Rapid Determination of Chlorogenic Acid, Luteoloside and 3,5-O-dicaffeoylquinic Acid in Chrysanthemum Using Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Spectrometric Measurements
2.3. Reference Analysis Methods
2.4. Spectral Preprocessing
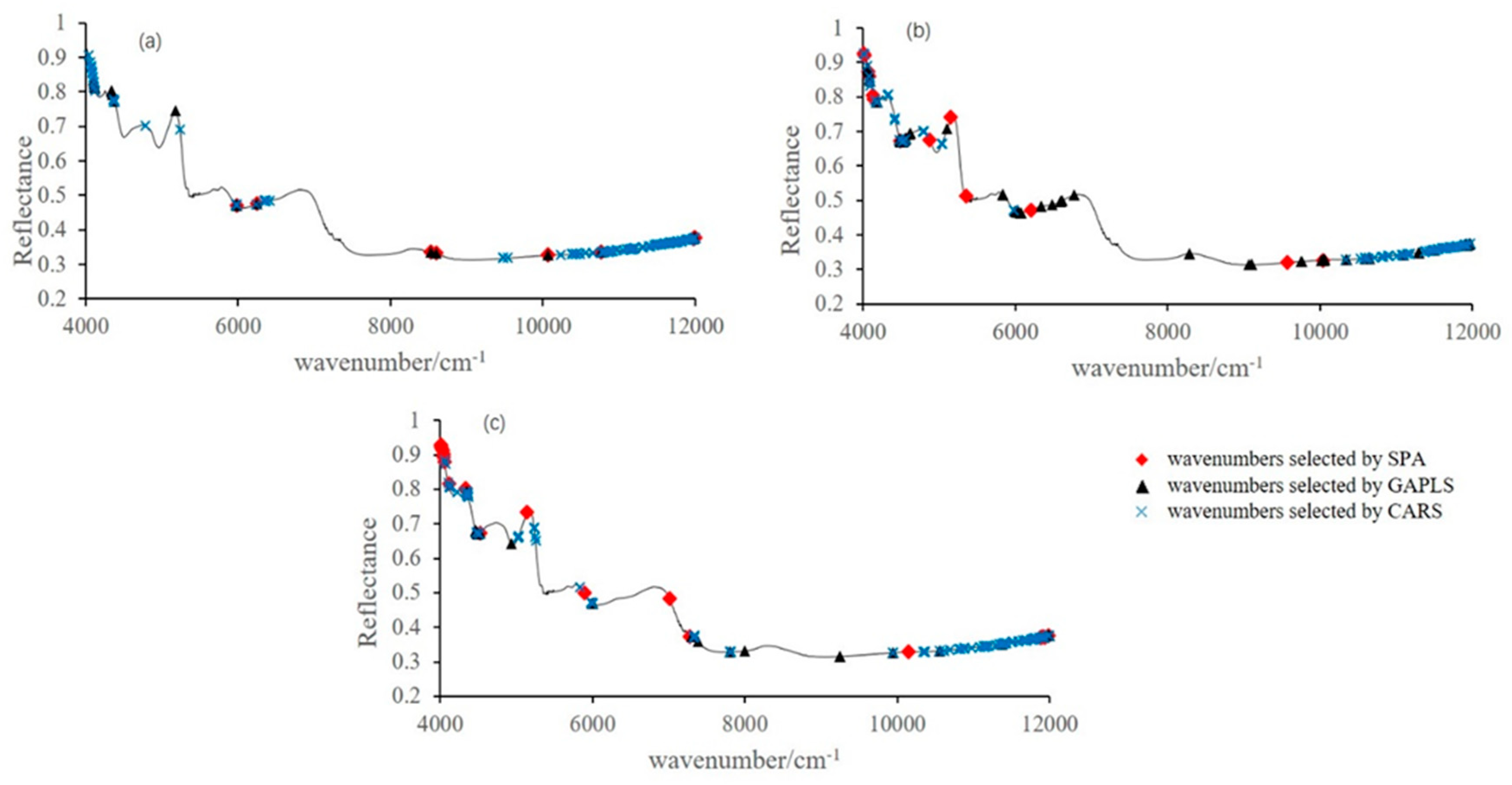
2.5. Sensitive Variables Selection
2.6. Chemometric Calibration Method
2.7. Model Evaluation and Softwares
3. Results and Discussion
3.1. Features of NIR Spectra and HPLC Analysis
3.2. Determination of the Best Preprocessing Algorithms
3.3. Sensitive Variables Selection
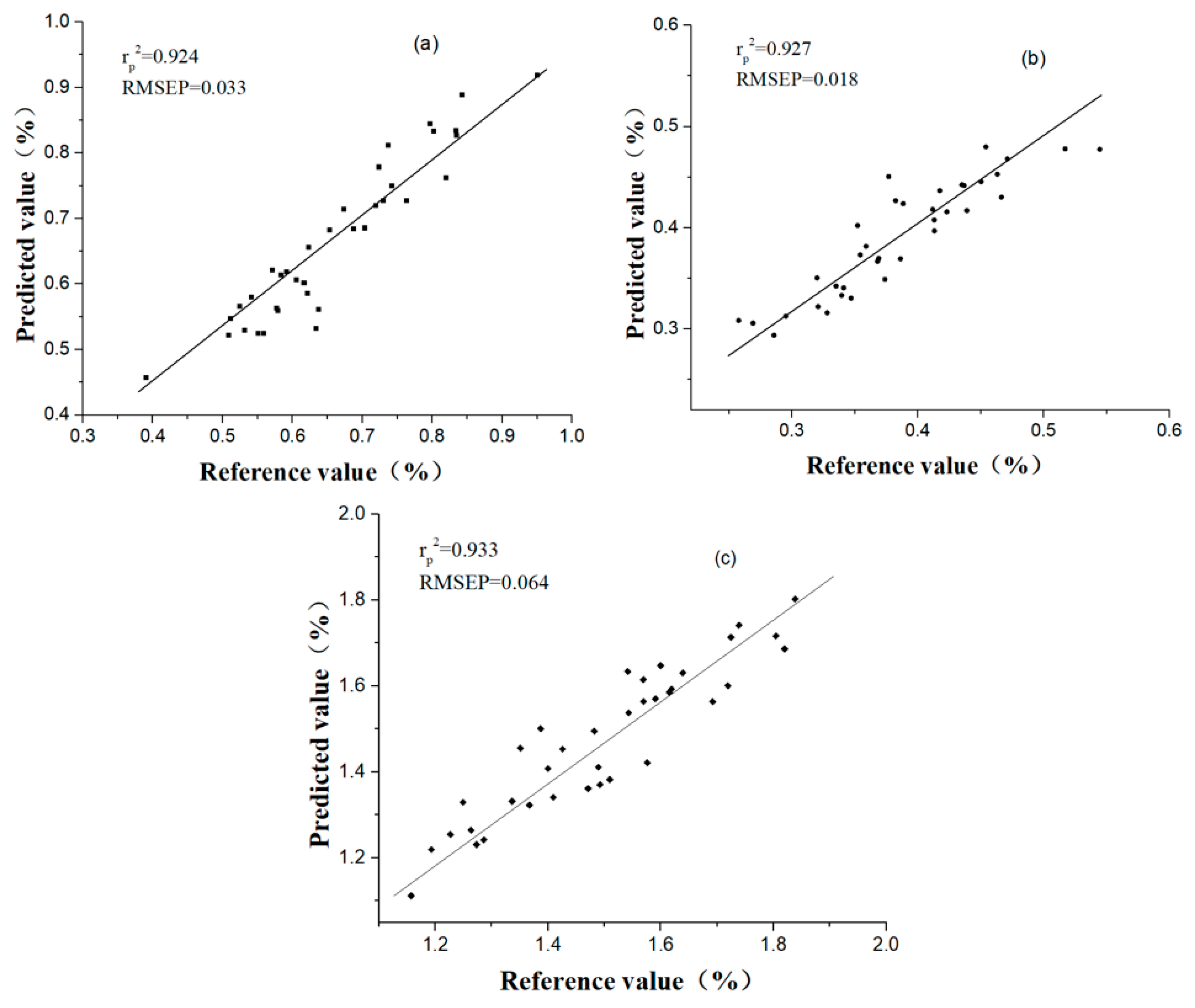
3.4. Model Calibration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, S.; Hao, L.J.; Zhu, J.J.; Wang, Z.M.; Zhang, X.; Song, X.M. Comparative Evaluation of Chrysanthemum Flos from Different Origins by HPLC-DAD-MS n and Relative Response Factors. Food Anal. Meth. 2015, 8, 40–51. [Google Scholar] [CrossRef]
- Chen, C.-W.; Yan, H.; Han, B.-X. Rapid identification of three varieties of chrysanthemum with near infrared spectroscopy. Rev. Bras. Farm. 2014, 24, 33–37. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. Identification of the phenolic components of chrysanthemum flower (Chrysanthemum morifolium Ramat). Food Chem. 2010, 120, 319–326. [Google Scholar] [CrossRef]
- Han, S.P. A successive projection method. Math. Program. 1988, 40, 1–14. [Google Scholar] [CrossRef]
- Upadhyay, R.; Rao, L.J.M. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial Activity and Mechanism of Action of Chlorogenic Acid. J. Food Sci. 2011, 76, 398–403. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef]
- Garbetta, A.; Capotorto, I.; Cardinali, A.; D’Antuono, I.; Linsalata, V.; Pizzi, F.; Minervini, F. Antioxidant activity induced by main polyphenols present in edible artichoke heads: influence of in vitro gastro-intestinal digestion. J. Funct. Foods 2014, 10, 456–464. [Google Scholar] [CrossRef]
- Zhao, H.E.; Liu, Z.H.; Hu, X.; Yin, J.L.; Li, W.; Rao, G.Y.; Zhang, X.H.; Huang, C.L.; Anderson, N.; Zhang, Q.X.; et al. Chrysanthemum genetic resources and related genera of Chrysanthemum collected in China. Genet. Resour. Evol. 2009, 56, 937–946. [Google Scholar] [CrossRef]
- Qin, M.-J.; Hong, J.-L.; He, D.-X.; Zhang, C.; Qin, M.; Shu, P.; Hong, J.; Lu, L.; He, D. Chemical Variations of the Essential Oils in Flower Heads of Chrysanthemum indicum L. from China. Chem. Biodivers. 2010, 7, 2951–2962. [Google Scholar]
- Guo, A.-L.; Chen, L.-M.; Wang, Y.-M.; Liu, X.-Q.; Zhang, Q.-W.; Gao, H.-M.; Wang, Z.-M.; Xiao, W.; Wang, Z.-Z.; McPhee, D.J. Influence of Sulfur Fumigation on the Chemical Constituents and Antioxidant Activity of Buds of Lonicera japonica. Molecules 2014, 19, 16640–16655. [Google Scholar] [CrossRef]
- Ding, G.; Li, B.; Han, Y.; Liu, A.; Zhang, J.; Peng, J.; Jiang, M.; Hou, Y.; Bai, G. A rapid integrated bioactivity evaluation system based on near-infrared spectroscopy for quality control of Flos Chrysanthemi. J. Pharm. Biomed. Anal. 2016, 131, 391–399. [Google Scholar] [CrossRef]
- Wang, D.-J.; Zhou, X.-Y.; Jin, T.-M.; Hu, X.-N.; Zhong, J.-E.; Wu, Q.-T. Application of near-infrared spectroscopy to agriculture and food analysis. Spectrosc. Spectr. Anal. 2004, 24, 447–450. [Google Scholar]
- Fu, H.-Y.; Huan, S.-Y.; Xu, L.; Tang, L.-J.; Jiang, J.-H.; Wu, H.-L.; Shen, G.-L.; Yu, R.-Q.; Tang, L.-J. Moving Window Partial Least-Squares Discriminant Analysis for Identification of Different Kinds of Bezoar Samples by near Infrared Spectroscopy and Comparison of Different Pattern Recognition Methods. J. Near Infrared Spectrosc. 2007, 15, 291–297. [Google Scholar] [CrossRef]
- Büning-Pfaue, H. Analysis of water in food by near infrared spectroscopy. Food Chem. 2003, 82, 107–115. [Google Scholar] [CrossRef]
- Zhang, C.; Su, J. Application of near infrared spectroscopy to the analysis and fast quality assessment of traditional Chinese medicinal products. Acta Pharm. Sin. B 2014, 4, 182–192. [Google Scholar] [CrossRef]
- Li, W.; Xing, L.; Fang, L.; Wang, J.; Qu, H. Application of near infrared spectroscopy for rapid analysis of intermediates of Tanreqing injection. J. Pharm. Biomed. Anal. 2010, 53, 350–358. [Google Scholar] [CrossRef]
- Tao, L.; Lin, Z.; Chen, J.; Wu, Y.; Liu, X. Mid-infrared and near-infrared spectroscopy for rapid detection of Gardeniae Fructus by a liquid-liquid extraction process. J. Pharm. Biomed. Anal. 2017, 145, 1–9. [Google Scholar] [CrossRef]
- Xiaobo, Z.; Jiewen, Z.; Povey, M.J.; Holmes, M.; Hanpin, M. Variables selection methods in near-infrared spectroscopy. Anal. Chim. Acta 2010, 667, 14–32. [Google Scholar] [CrossRef]
- Zhu, N.; Lin, M.; Nie, Y.; Wu, D.; Chen, K. Study on the quantitative measurement of firmness distribution maps at the pixel level inside peach pulp. Comput. Electron. Agric. 2016, 130, 48–56. [Google Scholar] [CrossRef]
- Ye, S.; Wang, D.; Min, S. Successive projections algorithm combined with uninformative variable elimination for spectral variable selection. Chemom. Intell. Lab. Syst. 2008, 91, 194–199. [Google Scholar] [CrossRef]
- Li, L.; Ustin, S.L.; Riano, D. Retrieval of Fresh Leaf Fuel Moisture Content Using Genetic Algorithm Partial Least Squares (GA-PLS) Modeling. IEEE Geosci. Sens. Lett. 2007, 4, 216–220. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key wavelengths screening using competitive adaptive reweighted sampling method for multivariate calibration. Anal. Chim. Acta 2009, 648, 77–84. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W. Application of visible and near infrared hyperspectral imaging for non-invasively measuring distribution of water-holding capacity in salmon flesh. Talanta 2013, 116, 266–276. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.-W. Potential of time series-hyperspectral imaging (TS-HSI) for non-invasive determination of microbial spoilage of salmon flesh. Talanta 2013, 111, 39–46. [Google Scholar] [CrossRef]
- Han, B.; Yan, H.; Chen, C.; Yao, H.; Dai, J.; Chen, N. A rapid identification of four medicinal chrysanthemum varieties with near infrared spectroscopy. Pharmacogn. Mag. 2014, 10, 353–358. [Google Scholar] [CrossRef]
- Saariaho, A.-M.; Argyropoulos, D.S.; Jääskeläinen, A.-S.; Vuorinen, T. Development of the partial least squares models for the interpretation of the UV resonance Raman spectra of lignin model compounds. Vib. Spectrosc. 2005, 37, 111–121. [Google Scholar] [CrossRef]
- Huang, L.; Liu, H.; Zhang, B.; Wu, D. Application of Electronic Nose with Multivariate Analysis and Sensor Selection for Botanical Origin Identification and Quality Determination of Honey. Food Bioprocess Technol. 2015, 8, 359–370. [Google Scholar] [CrossRef]
- Kumar, B.M.; Karthik, S. An efficient extreme learning machine (ELM) based classification of rice crop varieties using hyper-spectral image processing. Int. J. Appl. Eng. Res. 2014, 9, 28245–28260. [Google Scholar]
- Liu, G.-H.; Jiang, H.; Xiao, X.-H.; Zhang, D.-J.; Mei, C.-L.; Ding, Y.-H. Determination of process variable pH in solid-state fermentation by FT-NIR spectroscopy and extreme learning machine (ELM). Spectrosc. Spectr. Anal. 2012, 32, 970–973. [Google Scholar]
- Shi, H.; Lu, Y.; Du, J.; Du, W.; Ye, X.; Yu, X.; Ma, J.; Cheng, J.; Gao, Y.; Cao, Y.; et al. Application of Back Propagation Artificial Neural Network on Genetic Variants in Adiponectin ADIPOQ, Peroxisome Proliferator-Activated Receptor-γ, and Retinoid X Receptor-α Genes and Type 2 Diabetes Risk in a Chinese Han Population. Diabetes Technol. Ther. 2012, 14, 293–300. [Google Scholar] [CrossRef]
- Barma, S.D.; Das, B.; Giri, A.; Majumder, S.; Bose, P.K. Back propagation artificial neural network (BPANN) based performance analysis of diesel engine using biodiesel. J. Renew. Sustain. Energy 2011, 3, 13101. [Google Scholar] [CrossRef]
- Karegowda, A.G.; Nasiha, A.; Jayaram, M.; Manjunath, A. Exudates Detection in Retinal Images using Back Propagation Neural Network. Int. J. Comput. Appl. 2011, 25, 25–31. [Google Scholar] [CrossRef]

| Calibration Set | Prediction Set | |||||
|---|---|---|---|---|---|---|
| Range (%) | Mean (%) | S.D. 1 | Range (%) | Mean (%) | S.D. | |
| Chlorogenic acid | 0.388–0.961 | 0.648 | 0.121 | 0.390–0.950 | 0.660 | 0.121 |
| Luteoloside | 0.255–0.552 | 0.388 | 0.063 | 0.258–0.545 | 0.387 | 0.066 |
| 3,5-O-dicaffeoylquinic acid | 0.985–1.839 | 1.506 | 0.189 | 1.193–1.838 | 1.499 | 0.185 |
| Quality | Preprocessing | Number of Latent Variables | Calibration | Prediction | |||||
|---|---|---|---|---|---|---|---|---|---|
| rc2 | RMSEC | rp2 | RMSEP | Slope | Bias | RPD | |||
| Chlorogenic Acid | None | 9 | 0.906 | 0.036 | 0.797 | 0.054 | 0.775 | −0.014 | 2.241 |
| MAS | 6 | 0.841 | 0.047 | 0.841 | 0.047 | 0.727 | −0.011 | 2.574 | |
| SG | 6 | 0.839 | 0.047 | 0.843 | 0.047 | 0.728 | −0.010 | 2.574 | |
| SNV | 7 | 0.876 | 0.041 | 0.762 | 0.059 | 0.756 | −0.017 | 2.051 | |
| MSC | 9 | 0.878 | 0.041 | 0.767 | 0.057 | 0.768 | −0.017 | 2.123 | |
| 1-Der | 5 | 0.882 | 0.040 | 0.740 | 0.061 | 0.738 | −0.009 | 1.984 | |
| Detrend | 7 | 0.869 | 0.042 | 0.808 | 0.052 | 0.773 | −0.014 | 2.327 | |
| Luteoloside | None | 13 | 0.976 | 0.009 | 0.728 | 0.034 | 0.753 | 0.004 | 1.941 |
| MAS | 11 | 0.910 | 0.018 | 0.738 | 0.033 | 0.734 | −0.004 | 2.000 | |
| SG | 11 | 0.901 | 0.019 | 0.741 | 0.033 | 0.754 | 0.001 | 2.000 | |
| SNV | 12 | 0.974 | 0.010 | 0.741 | 0.033 | 0.761 | 0.005 | 2.000 | |
| MSC | 10 | 0.949 | 0.014 | 0.728 | 0.034 | 0.748 | 0.004 | 1.941 | |
| 1-Der | 8 | 0.918 | 0.018 | 0.650 | 0.039 | 0.624 | −0.006 | 1.692 | |
| Detrend | 11 | 0.964 | 0.012 | 0.691 | 0.036 | 0.731 | 0.003 | 1.833 | |
| 3,5-O-dicaffeoylquinic acid | None | 10 | 0.920 | 0.053 | 0.843 | 0.072 | 0.842 | 0.012 | 2.569 |
| MAS | 10 | 0.918 | 0.054 | 0.832 | 0.075 | 0.838 | 0.014 | 2.467 | |
| SG | 10 | 0.876 | 0.066 | 0.815 | 0.078 | 0.793 | 0.011 | 2.372 | |
| SNV | 8 | 0.846 | 0.073 | 0.766 | 0.088 | 0.754 | −0.004 | 2.102 | |
| MSC | 9 | 0.908 | 0.057 | 0.823 | 0.077 | 0.831 | −0.002 | 2.403 | |
| 1-Der | 5 | 0.803 | 0.083 | 0.605 | 0.114 | 0.791 | −0.003 | 1.623 | |
| Detrend | 9 | 0.918 | 0.054 | 0.814 | 0.079 | 0.843 | 0.015 | 2.342 | |
| Quality | Preprocessing | Variable Selection Methods | Models | Calibration | Prediction | |||
|---|---|---|---|---|---|---|---|---|
| rc2 | RMSEC | rp2 | RMSEP | RPD | ||||
| Chlorogenic Acid | SG | SPA | PLS | 0.859 | 0.044 | 0.843 | 0.047 | 2.574 |
| ELM | 0.846 | 0.046 | 0.876 | 0.047 | 2.574 | |||
| BP-ANN | 0.889 | 0.039 | 0.857 | 0.047 | 2.574 | |||
| GAPLS | PLS | 0.910 | 0.035 | 0.841 | 0.048 | 2.521 | ||
| ELM | 0.878 | 0.041 | 0.834 | 0.052 | 2.327 | |||
| BP-ANN | 0.885 | 0.039 | 0.874 | 0.044 | 2.750 | |||
| CARS | PLS | 0.970 | 0.020 | 0.899 | 0.038 | 3.184 | ||
| ELM | 0.972 | 0.020 | 0.882 | 0.041 | 2.951 | |||
| BP-ANN | 0.964 | 0.022 | 0.924 | 0.033 | 3.667 | |||
| Luteoloside | SNV | SPA | PLS | 0.803 | 0.027 | 0.736 | 0.033 | 2.000 |
| ELM | 0.856 | 0.023 | 0.738 | 0.033 | 2.000 | |||
| BP-ANN | 0.834 | 0.025 | 0.783 | 0.031 | 2.129 | |||
| GAPLS | PLS | 0.801 | 0.027 | 0.759 | 0.032 | 2.063 | ||
| ELM | 0.648 | 0.036 | 0.733 | 0.036 | 1.833 | |||
| BP-ANN | 0.846 | 0.024 | 0.814 | 0.028 | 2.357 | |||
| CARS | PLS | 0.976 | 0.009 | 0.910 | 0.020 | 3.300 | ||
| ELM | 0.998 | 0.000 | 0.819 | 0.041 | 1.610 | |||
| BP-ANN | 0.955 | 0.013 | 0.927 | 0.018 | 3.667 | |||
| 3,5-O-dicaffeoylquinic acid | None | SPA | PLS | 0.808 | 0.082 | 0.771 | 0.087 | 2.126 |
| ELM | 0.843 | 0.074 | 0.808 | 0.083 | 2.229 | |||
| BP-ANN | 0.848 | 0.074 | 0.850 | 0.072 | 2.569 | |||
| GAPLS | PLS | 0.869 | 0.068 | 0.856 | 0.069 | 2.681 | ||
| ELM | 0.904 | 0.058 | 0.878 | 0.065 | 2.846 | |||
| BP-ANN | 0.906 | 0.058 | 0.908 | 0.056 | 3.304 | |||
| CARS | PLS | 0.974 | 0.031 | 0.927 | 0.049 | 3.776 | ||
| ELM | 0.949 | 0.042 | 0.893 | 0.064 | 2.891 | |||
| BP-ANN | 0.962 | 0.039 | 0.933 | 0.064 | 2.891 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.; Sun, Y.; Cai, C.; He, Y.; Nie, P. Rapid Determination of Chlorogenic Acid, Luteoloside and 3,5-O-dicaffeoylquinic Acid in Chrysanthemum Using Near-Infrared Spectroscopy. Sensors 2019, 19, 1981. https://doi.org/10.3390/s19091981
Xia Z, Sun Y, Cai C, He Y, Nie P. Rapid Determination of Chlorogenic Acid, Luteoloside and 3,5-O-dicaffeoylquinic Acid in Chrysanthemum Using Near-Infrared Spectroscopy. Sensors. 2019; 19(9):1981. https://doi.org/10.3390/s19091981
Chicago/Turabian StyleXia, Zhengyan, Yiming Sun, Chengyong Cai, Yong He, and Pengcheng Nie. 2019. "Rapid Determination of Chlorogenic Acid, Luteoloside and 3,5-O-dicaffeoylquinic Acid in Chrysanthemum Using Near-Infrared Spectroscopy" Sensors 19, no. 9: 1981. https://doi.org/10.3390/s19091981
APA StyleXia, Z., Sun, Y., Cai, C., He, Y., & Nie, P. (2019). Rapid Determination of Chlorogenic Acid, Luteoloside and 3,5-O-dicaffeoylquinic Acid in Chrysanthemum Using Near-Infrared Spectroscopy. Sensors, 19(9), 1981. https://doi.org/10.3390/s19091981






