pothier, C.E.; Lauer, M.S. Heart rate recovery after exercise is a predictor of mortality, independent of the angiographic severity of coronary disease. J. Am. Coll. Cardiol. 2003, 42, 831–838. [Google Scholar] [CrossRef]
- Jouven, X.; Empana, J.P.; Schwartz, P.J.; Desnos, M.; Courbon, D.; Ducimetire, P. Heart-rate profile during exercise as a predictor of sudden death. N. Engl. J. Med. 2005, 352, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, T.; Silva-Júnior, N.D.; Forjaz, C.L. Heart rate recovery: Autonomic determinants, methods of assessment and association with mortality and cardiovascular diseases. Clin. Physiol. Funct. Imaging 2014, 34, 327–339. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Mora, S.; Redberg, R.F.; Cui, Y.; Whiteman, M.K.; Flaws, J.A.; Sharrett, A.R.; Blumenthal, R.S. Ability of exercise testing to predict cardiovascular and all-cause death in asymptomatic women. A 20-year follow-up of the Lipid Research Clinics Prevalence Study. JAMA 2003, 290, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Aktas, M.; Ozduran, V.; Pothier, C.; Lang, R.; Lauer, M. Global risk scores and exercise testing for predicting all-cause mortality in a preventive medicine program. JAMA 2004, 292, 1462–1468. [Google Scholar] [CrossRef]
- Savonen, K.P.; Kiviniemi, V.; Laaksonen, D.E.; Lakka, T.A.; Laukkanen, J.A.; Tuomainen, T.P.; Rauramaa, R. Two-minute heart rate recovery after cycle ergometer exercise and all-cause mortality in middle-aged men. J. Intern. Med. 2011, 270, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.P.; Goldberger, J.J. Prognostic value of late heart rate recovery after treadmill exercise. Am. J. Cardiol. 2012, 110, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Sun, Z.; Li, L.; Zuegel, M.; Steinacker, J.M.; Schumann, U. Heart rate recovery and risk of cardiovascular events and all-cause mortality: A meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2017, 6, e005505. [Google Scholar] [CrossRef]
- Okutucu, S.; Karakulak, U.N.; Aytemir, K.; Oto, A. Heart rate recovery: A practical clinical indicator of abnormal cardiac autonomic function. Expert Rev. Cardiovasc. Ther. 2011, 9, 1417–1430. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016, 133, 694–711. [Google Scholar] [CrossRef]
- Streuber, S.D.; Amsterdam, E.A.; Stebbins, C.L. Heart rate recovery in heart failure patients after a 12-week cardiac rehabilitation program. Am. J. Cardiol. 2006, 97, 694–698. [Google Scholar] [CrossRef]
- Adams, B.J.; Carr, J.G.; Ozonoff, A.; Lauer, M.S.; Balady, G.J. Effect of exercise training in supervised cardiac rehabilitation programs on prognostic variables from the exercise tolerance test. Am. J. Cardiol. 2008, 101, 1403–1407. [Google Scholar] [CrossRef]
- Hai, J.J.; Siu, C.W.; Ho, H.H.; Li, S.W.; Lee, S.; Tse, H.F. Relationship between changes in heart rate recovery after cardiac rehabilitation on cardiovascular mortality in patients with myocardial infarction. Heart Rhythm 2010, 7, 929–936. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Clifton, P.M. Weight loss improves heart rate recovery in overweight and obese men with features of the metabolic syndrome. Am. Heart J. 2006, 152, 693.e1–693.e6. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.L.; Buckley, J.D.; Noakes, M.; Clifton, P.M.; Norman, R.J.; Brinkworth, G.D. Heart rate recovery improves after weight loss in overweight and obese women with polycystic ovary syndrome. Fertil. Steril. 2010, 93, 1173–1178. [Google Scholar] [CrossRef]
- Wasmund, S.L.; Owan, T.; Yanowitz, F.G.; Adams, T.D.; Hunt, S.C.; Hamdan, M.H.; Litwin, S.E. Improved heart rate recovery after marked weight loss induced by gastric bypass surgery: Two-year follow up in the Utah Obesity Study. Heart Rhythm 2011, 8, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, T.; Bartels, R.; Brito, L. Methods of assessment of the post-exercise cardiac autonomic recovery: A methodological review. Int. J. Cardiol. 2017, 227, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Lester, J.; Choudhury, T.; Borriello, G. A practical approach to recognizing physical activities. In Proceedings of the International Conference on Pervasive Computing, Dublin, Ireland, 7–10 May 2006; pp. 1–16. [Google Scholar]
- Nam, Y.; Park, J.W. Child activity recognition based on cooperative fusion model of a triaxial accelerometer and a barometric pressure sensor. IEEE J. Biomed. Health 2013, 17, 420–426. [Google Scholar]
- Moncada-Torres, A.; Leuenberger, K.; Gonzenbach, R.; Luft, A.; Gassert, R. Activity classification based on inertial and barometric pressure sensors at different anatomical locations. Physiol. Meas. 2014, 35, 1245–1263. [Google Scholar] [CrossRef] [PubMed]
- Massé, F.; Gonzenbach, R.R.; Arami, A.; Paraschiv-Ionescu, A.; Luft, A.R.; Aminian, K. Improving activity recognition using a wearable barometric pressure sensor in mobility-impaired stroke patients. J. Neuroeng. Rehabil. 2015, 12, 72. [Google Scholar] [CrossRef]
- Sokas, D.; Petrėnas, A.; Daukantas, S.; Rapalis, A.; Marozas, V. Photoplethysmography-based estimation of heart rate recovery using a wrist-worn device. In Proceedings of the 16th Biennial Baltic Electronics Conference (BEC), Tallinn, Estonia, 8–10 October 2018; pp. 1–4. [Google Scholar]
- Aboy, M.; McNames, J.; Tsunami, D.; Ellenby, M.S.; Goldstein, B. An automatic beat detection algorithm for pressure signals. IEEE Trans. Biomed. Eng. 2005, 52, 1662–1670. [Google Scholar] [CrossRef]
- Gil, E.; Orini, M.; Bailón, R.; Vergara, J.M.; Mainardi, L.; Laguna, P. Photoplethysmography pulse rate variability as a surrogate measurement of heart rate variability during non-stationary conditions. Physiol. Meas. 2010, 31, 1271–1290. [Google Scholar] [CrossRef]
- Scafer, A.; Vagedes, J. How accurate is pulse rate variability as an estimate of heart rate variability?: A review on studies comparing photoplethysmographic technology with an electrocardiogram. Int. J. Cardiol. 2013, 166, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Hernando, D.; Roca, S.; Sancho, J.; Alesanco, Á.; Bailón, R. Validation of the Apple Watch for heart rate variability measurements during relax and mental stress in healthy subjects. Sensors 2018, 18, 2619. [Google Scholar] [CrossRef] [PubMed]
- Golding, L.A. (Ed.) YMCA Fitness Testing and Assessment Manual, 4th ed.; Human Kinetics Publishers: Champaign, IL, USA, 2000. [Google Scholar]
- Pierpont, G.L.; Stolpman, D.R.; Gornick, C.C. Heart rate recovery post-exercise as an index of parasympathetic activity. J. Auton. Nervous Syst. 2000, 80, 169–174. [Google Scholar] [CrossRef]
- Bartels-Ferreira, R.; de Sousa, E.D.; Trevizani, G.A.; Silva, L.P.; Nakamura, F.Y.; Forjaz, C.L.; Lima, J.R.; Peçanha, T. Can a first-order exponential decay model fit heart rate recovery after resistance exercise? Clin. Physiol. Funct. Imaging 2015, 35, 98–103. [Google Scholar] [CrossRef]
- Imai, K.; Saito, H. Vagally mediated heart rate recovery after exercise is accelerated in athletes but blunted in patients with chronic heart failure. J. Am. Coll. Cardiol. 1994, 24, 1529–1535. [Google Scholar] [CrossRef]
- Arduini, A.; Gomez-Cabrera, M.C.; Romagnoli, M. Reliability of different models to assess heart rate recovery after submaximal bicycle exercise. J. Sci. Med. Sport 2011, 14, 352–357. [Google Scholar] [CrossRef]
- Bartlett, J.W.; Frost, C. Reliability, repeatability and reproducibility: Analysis of measurement errors in continuous variables. Ultrasound Obstet. Gynecol. 2008, 31, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kannankeril, P.J.; Le, F.K.; Kadish, A.H.; Goldberger, J.J. Parasympathetic effects on heart rate recovery after exercise. J. Investig. Med. 2004, 52, 394–401. [Google Scholar] [CrossRef]
- Coote, J.H. Recovery of heart rate following intense dynamic exercise. Exp. Physiol. 2010, 95, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Nanas, S.; Anastasiou, M. Early heart rate recovery after exercise predicts mortality in patients with chronic heart failure. Int. J. Cardiol. 2006, 2, 393–400. [Google Scholar] [CrossRef]
- Maddox, T.M.; Ross, C.; Ho, P.M.; Masoudi, F.A.; Magid, D.; Daugherty, S.L.; Peterson, P.; Rumsfeld, J.S. The prognostic importance of abnormal heart rate recovery and chronotropic response among exercise treadmill test patients. Am. Heart J. 2008, 156, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Peçanha, T.; Prodel, E.; Bartels, R.; Nasario-Junior, O.; Paula, R.B.; Silva, L.P.; Laterza, M.C.; Lima, J.R. 24-h cardiac autonomic profile after exercise in sedentary subjects. Int. J. Sport Med. 2014, 35, 245–252. [Google Scholar] [CrossRef]
- Al Haddad, H.; Laursen, P.B.; Chollet, D.; Ahmaidi, S.; Buchheit, M. Reliability of resting and postexercise heart rate measures. Int. J. Sport Med. 2011, 32, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, O.; Mekary, S.; Berryman, N.; Bherer, L.; Audiffren, M.; Bosquet, L. Reliability of heart rate measures used to assess post-exercise parasympathetic reactivation. Clin. Physiol. Funct. Imaging 2012, 32, 296–304. [Google Scholar] [CrossRef]
- Boullosa, D.A.; Barros, E.S.; del Rosso, S.; Nakamura, F.Y.; Leicht, A.S. Reliability of heart rate measures during walking before and after running maximal efforts. Int. J. Sport Med. 2014, 35, 999–1005. [Google Scholar] [CrossRef]
- Fecchio, R.Y.; Chehuen, M.; PeƧanha, T.; Cucato, G.G.; Costa, L.A.R.; Leicht, A.S.; Ritti-Dias, R.M.; Wolosker, N.; de Moraes Forjaz, C.L. Reproducibility of heart rate recovery in patients with intermittent claudication. Clin. Physiol. Funct. Imaging 2018, 38, 603–609. [Google Scholar] [CrossRef]
- Johar, P.; Grover, V.; DiSanto, M.C.; Button, D.C.; Behm, D.G. A rapid rotation to an inverted seated posture inhibits muscle force, activation, heart rate and blood pressure. Eur. J. Appl. Physiol. 2013, 113, 2005–2013. [Google Scholar] [CrossRef]
- Rapalis, A.; Petrėnas, A.; Šimaitytė, M.; Bailón, R.; Marozas, V. Towards pulse rate parametrization during free-living activities using smart wristband. Physiol. Meas. 2018, 39, 055007. [Google Scholar] [CrossRef] [PubMed]
- Bean, J.F.; Kiely, D.K.; LaRose, S.; Alian, J.; Frontera, W.R. Is stair climb power a clinically relevant measure of leg power impairments in at-risk older adults? Arch. Phys. Med. Rehabil. 2007, 88, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.; Ng, H.; Chan, K.; Lai, J.; To, A.; Yeung, C. Reliability of the 12-step ascend and descend test and its correlation with motor function in people with chronic stroke. J. Rehabil. Med. 2013, 45, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.; Peake, J.M.; Buchheit, M. Cardiac parasympathetic reactivation following exercise: Implications for training prescription. Sport Med. 2013, 43, 1259–1277. [Google Scholar] [CrossRef]

| Reference ECG | |||||
| Stair climbing rate | > 0.5 | < 0.5 | > 100 s | Corrupted | ( > 0.5) |
| 48 steps/min | 33/54 (61.1%) | 11/54 (20.4%) | 6/54 (11.1%) | 4/54 (7.4%) | 0.64 ± 0.12 |
| 72 steps/min | 38/54 (70.3%) | 7/54 (13.0%) | 4/54 (7.4%) | 5/54 (9.3%) | 0.64 ± 0.06 |
| 96 steps/min | 112/162 (69.1%) | 12/162 (7.4%) | 17/162 (10.5%) | 21/162 (13.0%) | 0.68 ± 0.11 |
| Wrist-Worn Device | |||||
| Stair climbing rate | > 0.5 | < 0.5 | > 100 s | Corrupted | ( > 0.5) |
| 48 steps/min | 26/54 (48.1%) | 16/54 (29.6%) | 8/54 (14.8%) | 4/54 (7.4%) | 0.65 ± 0.09 |
| 72 steps/min | 32/54 (59.3%) | 14/54 (25.9%) | 4/54 (7.4%) | 4/54 (7.4%) | 0.67 ± 0.16 |
| 96 steps/min | 86/162 (53.1%) | 45/162 (27.8%) | 16/162 (9.9%) | 15/162 (9.3%) | 0.66 ± 0.12 |
| Smart Wristband | |||||
| Stair climbing rate | > 0.5 | < 0.5 | > 100 s | Corrupted | ( > 0.5) |
| 48 steps/min | 34/54 (62.9%) | 8/54 (14.8%) | 5/54 (9.3%) | 7/54 (13.0%) | 0.78 ± 0.13 |
| 72 steps/min | 35/54 (64.8%) | 3/54 (5.6%) | 6/54 (11.1%) | 10/54 (18.5%) | 0.79 ± 0.11 |
| 96 steps/min | 104/162 (64.2%) | 33/162 (20.4%) | 16/162 (9.9%) | 9/162 (5.6%) | 0.84 ± 0.11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokas, D.; Petrėnas, A.; Daukantas, S.; Rapalis, A.; Paliakaitė, B.; Marozas, V. Estimation of Heart Rate Recovery after Stair Climbing Using a Wrist-Worn Device. Sensors 2019, 19, 2113. https://doi.org/10.3390/s19092113
Sokas D, Petrėnas A, Daukantas S, Rapalis A, Paliakaitė B, Marozas V. Estimation of Heart Rate Recovery after Stair Climbing Using a Wrist-Worn Device. Sensors. 2019; 19(9):2113. https://doi.org/10.3390/s19092113
Chicago/Turabian StyleSokas, Daivaras, Andrius Petrėnas, Saulius Daukantas, Andrius Rapalis, Birutė Paliakaitė, and Vaidotas Marozas. 2019. "Estimation of Heart Rate Recovery after Stair Climbing Using a Wrist-Worn Device" Sensors 19, no. 9: 2113. https://doi.org/10.3390/s19092113
APA StyleSokas, D., Petrėnas, A., Daukantas, S., Rapalis, A., Paliakaitė, B., & Marozas, V. (2019). Estimation of Heart Rate Recovery after Stair Climbing Using a Wrist-Worn Device. Sensors, 19(9), 2113. https://doi.org/10.3390/s19092113





