Feasible Classified Models for Parkinson Disease from 99mTc-TRODAT-1 SPECT Imaging
Abstract
1. Introduction
2. Materials and Methodology
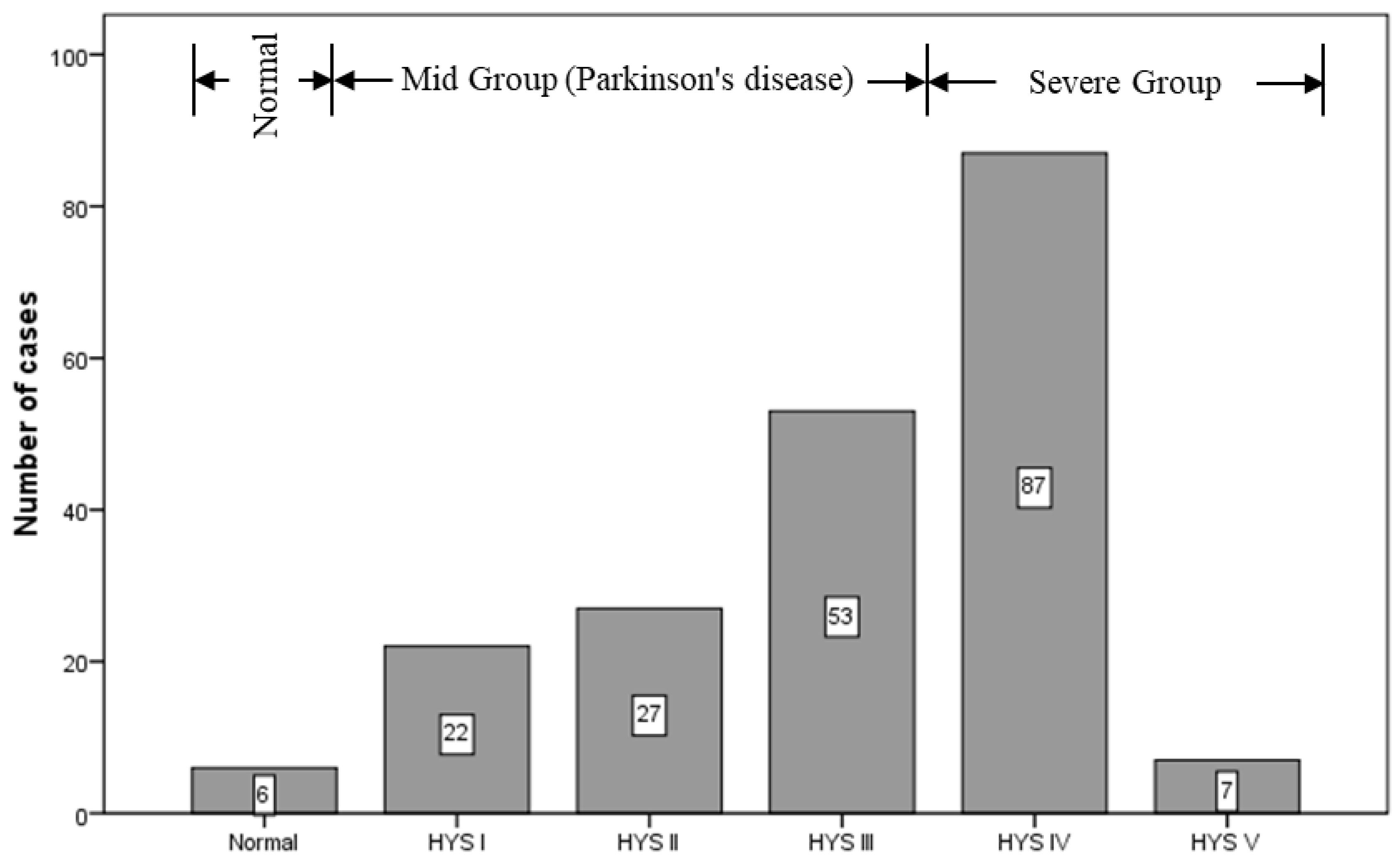
2.1. Subjects
2.2. Imaging Instrument and Criteria
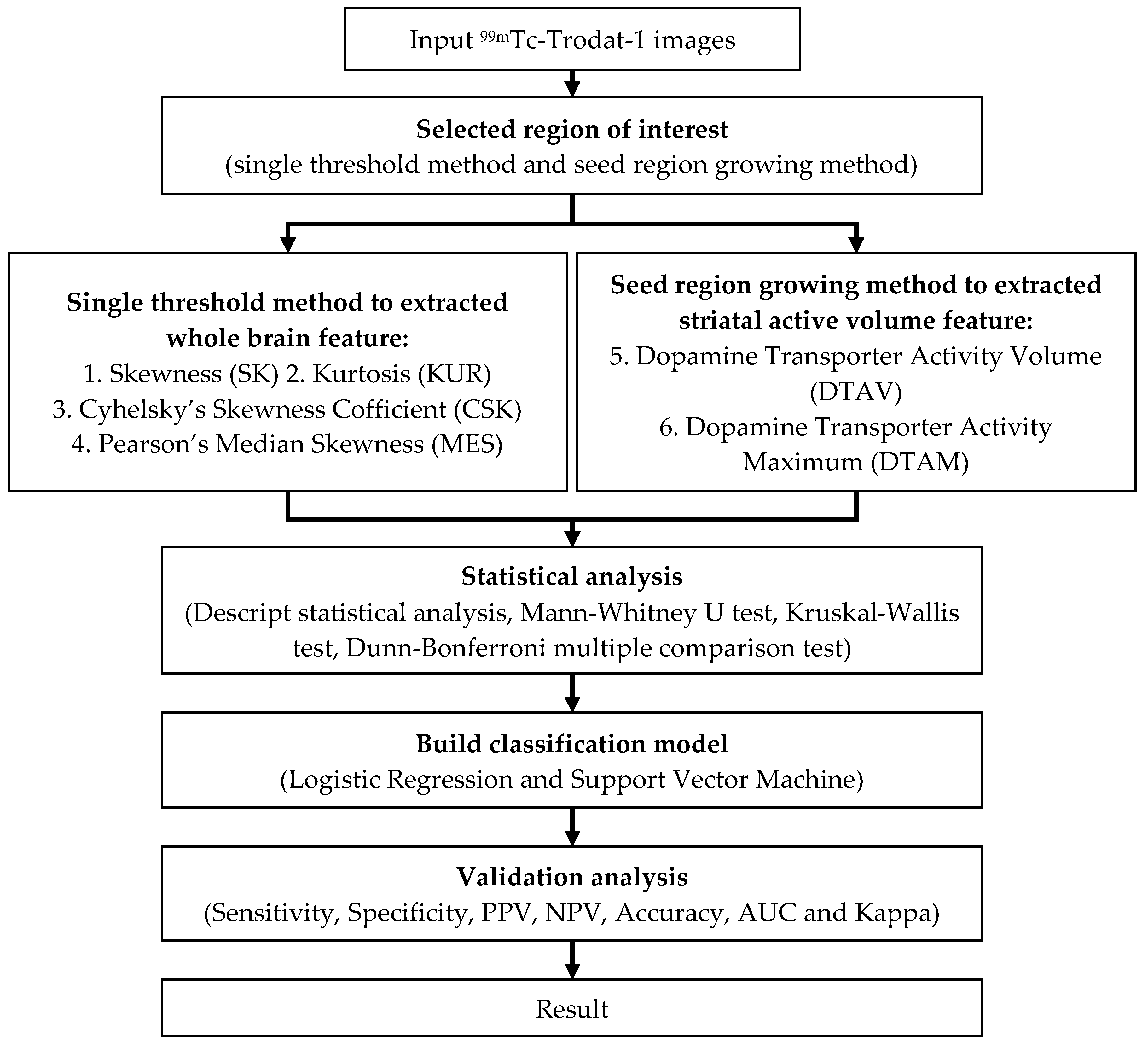
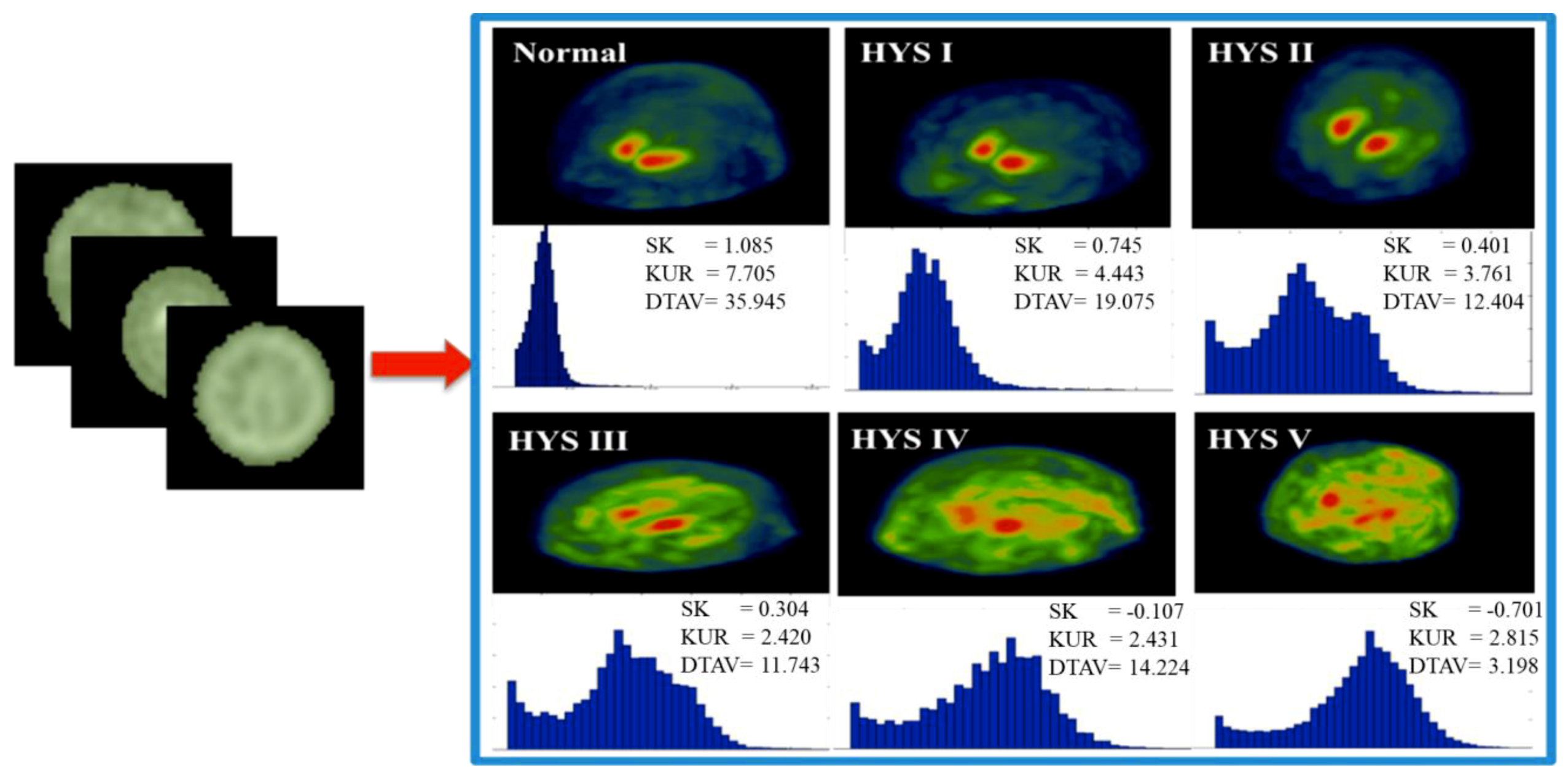
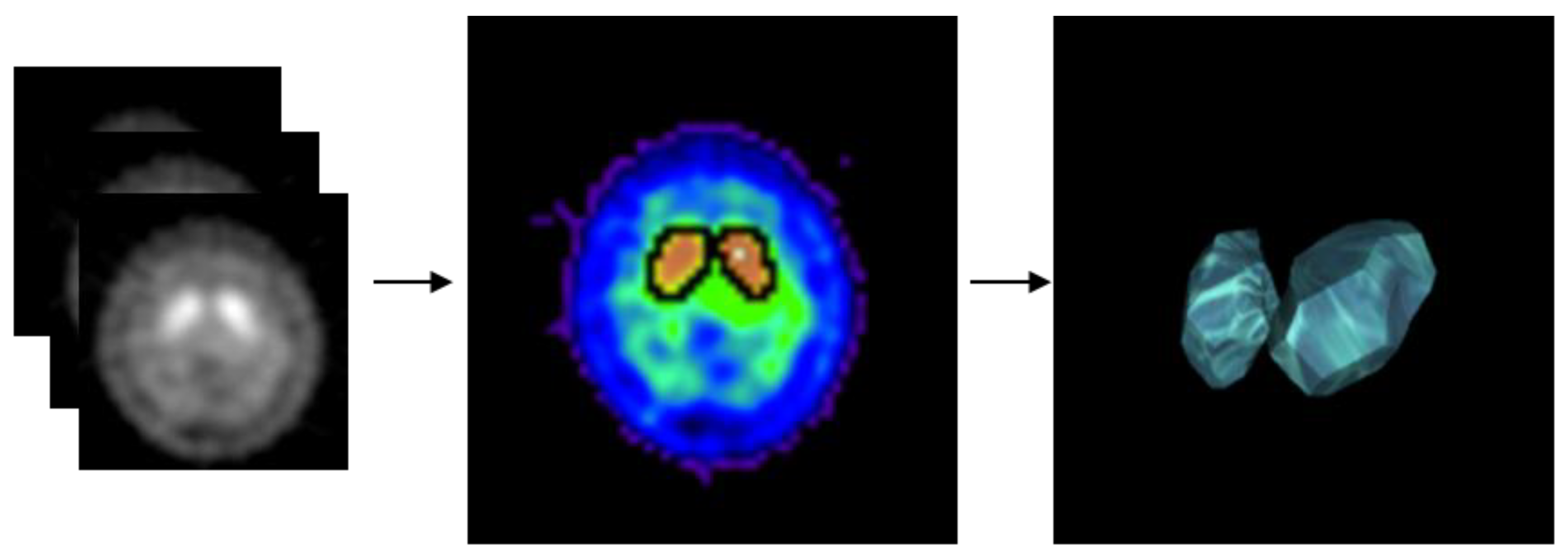
2.3. Experimental Design
2.4. Classification Implementation
3. Results
4. Conclusions
5. Research Limitations and Future Work
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vaughan, R.A.; Foster, J.D. Mechanisms of Dopamine Transporter Regulation in Normal and Disease States. Trends Pharmacol. Sci. 2013, 34, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Merchant, B.A.; Madura, J.D. Insights from Molecular Dynamics: The Binding Site of Cocaine in the Dopamine Transporter and Permeation Pathways of Substrates in the Leucine and Dopamine Transporters. J. Mol. Graph. Model. 2012, 38, 1–12. [Google Scholar] [CrossRef]
- Kordower, J.H.; Olanow, C.W.; Dodiya, H.B.; Chu, Y.; Beach, T.G.; Adler, C.H.; Halliday, G.M.; Bartus, R.T. Disease Duration and the Integrity of the Nigrostriatal System in Parkinson’s Disease. Brain 2013, 136, 2419–2431. [Google Scholar] [CrossRef]
- Gelb, D.J.; Oliver, E.; Gilman, S. Diagnostic Criteria for Parkinson Disease. Arch. Neurol. 1999, 56, 33–39. [Google Scholar] [CrossRef]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, Progression, and Mortality. Neurology 1998, 50, 318–318. [Google Scholar] [CrossRef]
- Leverenz, J.B.; Quinn, J.F.; Zabetian, C.; Zhang, J.; Montine, K.S.; Montine, T.J. Cognitive Impairment and Dementia in Patients with Parkinson Disease. Curr. Top. Med. Chem. 2009, 9, 903–912. [Google Scholar] [PubMed]
- Chou, K.L.; Amick, M.M.; Brandt, J.; Camicioli, R.; Frei, K.; Gitelman, D.; Goldman, J.; Growdon, J.; Hurtig, H.I.; Levin, B. A Recommended Scale for Cognitive Screening in Clinical Trials of Parkinson’s Disease. Mov. Disord. 2010, 25, 2501–2507. [Google Scholar] [CrossRef]
- Suwijn, S.R.; van Boheemen, C.J.; de Haan, R.J.; Tissingh, G.; Booij, J.; de Bie, R.M. The Diagnostic Accuracy of Dopamine Transporter Spect Imaging to Detect Nigrostriatal Cell Loss in Patients with Parkinson’s Disease or Clinically Uncertain Parkinsonism: A Systematic Review. Ejnmmi Res. 2015, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Politis, M. Neuroimaging in Parkinson Disease: From Research Setting to Clinical Practice. Nat. Rev. Neurol. 2014, 10, 708. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Q.; Li, H.; Zhang, H. Spect Molecular Imaging in Parkinson’s Disease. BioMed Res. Int. 2012, 2012, 412486. [Google Scholar] [CrossRef]
- Roussakis, A.; Towey, D.; Piccini, P. Longitudinal Single-Photon Emitted Computed Tomography (Spect) Study of Striatal Dopamine Transporter (Dat) Density: Relevance to Levodopa-Induced Dyskinesias in Parkinson’s Disease. J. Neurol. Sci. 2017, 381, 949. [Google Scholar] [CrossRef]
- Prashanth, R.; Roy, S.D.; Mandal, P.K.; Ghosh, S. Automatic Classification and Prediction Models for Early Parkinson’s Disease Diagnosis from Spect Imaging. Expert Syst. Appl. 2014, 41, 3333–3342. [Google Scholar] [CrossRef]
- Oliveira, F.P.; Castelo-Branco, M. Computer-Aided Diagnosis of Parkinson’s Disease Based on [123i] Fp-Cit Spect Binding Potential Images, Using the Voxels-as-Features Approach and Support Vector Machines. J. Neural Eng. 2015, 12, 026008. [Google Scholar] [CrossRef]
- Tinaz, S.; Chow, C.; Kuo, P.H.; Krupinski, E.A.; Blumenfeld, H.; Louis, E.D.; Zubal, G. Semiquantitative Analysis of Dopamine Transporter Scans in Patients with Parkinson Disease. Clin. Nucl. Med. 2018, 43, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Ueda, J.; Yoshimura, H.; Shimizu, K.; Hino, M.; Kohara, N. Combined Visual and Semi-Quantitative Assessment of 123i-Fp-Cit Spect for the Diagnosis of Dopaminergic Neurodegenerative Diseases. Neurol. Sci. 2017, 38, 1187–1191. [Google Scholar] [CrossRef]
- Davidsson, A.; Georgiopoulos, C.; Dizdar, N.; Granerus, G.; Zachrisson, H. Comparison between Visual Assessment of Dopaminergic Degeneration Pattern and Semi-Quantitative Ratio Calculations in Patients with Parkinson’s Disease and Atypical Parkinsonian Syndromes Using Datscan® Spect. Ann. Nucl. Med. 2014, 28, 851–859. [Google Scholar] [CrossRef]
- Sasannezhad, P.; Juibary, A.G.; Sadri, K.; Sadeghi, R.; Sabour, M.; Kakhki, V.R.D.; Alizadeh, H. 99mtc-Trodat-1 Spect Imaging in Early and Late Onset Parkinson’s Disease. Asia Ocean. J. Nucl. Med. Biol. 2017, 5, 114. [Google Scholar] [PubMed]
- Chang, N.-W.T.; Huang, C.-C.; Kung, C.-T.; Wang, H.-C.; Su, Y.-J.; Chang, Y.-T.; Cheng, B.-C.; Su, C.-M.; Hsiao, S.-Y.; Hsu, C.-W. Clinical Factors Associated with the Risks of Depression in Patients with Parkinson’s Disease. Neuropsychiatry 2017, 7, 416–423. [Google Scholar]
- Aguadé-Bruix, S.; Romero-Farina, G.; Candell-Riera, J.; Pizzi, M.N.; García-Dorado, D. Mechanical Dyssynchrony According to Validated Cut-Off Values Using Gated Spect Myocardial Perfusion Imaging. J. Nucl. Cardiol. 2016. [Google Scholar] [CrossRef]
- Newberg, A.B.; Serruya, M.; Gepty, A.; Intenzo, C.; Lewis, T.; Amen, D.; Russell, D.S.; Wintering, N. Clinical Comparison of 99mtc Exametazime and 123i Ioflupane Spect in Patients with Chronic Mild Traumatic Brain Injury. PLoS ONE 2014, 9, e87009. [Google Scholar] [CrossRef] [PubMed]
- Garn, H.; Coronel, C.; Waser, M.; Caravias, G.; Ransmayr, G. Differential Diagnosis between Patients with Probable Alzheimer’s Disease, Parkinson’s Disease Dementia, or Dementia with Lewy Bodies and Frontotemporal Dementia, Behavioral Variant, Using Quantitative Electroencephalographic Features. J. Neural Transm. 2017, 124, 569–581. [Google Scholar] [CrossRef]
- Górriz, J.; Ramírez, J.; Suckling, J.; Illán, I.A.; Ortiz, A.; Martinez-Murcia, F.; Segovia, F.; Salas-González, D.; Wang, S. Case-Based Statistical Learning: A Non-Parametric Implementation with a Conditional-Error Rate Svm. IEEE Access 2017, 5, 11468–11478. [Google Scholar] [CrossRef]
- Lahmiri, S.; Dawson, D.A.; Shmuel, A. Performance of Machine Learning Methods in Diagnosing Parkinson’s Disease Based on Dysphonia Measures. Biomed. Eng. Lett. 2018, 8, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Alvi, R.; Miller, E.J.; Zonouz, T.H.; Sandoval, V.; Tariq, N.; Lampert, R.; Sinusas, A.J.; Liu, Y.-H. Quantification and Determination of Normal 123i-Meta Iodobenzylguanidine Heart-to-Mediastinum Ratio (HMR) from Cardiac SPECT/CT and Correlation with Planar HMR. J. Nucl. Med. 2018, 59, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.A.; Mini, M. Pet Image Segmentation Based on Gabor Annulus Filtering and Region Growing. Int. J. Comput. Theory Eng. 2016, 8, 409. [Google Scholar] [CrossRef]
| Features | Formula | Location |
|---|---|---|
| SK | Whole-brain | |
| KUR | Whole-brain | |
| CSK | Whole-brain | |
| MES | Whole-brain | |
| DTAV | striatal | |
| DTAM | striatal |
| Features | Group | Cases | Mean | 95% Confidence Index | Standard Deviation | Minimum | Maximum | Kruskal–Wallis Test (P-value) | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| SK | Healthy | 6 | 0.520 | 0.003 | 1.041 | 0.490 | −0.330 | 1.090 | <0.001 |
| Mild | 102 | 0.050 | −0.009 | 0.108 | 0.290 | −0.550 | 0.750 | ||
| Severe | 94 | −0.210 | −0.263 | −0.157 | 0.260 | −0.870 | 0.380 | ||
| KUR | Healthy | 6 | 4.980 | 3.383 | 6.574 | 1.520 | 3.530 | 7.710 | <0.001 |
| Mild | 102 | 3.270 | 3.155 | 3.375 | 0.560 | 2.390 | 5.040 | ||
| Severe | 94 | 2.670 | 2.602 | 2.736 | 0.330 | 2.100 | 4.050 | ||
| CSK | Healthy | 6 | −0.010 | −0.111 | 0.087 | 0.090 | −0.200 | 0.070 | 0.046 |
| Mild | 102 | −0.050 | −0.068 | −0.034 | 0.090 | −0.260 | 0.130 | ||
| Severe | 94 | −0.070 | −0.091 | −0.058 | 0.080 | −0.260 | 0.110 | ||
| MES | Healthy | 6 | −0.040 | −0.334 | 0.256 | 0.280 | −0.550 | 0.220 | 0.033 |
| Mild | 102 | −0.170 | −0.233 | −0.112 | 0.310 | −0.890 | 0.570 | ||
| Severe | 94 | −0.260 | −0.316 | −0.205 | 0.270 | −0.810 | 0.440 | ||
| DTAV | Healthy | 6 | 33.680 | 26.780 | 40.582 | 6.580 | 24.290 | 40.470 | <0.001 |
| Mild | 102 | 15.850 | 14.940 | 16.753 | 4.630 | 7.390 | 28.940 | ||
| Severe | 94 | 10.090 | 9.178 | 10.993 | 4.430 | 0.720 | 17.640 | ||
| DTAM | Healthy | 6 | 291.330 | 147.010 | 435.660 | 137.530 | 95.000 | 459.000 | <0.001 |
| Mild | 102 | 364.900 | 347.280 | 382.570 | 89.830 | 230.000 | 739.000 | ||
| Severe | 94 | 298.240 | 280.660 | 315.830 | 85.840 | 141.000 | 509.000 | ||
| Feature | Control Group | Compare Group | Dunn–Bonferroni Test (P-value) | Feature | Control Group | Compare Group | Dunn–Bonferroni Test(P-value) |
|---|---|---|---|---|---|---|---|
| SK | Healthy | Mild | 0.170 | MES | Healthy | Mild | 0.704 |
| Severe | 0.001 | Severe | 0.172 | ||||
| Mild | Healthy | 0.170 | Mild | Healthy | 0.704 | ||
| Severe | 0.001 | Severe | 0.106 | ||||
| Severe | Healthy | 0.001 | Severe | Healthy | 0.172 | ||
| Mild | 0.001 | Mild | 0.106 | ||||
| KUR | Healthy | Mild | 0.038 | DTAV | Healthy | Mild | 0.012 |
| Severe | 0.001 | Severe | 0.001 | ||||
| Mild | Healthy | 0.038 | Mild | Healthy | 0.012 | ||
| Severe | 0.001 | Severe | 0.001 | ||||
| Severe | Healthy | 0.001 | Severe | Healthy | 0.001 | ||
| Mild | 0.001 | Mild | 0.001 | ||||
| CSK | Healthy | Mild | 0.553 | DTAM | Healthy | Mild | 0.596 |
| Severe | 0.153 | Severe | 0.999 | ||||
| Mild | Healthy | 0.553 | Mild | Healthy | 0.596 | ||
| Severe | 0.193 | Severe | 0.001 | ||||
| Severe | Healthy | 0.153 | Severe | Healthy | 0.999 | ||
| Mild | 0.193 | Mild | 0.001 |
| Item | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 |
|---|---|---|---|---|---|
| Variable | SK | KUR | DTAV | SK, KUR | SK, KUR, DTAV |
| Name | FAV | FAD | FADV |
| Validation | LR | SVM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SK | KUR | FAV | FAD | FADV | SK | KUR | FAV | FAD | FADV | |
| Sensitivity | 0.667 | 0.737 | 0.747 | 0.768 | 0.818 | 0.657 | 0.747 | 0.747 | 0.788 | 0.828 |
| Specificity | 0.500 | 0.500 | 1.000 | 0.500 | 1.000 | 0.500 | 0.500 | 1.000 | 0.500 | 1.000 |
| PPV | 0.660 | 0.730 | 0.747 | 0.760 | 0.827 | 0.650 | 0.755 | 0.747 | 0.780 | 0.837 |
| NPV | 1.000 | 1.000 | 1.000 | 1.000 | 0.667 | 1.000 | 0.333 | 1.000 | 1.000 | 0.667 |
| Accuracy | 0.663 | 0.733 | 0.752 | 0.762 | 0.822 | 0.653 | 0.753 | 0.752 | 0.782 | 0.832 |
| AUC | 0.738 | 0.840 | 0.845 | 0.866 | 0.905 | 0.739 | 0.790 | 0.768 | 0.865 | 0.845 |
| Kappa | 0.344 | 0.482 | 0.527 | 0.539 | 0.661 | 0.326 | 0.509 | 0.523 | 0.578 | 0.680 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, S.-Y.; Lin, H.-C.; Chen, T.-B.; Du, W.-C.; Hsu, Y.-H.; Wu, Y.-C.; Tu, P.-W.; Huang, Y.-H.; Chen, H.-Y. Feasible Classified Models for Parkinson Disease from 99mTc-TRODAT-1 SPECT Imaging. Sensors 2019, 19, 1740. https://doi.org/10.3390/s19071740
Hsu S-Y, Lin H-C, Chen T-B, Du W-C, Hsu Y-H, Wu Y-C, Tu P-W, Huang Y-H, Chen H-Y. Feasible Classified Models for Parkinson Disease from 99mTc-TRODAT-1 SPECT Imaging. Sensors. 2019; 19(7):1740. https://doi.org/10.3390/s19071740
Chicago/Turabian StyleHsu, Shih-Yen, Hsin-Chieh Lin, Tai-Been Chen, Wei-Chang Du, Yun-Hsuan Hsu, Yi-Chen Wu, Po-Wei Tu, Yung-Hui Huang, and Huei-Yung Chen. 2019. "Feasible Classified Models for Parkinson Disease from 99mTc-TRODAT-1 SPECT Imaging" Sensors 19, no. 7: 1740. https://doi.org/10.3390/s19071740
APA StyleHsu, S.-Y., Lin, H.-C., Chen, T.-B., Du, W.-C., Hsu, Y.-H., Wu, Y.-C., Tu, P.-W., Huang, Y.-H., & Chen, H.-Y. (2019). Feasible Classified Models for Parkinson Disease from 99mTc-TRODAT-1 SPECT Imaging. Sensors, 19(7), 1740. https://doi.org/10.3390/s19071740





