Multifunctional UV and Gas Sensors Based on Vertically Nanostructured Zinc Oxide: Volume Versus Surface Effect
Abstract
1. Introduction
2. Experimental
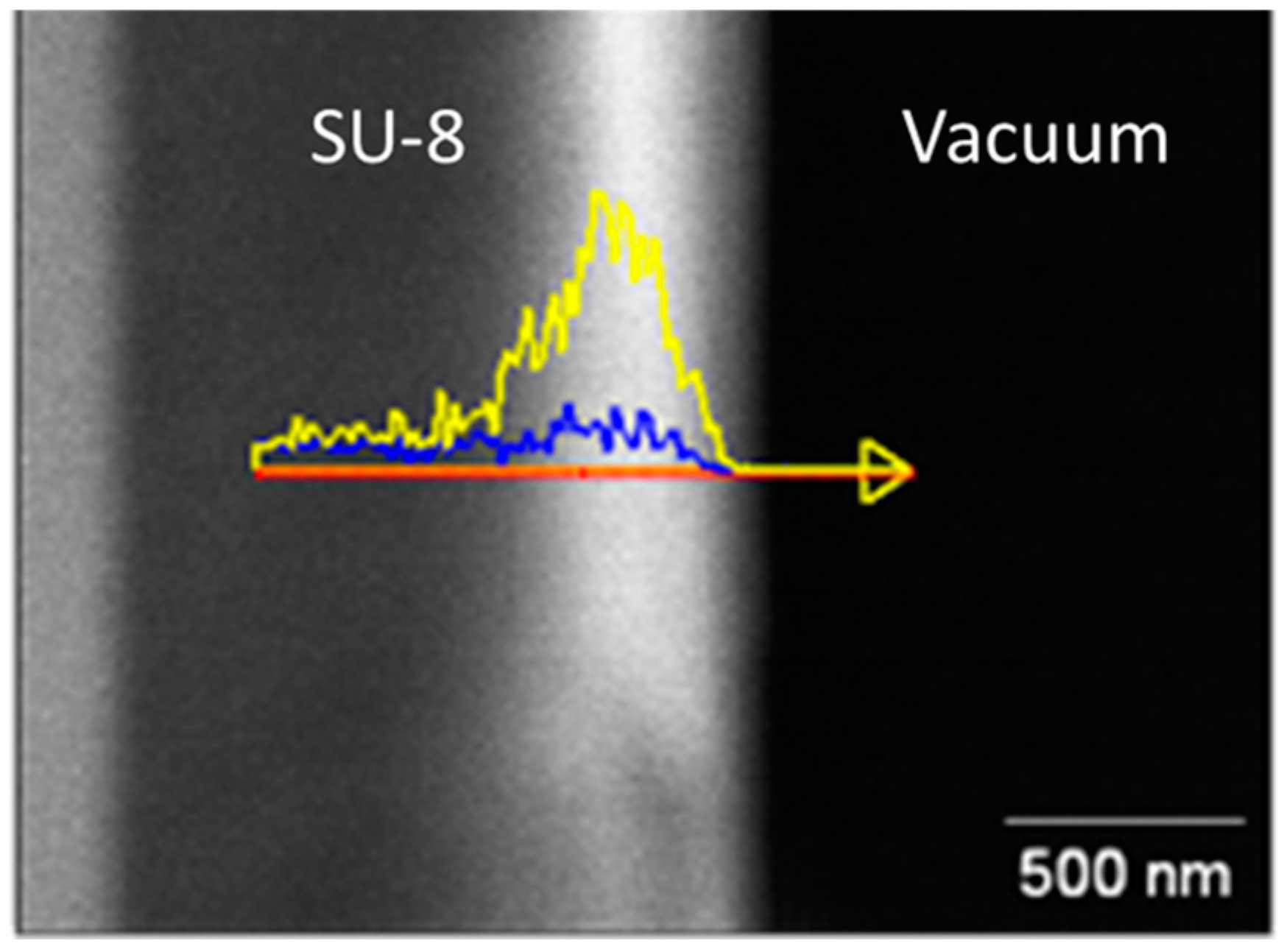
2.1. Energy-Dispersive X-Ray Spectroscopy (EDS) Characterization
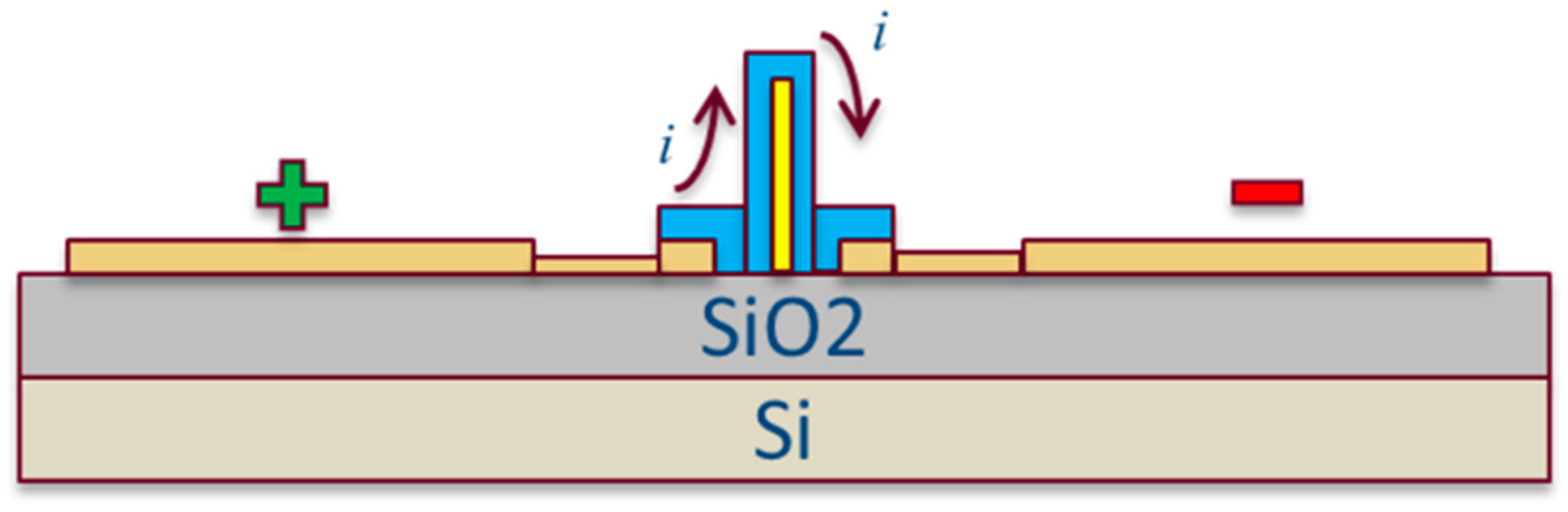
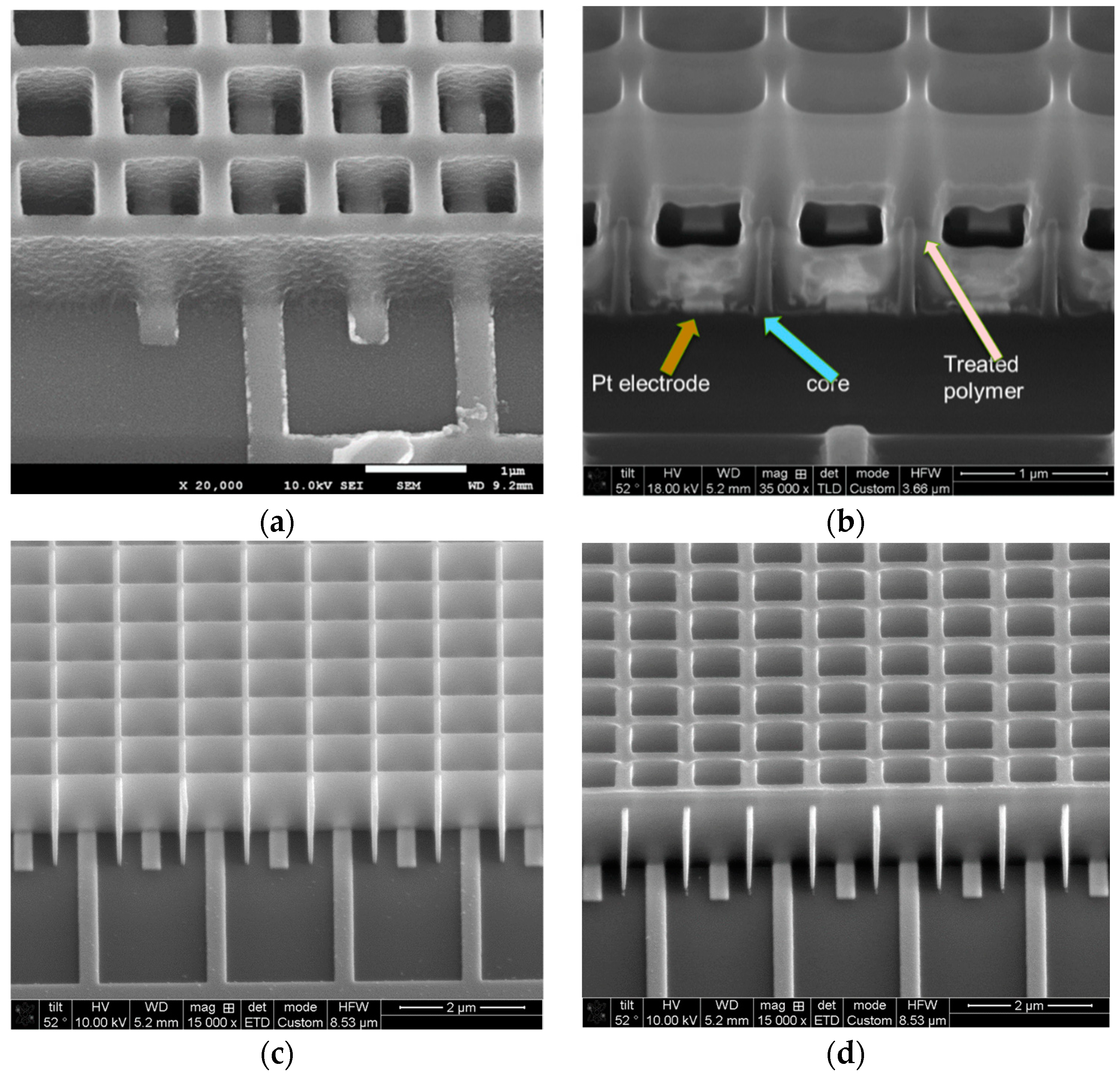
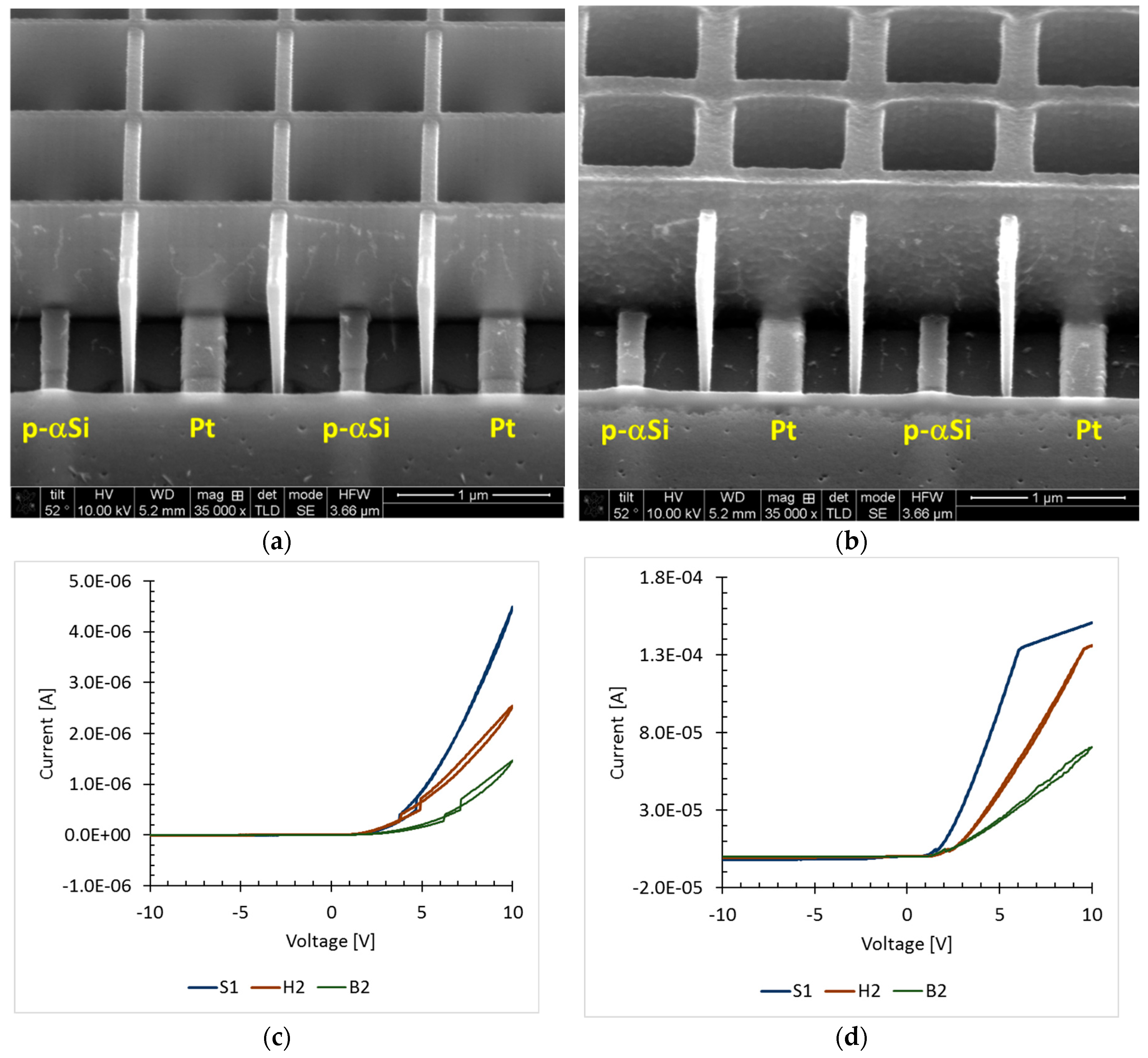
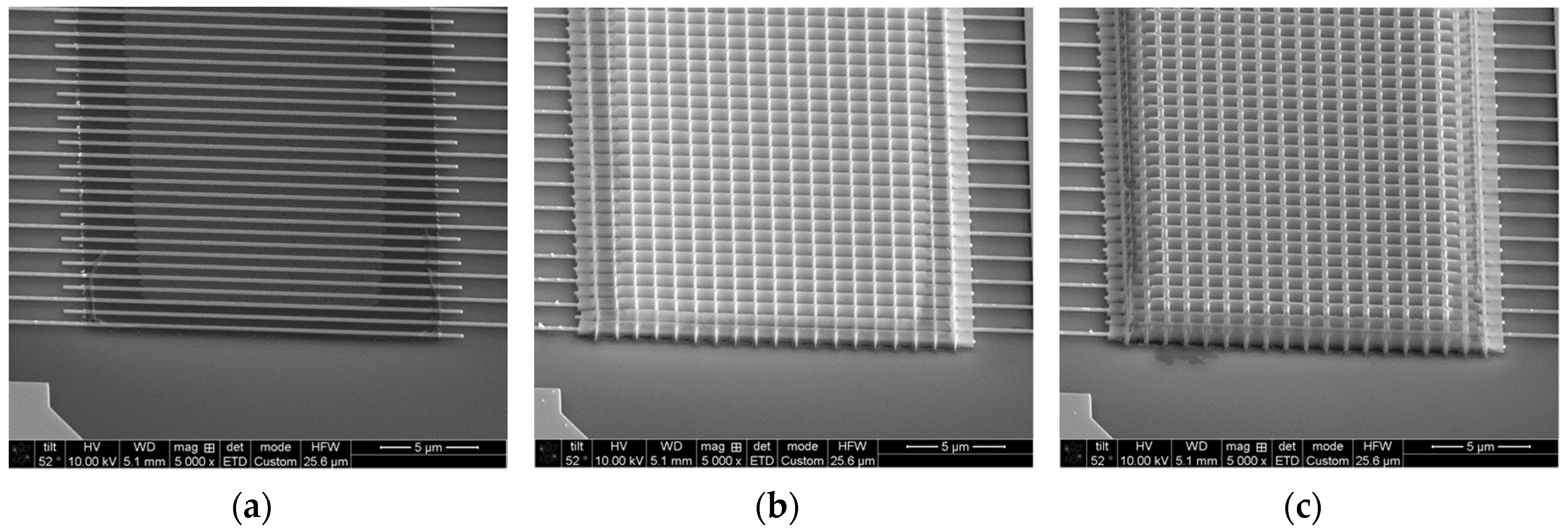
2.2. Sensor Fabrication
3. Results and Discussions
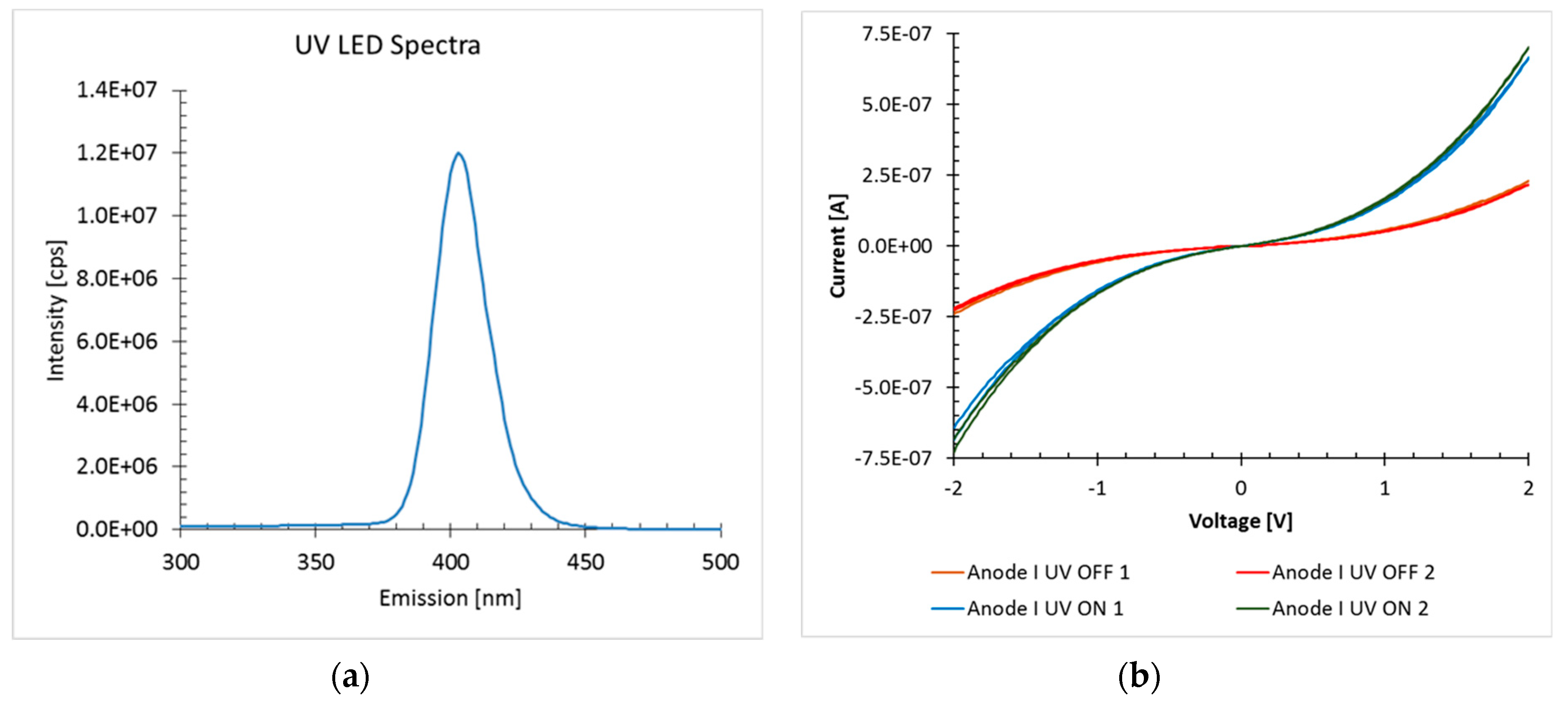
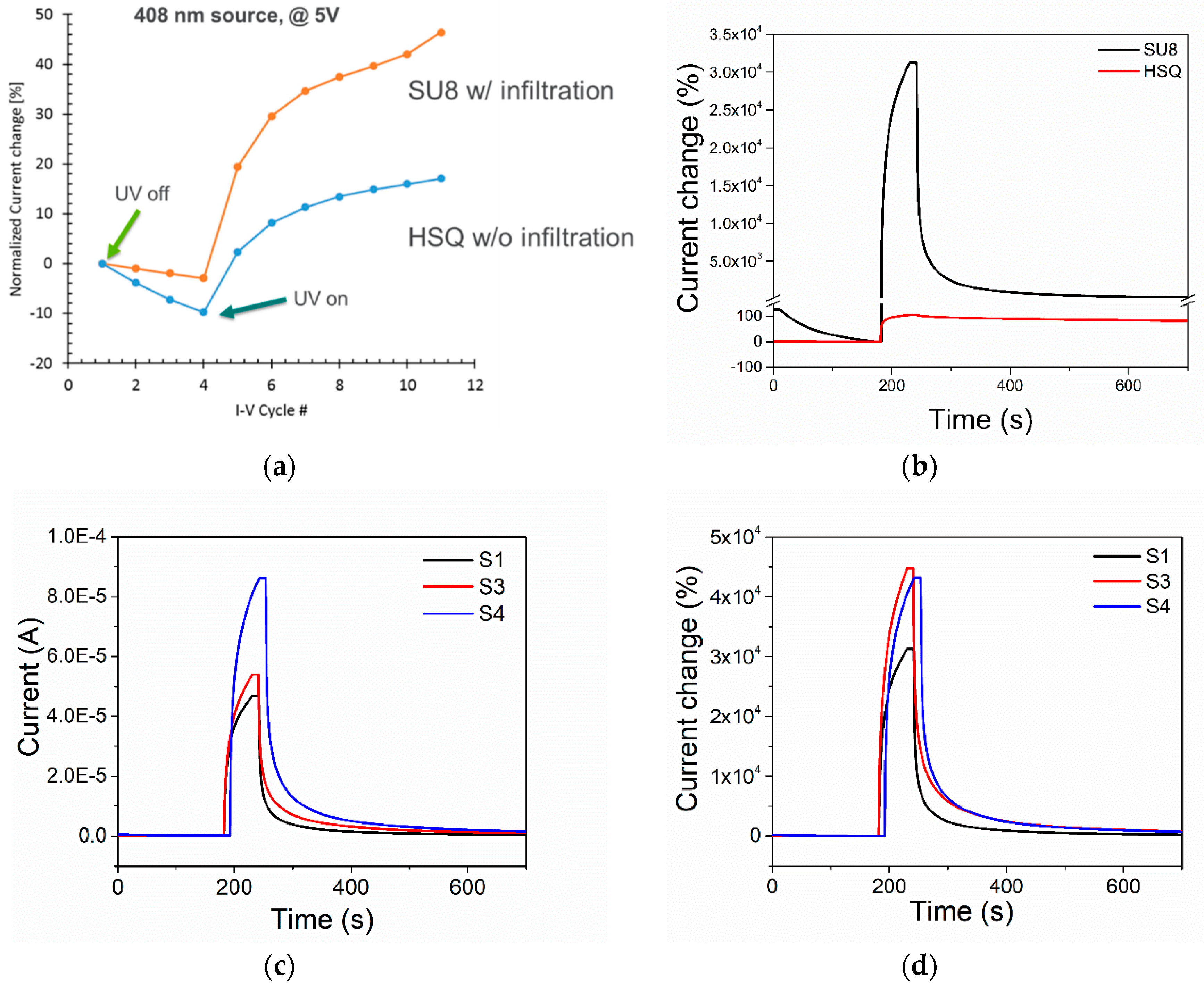
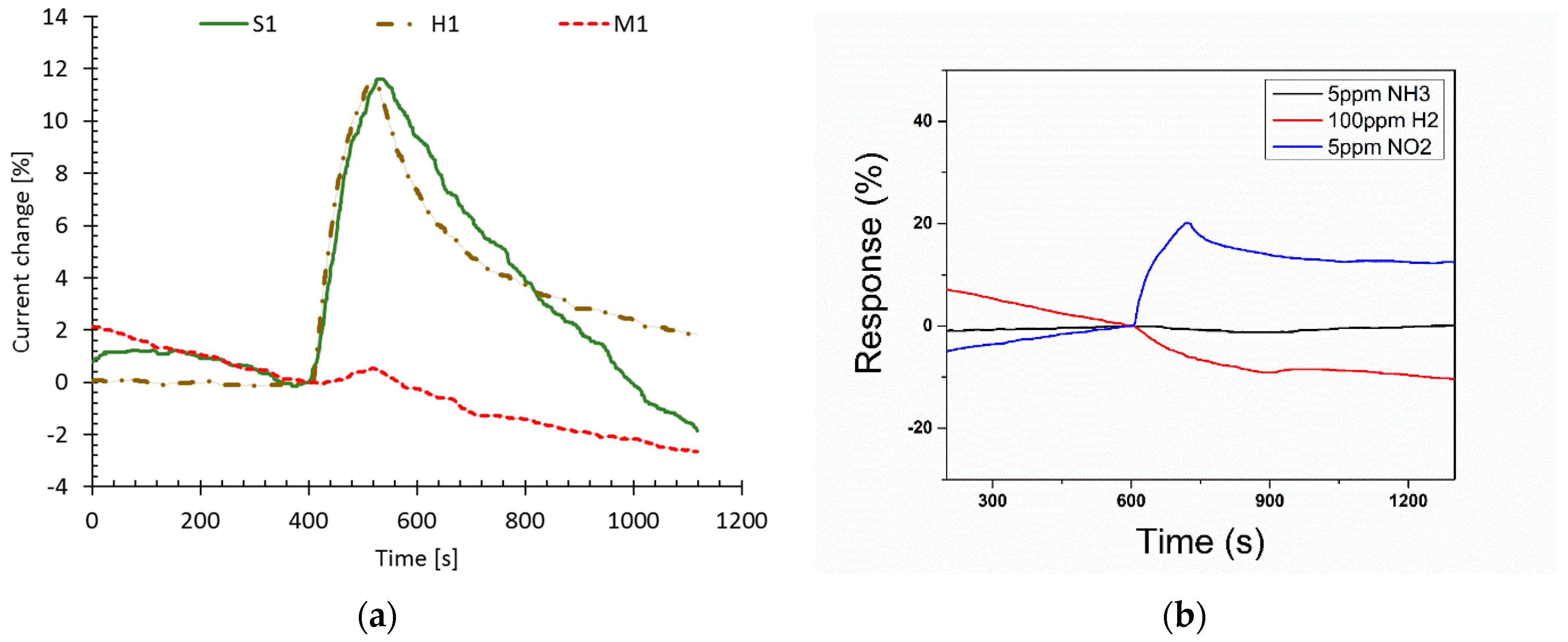
3.1. Response to UV Light
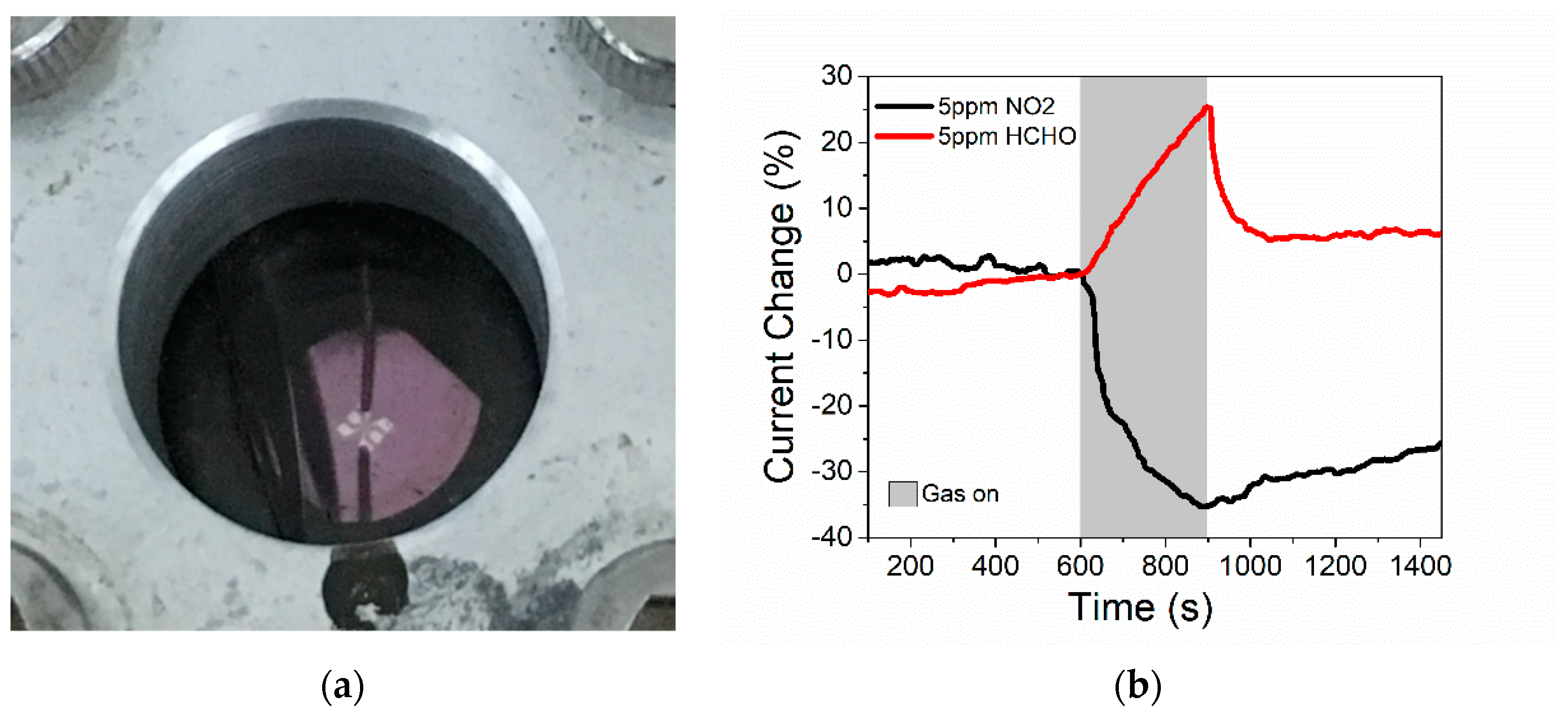
3.2. Response to Formaldehyde and NO2
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Liu, M.; Nam, C.-Y.; Black, C.T.; Kamcev, J.; Zhang, L. Enhancing water splitting activity and chemical stability of zinc oxide nanowire photoanodes with ultrathin titania shells. J. Phys. Chem. C 2013, 117, 13396–13402. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y. Understanding the reaction mechanism of photocatalytic reduction of CO2 with H2O on TiO2-based photocatalysts: A Review. Aerosol Air Qual. Res. 2014, 14, 453–469. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Fan, J.C.; Chang, S.L.; Xie, Z. ZnO-Based Light-Emitting Diodes. Optoelectronics—Advanced Materials and Devices; Pyshkin, S.L., Ballato, J.M., Eds.; InTech: Rijeka, Croatia, 2013; Chapter 2; p. 25. [Google Scholar]
- Chorfi, H.; Saadoun, M.; Bousselmi, L.; Bessais, B. TiO2-ITO and TiO2-ZnO nanocomposites: Application on water treatment. EPJ Web Conf. 2012, 29, 00015. [Google Scholar] [CrossRef]
- Comarmond, M.J.; Payne, T.E.; Harrison, J.J.; Thiruvoth, S.; Wong, H.K.; Aughterson, R.D.; Lumpkin, G.R.; Müller, K.; Foerstendorf, H. Uranium sorption on various forms of titanium dioxide—Influence of surface area, surface charge and impurities. Environ. Sci. Technol. 2011, 45, 5536–5542. [Google Scholar] [CrossRef]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent advances in ZnO nanostructures and thin films for biosensor applications: Review. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, E.; Barquinha, P.; Martins, R. Oxide Semiconductor Thin-Film Transistors: A Review of Recent Advances. Adv. Mater. 2012, 24, 2945–2986. [Google Scholar] [CrossRef]
- Chaieb, A.; Chari, A.; Sahraoui, B. Optical properties of ZnO nanocrystals embedded in PMMA. Opt. Quantum Electron. 2014, 46, 39–46. [Google Scholar] [CrossRef][Green Version]
- Soumya, S.; Mohamed, A.P.; Paul, L.; Mohan, K.; Ananthakumar, S. Near IR reflectance characteristics of PMMA/ZnO nanocomposites for solar thermal control interface films. Sol. Energy Mater. Sol. Cells 2014, 125, 102–112. [Google Scholar] [CrossRef]
- Paramo, J.A.; Strzhemechny, Y.M.; Anžlovar, A.; Žigon, M.; Orel, Z.C. Enhanced room temperature excitonic luminescence in ZnO/polymethyl methacrylate nanocomposites prepared by bulk polymerization. J. Appl. Phys. 2010, 108, 023517. [Google Scholar] [CrossRef]
- Japić, D.; Djerdj, I.; Marinšek, M.; Orel, Z.C. In situ and ex situ TEOS coating of ZnO nanoparticles and the preparation of composite ZnO/PMMA for UV-VIS absorbers. Acta Chim. Slov. 2013, 60, 797–806. [Google Scholar]
- Peng, Q.; Tseng, Y.-C.; Darling, S.B.; Elam, J.W. A route to nanoscopic materials via sequential infiltration synthesis on block copolymer templates. ACS Nano 2011, 5, 4600–4606. [Google Scholar] [CrossRef]
- Ocola, L.E.; Connolly, A.D.; Gosztola, J.; Schaller, R.D.; Yanguas-Gil, A. Infiltrated Zinc Oxide in Poly(methyl methacrylate): An Atomic Cycle Growth Study. J. Phys. Chem. C 2017, 121, 1893–1903. [Google Scholar] [CrossRef]
- Ye, X.; Kestell, J.; Kisslinger, K.; Liu, M.; Grubbs, R.B.; Boscoboinik, J.A.; Nam, C.-Y. Effects of Residual Solvent Molecules Facilitating the Infiltration Synthesis of ZnO in a Nonreactive Polymer. Chem. Mater. 2017, 29, 4535–4545. [Google Scholar] [CrossRef]
- Weber, M.; Julbe, A.; Ayral, A.; Miele, P.; Bechelany, M. Atomic Layer Deposition for Membranes: Basics, Challenges, and Opportunities. Chem. Mater. 2018, 30, 7368–7390. [Google Scholar] [CrossRef]
- Nam, C.-Y.; Stein, A.; Kisslinger, K. Direct fabrication of high aspect-ratio metal oxide nanopatterns via sequential infiltration synthesis in lithographically defined SU-8 templates. J. Vac. Sci. Technol. B 2015, 33, 06F201. [Google Scholar] [CrossRef]
- Nam, C.-Y.; Stein, A.; Kisslinger, K.; Black, C.T. Electrical and structural properties of ZnO synthesized via infiltration of lithographically defined polymer templates. Appl. Phys. Lett. 2015, 107, 203106. [Google Scholar] [CrossRef]
- Ocola, L.E.; Gosztola, D.J.; Yanguas-Gil, A. A Polymer-Hybrid Electro-Optic Devices and Method of Fabricating Polymer-Hybrid Electro-Optic Devices. US Patent 10164188, 25 December 2018. [Google Scholar]
- Lu, G.H.; Ocola, L.E.; Chen, J.H. Room-Temperature Gas Sensing through Electronic Transfer between Discrete Tin Oxide Nanocrystal and Multiwalled Carbon Nanotube. Adv. Mater. 2009, 21, 2487–2491. [Google Scholar] [CrossRef]
- Wang, R.; Tan, H.; Zhao, Z.; Zhang, G.; Song, L.; Dong, W.; Sun, Z. Stable ZnO@TiO2 core/shell nanorod arrays with exposed high energy facets for self-cleaning coatings with anti-reflective properties. J. Mater. Chem. A 2014, 2, 7313–7318. [Google Scholar] [CrossRef]
- Dai, J.; Xu, C.; Xu, X.; Guo, J.; Li, J.; Zhu, G.; Lin, Y. Single ZnO microrod ultraviolet photodetector with high photocurrent gain. ACS Appl. Mater. Interfaces 2013, 5, 9344–9348. [Google Scholar] [CrossRef] [PubMed]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO Nanowire UV Photodetectors with High Internal Gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- ThermoFisher Scientific Safety Data Sheet Formaldehyde solution 37%. Available online: https://www.fishersci.com/store/msds?partNumber=S25329&productDescription=formaldehyde-solution-&vendorId=VN00115888&keyword=true&countryCode=US&language=en (accessed on 28 April 2019).
- Praxair, Nitrogen dioxide, Safety Data Sheet. Available online: https://www.praxair.com/-/media/corporate/praxairus/documents/sds/nitrogen/nitrogen-dioxide-no2-and-n2o4-safety-data-sheet-sds-p4633.pdf?rev=a50e30f97ceb40c7a8340ecb79d2ec14 (accessed on 28 April 2019).
- Chougule, M.A.; Dalavi, D.S.; Mali, S.; Patil, P.S.; Moholkar, A.V.; Agawane, G.L.; Kim, J.H.; Sen, S.; Patil, V.B. Novel method for fabrication of room temperature polypyrrole-ZnO nanocomposite NO2 sensor. Measurement J. Int. Meas. Confed. 2012, 45, 1989–1996. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Xia, Y.; Komarneni, S.; Chen, H.; Xu, J.; Xiang, L.; Xie, D. Hierarchical ZnO Nanosheet-Nanorod Architectures for Fabrication of Poly(3-hexylthiophene)/ZnO Hybrid NO2 Sensor. ACS Appl. Mater. Interfaces 2016, 8, 8600–8607. [Google Scholar] [CrossRef]
- Kannan, P.K.; Saraswathi, R. An impedance sensor for the detection of formaldehyde vapor using ZnO nanoparticles. J. Mater. Res. 2017, 82, 2800–2809. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Li, X.; Xie, D.; Zhou, D.; Xiang, L.; Komarneni, S. Nanoseed-assisted rapid formation of ultrathin ZnO nanorods for efficient room temperature NO2 detection. Ceram. Int. 2016, 42, 15876–15880. [Google Scholar] [CrossRef]
- Zou, Z.; Qiu, Y.; Xu, J.; Guo, P.; Luo, Y.; Wang, C. Enhanced formaldehyde photoelectric response on ZnO film illuminated with visible light. J. Alloys Compd. 2017, 695, 2117–2123. [Google Scholar] [CrossRef]
- Su, X.; Gao, L.; Zhou, F.; Cai, W.; Duan, G. “Close network” effect of a ZnO micro/nanoporous array allows high UV-irradiated NO2 sensing performance. RSC Adv. 2017, 7, 21054–21060. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocola, L.E.; Wang, Y.; Divan, R.; Chen, J. Multifunctional UV and Gas Sensors Based on Vertically Nanostructured Zinc Oxide: Volume Versus Surface Effect. Sensors 2019, 19, 2061. https://doi.org/10.3390/s19092061
Ocola LE, Wang Y, Divan R, Chen J. Multifunctional UV and Gas Sensors Based on Vertically Nanostructured Zinc Oxide: Volume Versus Surface Effect. Sensors. 2019; 19(9):2061. https://doi.org/10.3390/s19092061
Chicago/Turabian StyleOcola, Leonidas E., Yale Wang, Ralu Divan, and Junhong Chen. 2019. "Multifunctional UV and Gas Sensors Based on Vertically Nanostructured Zinc Oxide: Volume Versus Surface Effect" Sensors 19, no. 9: 2061. https://doi.org/10.3390/s19092061
APA StyleOcola, L. E., Wang, Y., Divan, R., & Chen, J. (2019). Multifunctional UV and Gas Sensors Based on Vertically Nanostructured Zinc Oxide: Volume Versus Surface Effect. Sensors, 19(9), 2061. https://doi.org/10.3390/s19092061





