A Polyamidoamine Dendrimer-Based Electrochemical Immunosensor for Label-Free Determination of Epithelial Cell Adhesion Molecule- Expressing Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Apparatus
2.2. Pre-Preparation of Gold Electrode
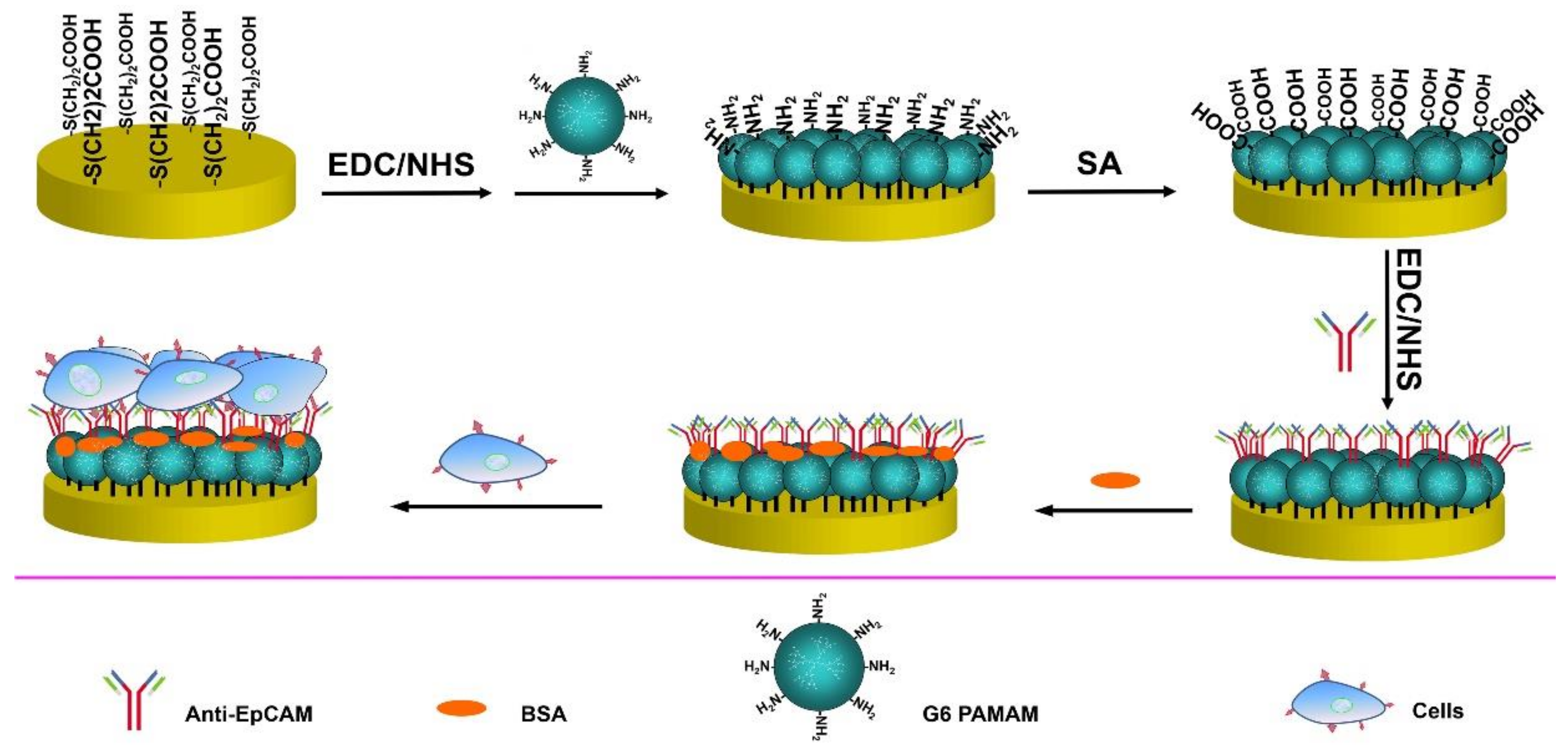
2.3. Modification of Gold Electrode
2.4. Cell Culture and Treatment
3. Results and Discussion
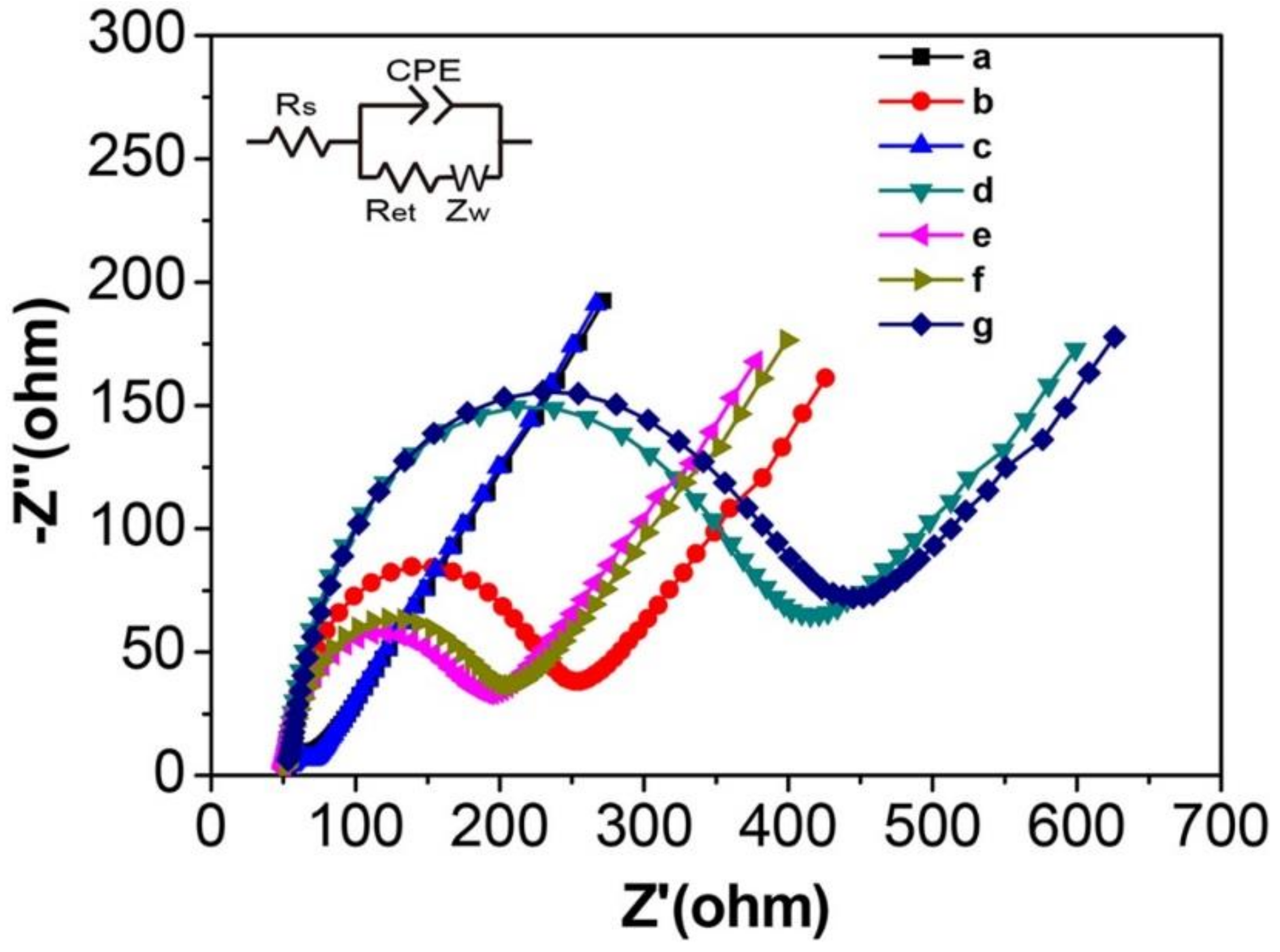
3.1. EIS Characterization of the Modified Electrode
3.2. Cyclic Voltammetry Behavior of the Modified Electrode
3.3. Experimental Conditions Optimization
3.4. Specificity and Interference Study
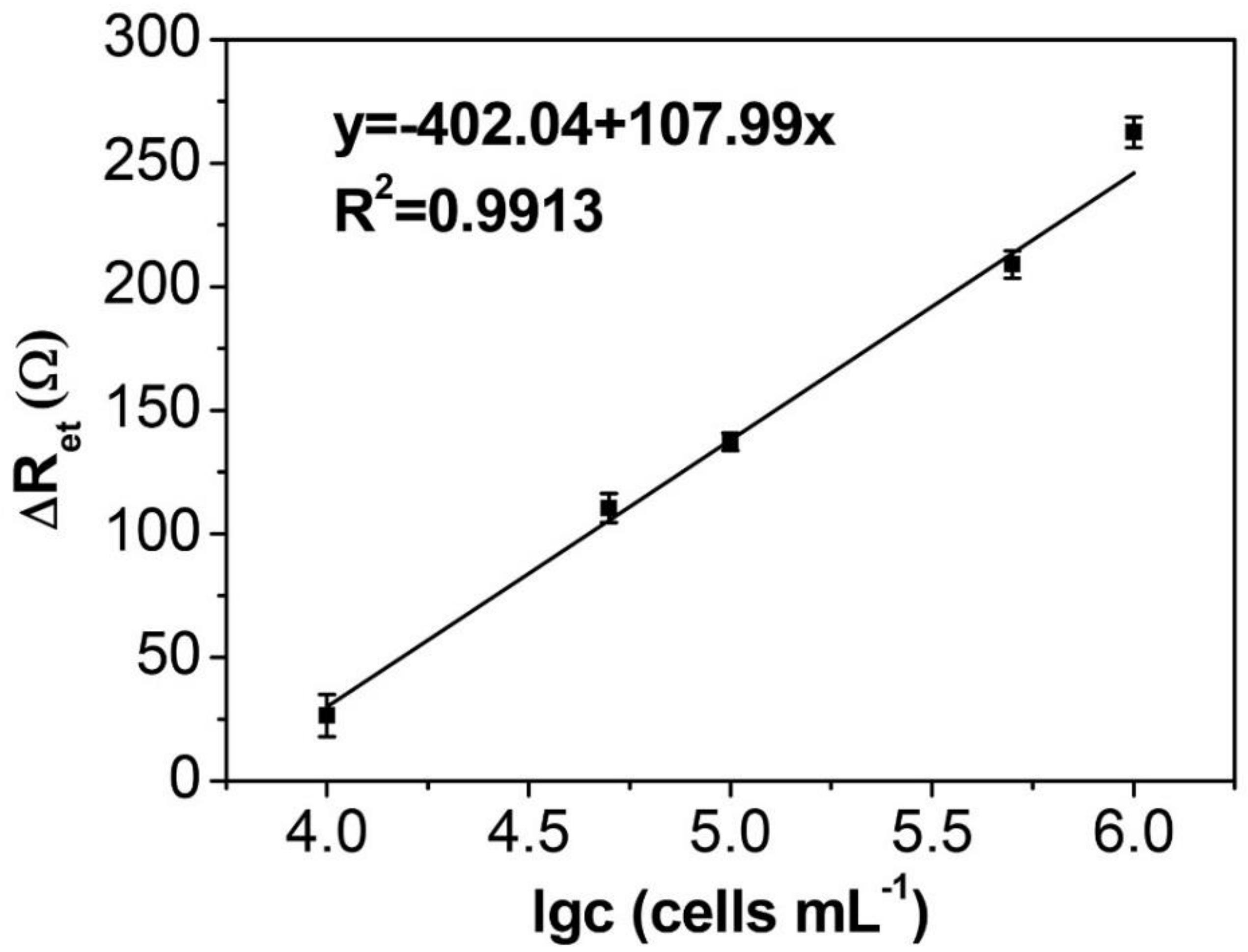
3.5. EIS Detection of HepG2 Cells
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jiang, W.; Zhao, S.; Jiang, X.; Zhang, E.; Hu, G.; Hu, B.; Ping, Z.; Xiao, J.; Lu, Z.; Lu, Y. The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett. 2016, 371, 314–325. [Google Scholar] [CrossRef]
- Deng, R.; Zhang, K.; Wang, L.; Ren, X.; Sun, Y.; Li, J. DNA-Sequence-Encoded Rolling Circle Amplicon for Single-Cell RNA Imaging. Chem 2018, 4, 1373–1386. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Gui, D.Y.; Heiden, M.G.V. Altered metabolite levels in cancer: Implications for tumour biology and cancer therapy. Nat. Rev. Cancer 2016, 16, 680. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, Z.S.; Wang, Z.; Le, J.; Zheng, T.; Jia, L. Autonomous assembly of ordered metastable DNA nanoarchitecture and in situ visualizing of intracellular microRNAs. Biomaterials 2017, 120, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wu, Z.S.; Wang, Z.; Li, H.; Le, J.; Jia, L. Two-wheel drive-based DNA nanomachine and its sensing potential for highly sensitive analysis of cancer-related gene. Biomaterials 2016, 100, 110–117. [Google Scholar] [CrossRef]
- Xia, X.; Wang, H.; Yang, H.; Deng, S.; Deng, R.; Dong, Y.; He, Q. Dual-Terminal Stemmed Aptamer Beacon for Label-Free Detection of Aflatoxin B1 in Broad Bean Paste and Peanut Oil Via Aggregation-Induced Emission. J. Agric. Food Chem. 2018, 66, 12431–12438. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-J.; Mannan, P.; Lu, M.; Udey, M.C. Epithelial Cell Adhesion Molecule (EpCAM) Regulates Claudin Dynamics and Tight Junctions. J. Biol. Chem. 2013, 288, 12253–12268. [Google Scholar] [CrossRef]
- Myung, J.H.; Gajjar, K.A.; Saric, J.; Eddington, D.T.; Hong, S. Dendrimer-Mediated Multivalent Binding for the Enhanced Capture of Tumor Cells. Angew. Chem. 2011, 123, 11973–11976. [Google Scholar] [CrossRef]
- Henrich, C.J.; Budhu, A.; Yu, Z.; Evans, J.R.; Goncharova, E.I.; Ransom, T.T.; Wang, X.W.; McMahon, J.B. High-throughput Screening for Identification of Inhibitors of EpCAM-Dependent Growth of Hepatocellular Carcinoma Cells. Chem. Biol. Drug Des. 2013, 82, 131–139. [Google Scholar] [CrossRef]
- Tomasz, S.; Gra?Yna, H.; Jerzy, K.; Dariusz, D.; Joanna, D.A.K. Flow cytometric analysis of CD133- and EpCAM-positive cells in the peripheral blood of patients with lung cancer. Arch. Immunol. Et. Ther. Exp. 2014, 62, 67–75. [Google Scholar]
- Awasthi, N.P.; Kumari, S.; Neyaz, A.; Gupta, S.; Agarwal, A.; Singhal, A.; Husain, N. EpCAM-based Flow Cytometric Detection of Circulating Tumor Cells in Gallbladder Carcinoma Cases. Asian Pac. J. Cancer Prev. 2017, 18, 3429. [Google Scholar]
- Ntouroupi, T.; Ashraf, S.; McGregor, S.; Turney, B.; Seppo, A.; Kim, Y.; Wang, X.; Kilpatrick, M.; Tsipouras, P.; Tafas, T. Detection of circulating tumour cells in peripheral blood with an automated scanning fluorescence microscope. Br. J. Cancer 2008, 99, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xiao, T.; Zhang, Z.; He, R.; Wen, D.; Cao, Y.; Zhang, W.; Chen, Y. A 3D graphene oxide microchip and a Au-enwrapped silica nanocomposite-based supersandwich cytosensor toward capture and analysis of circulating tumor cells. Nanoscale 2015, 7, 16354. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.; Huang, H.; Deng, X.; Chen, Y.; Jiang, Z.; Min, X.; Liu, S.; Huang, W.; Xiang, Z. Programmable DNA-responsive microchip for the capture and release of circulating tumor cells by nucleic acid hybridization. Nano Res. 2018, 11, 1–13. [Google Scholar]
- Qiu, L.; Qiu, L.; Wu, Z.-S.; Shen, G.; Yu, R.-Q. Cooperative Amplification-Based Electrochemical Sensor for the Zeptomole Detection of Nucleic Acids. Anal. Chem. 2013, 85, 8225–8231. [Google Scholar] [CrossRef] [PubMed]
- Ciani, I.; Schulze, H.; Corrigan, D.K.; Henihan, G.; Giraud, G.; Terry, J.G.; Walton, A.J.; Pethig, R.; Ghazal, P.; Crain, J. Development of immunosensors for direct detection of three wound infection biomarkers at point of care using electrochemical impedance spectroscopy. Biosens. Bioelectron. 2012, 31, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhu, X.; Liu, Q.; Lin, Z.; Qiu, B.; Chen, G. Label-free detection of telomerase activity in HeLa cells using electrochemical impedance spectroscopy. Chem. Commun. 2011, 47, 3129–3131. [Google Scholar] [CrossRef]
- Wang, R.; Di, J.; Ma, J.; Ma, Z. Highly sensitive detection of cancer cells by electrochemical impedance spectroscopy. Electrochim. Acta 2012, 61, 179–184. [Google Scholar] [CrossRef]
- Evtugyn, G.; Hianik, T. Electrochemical DNA sensors and aptasensors based on electropolymerized materials and polyelectrolyte complexes. TRAC Trends Anal. Chem. 2016, 79, 168–178. [Google Scholar] [CrossRef]
- Karapetis, S.; Nikoleli, G.-P.; Siontorou, C.G.; Nikolelis, D.P.; Tzamtzis, N.; Psaroudakis, N. Development of an Electrochemical Biosensor for the Rapid Detection of Cholera Toxin Based on Air Stable Lipid Films with Incorporated Ganglioside GM1 Using Graphene Electrodes. Electroanalysis 2016, 28, 1584–1590. [Google Scholar] [CrossRef]
- Sun, X.; Ji, J.; Jiang, D.; Li, X.; Zhang, Y.; Li, Z.; Wu, Y. Development of a novel electrochemical sensor using pheochromocytoma cells and its assessment of acrylamide cytotoxicity. Biosens. Bioelectron. 2013, 44, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Cheng, W.; Wang, X.; Xue, Y.; Lei, J.; Yin, Y.; Ju, H. A label-free strategy for facile electrochemical analysis of dynamic glycan expression on living cells. Chem. Commun. 2009, 7161–7163. [Google Scholar] [CrossRef] [PubMed]
- Satija, J.; Sai, V.; Mukherji, S. Dendrimers in biosensors: Concept and applications. J. Mater. Chem. 2011, 21, 14367–14386. [Google Scholar] [CrossRef]
- Castillo, G.; Spinella, K.; Poturnayová, A.; Šnejdárková, M.; Mosiello, L.; Hianik, T. Detection of aflatoxin B 1 by aptamer-based biosensor using PAMAM dendrimers as immobilization platform. Food Control 2015, 52, 9–18. [Google Scholar] [CrossRef]
- Deng, S.; Lei, J.; Liu, Y.; Huang, Y.; Ju, H. A ferrocenyl-terminated dendrimer as an efficient quencher via electron and energy transfer for cathodic electrochemiluminescent bioanalysis. Chem. Commun. 2013, 49, 2106–2108. [Google Scholar] [CrossRef]
- Zhuo, Y.; Gui, G.; Chai, Y.; Liao, N.; Xiao, K.; Yuan, R. Sandwich-format electrochemiluminescence assays for tumor marker based on PAMAM dendrimer-l-cysteine-hollow gold nanosphere nanocomposites. Biosens. Bioelectron. 2014, 53, 459–464. [Google Scholar] [CrossRef]
- Guichard, C.; Amaddeo, G.; Imbeaud, S.; Ladeiro, Y.; Pelletier, L.; Maad, I.B.; Calderaro, J.; Bioulac-Sage, P.; Letexier, M.; Degos, F. Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat. Genet. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Yamashita, T.; Budhu, A.; Forgues, M.; Wang, X.W. Activation of hepatic stem cell marker EpCAM by Wnt–β-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007, 67, 10831–10839. [Google Scholar] [CrossRef]
- Chung, Y.-K.; Reboud, J.; Lee, K.C.; Lim, H.M.; Lim, P.Y.; Wang, K.Y.; Tang, K.C.; Ji, H.; Chen, Y. An electrical biosensor for the detection of circulating tumor cells. Biosens. Bioelectron. 2011, 26, 2520–2526. [Google Scholar] [CrossRef]
- Li, G.; Li, X.; Wan, J.; Zhang, S. Dendrimers-based DNA biosensors for highly sensitive electrochemical detection of DNA hybridization using reporter probe DNA modified with Au nanoparticles. Biosens. Bioelectron. 2009, 24, 3281–3287. [Google Scholar] [CrossRef] [PubMed]
- Buis, B.; Wever, P.; Koomen, G.; Van Acker, B.; Groothoff, J.; Krediet, R.; Arisz, L. Clearance ratios of amylase isoenzymes and IgG subclasses: Do they reflect glomerular charge selectivity? Nephron 1997, 75, 444–450. [Google Scholar] [CrossRef]
- Zandberg, W.F.; Kumarasamy, J.; Pinto, B.M.; Vocadlo, D.J. Metabolic inhibition of sialyl-Lewis X biosynthesis by 5-thiofucose remodels the cell surface and impairs selectin-mediated cell adhesion. J. Biol. Chem. 2012, 287, 40021–40030. [Google Scholar] [CrossRef]
- Ohyama, C.; Tsuboi, S.; Fukuda, M. Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. EMBO J. 1999, 18, 1516–1525. [Google Scholar] [CrossRef]
- Jiang, X.; Tan, L.; Zhang, B.; Zhang, Y.; Tang, H.; Xie, Q.; Yao, S. Detection of adherent cells using electrochemical impedance spectroscopy based on molecular recognition of integrin β1. Sens. Actuators B Chem. 2010, 149, 87–93. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.; Jia, N.; Huang, B.; Tan, L.; Yang, S.; Tang, H.; Xie, Q.; Yao, S. Quantification of Bax protein on tumor cells based on electrochemical immunoassay. Sens. Actuators B Chem. 2013, 186, 506–514. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Wang, X.; Yan, C.; Chen, W. A Polyamidoamine Dendrimer-Based Electrochemical Immunosensor for Label-Free Determination of Epithelial Cell Adhesion Molecule- Expressing Cancer Cells. Sensors 2019, 19, 1879. https://doi.org/10.3390/s19081879
Xu J, Wang X, Yan C, Chen W. A Polyamidoamine Dendrimer-Based Electrochemical Immunosensor for Label-Free Determination of Epithelial Cell Adhesion Molecule- Expressing Cancer Cells. Sensors. 2019; 19(8):1879. https://doi.org/10.3390/s19081879
Chicago/Turabian StyleXu, Jianguo, Xinxin Wang, Chao Yan, and Wei Chen. 2019. "A Polyamidoamine Dendrimer-Based Electrochemical Immunosensor for Label-Free Determination of Epithelial Cell Adhesion Molecule- Expressing Cancer Cells" Sensors 19, no. 8: 1879. https://doi.org/10.3390/s19081879
APA StyleXu, J., Wang, X., Yan, C., & Chen, W. (2019). A Polyamidoamine Dendrimer-Based Electrochemical Immunosensor for Label-Free Determination of Epithelial Cell Adhesion Molecule- Expressing Cancer Cells. Sensors, 19(8), 1879. https://doi.org/10.3390/s19081879




